Abstract
The effect of seven heavy metals on the motility parameter of zebrafish sperm was tested in order to develop an in vitro toxicological test system as an alternative to live animal testing. In vitro test systems are currently preferred in ecotoxicology due to their practical and ethical advantages and fish sperm can be a suitable model. A number of studies had been carried out previously on this topic, but the described methods had not been standardized in numerous aspects (donor species, measured endpoint, etc.). In this study, heavy metals (mercury, arsenic, chromium, zinc, nickel, copper, cadmium) were used as reference toxicants with known toxicity to develop a standardized fish sperm in vitro assay. The tested concentrations were determined based on preliminary range finding tests. The endpoints were progressive motility (PMOT, %), curvilinear velocity (VCL, μm/s), and linearity (LIN, %) measured by a computer-assisted sperm analysis (CASA) system. According to our results, PMOT was the most sensitive of the three investigated parameters: dose-response curves were observed for each metal at relatively low concentrations. VCL values were less sensitive: higher concentrations were needed to observe changes. Of the three parameters, LIN was the least affected: dose-response relationship was observed only in the case of mercury (e.g., lowest observed effect concentration (LOEC) of Hg at 120 min: 1 mg/L for PMOT, 2.5 mg/L for VCL, 5 mg/L for LIN; LOEC of Cu at 120 min: 1 mg/L for PMOT, 5 mg/L for VCL, any for LIN). The order of toxicity as determined by PMOT was as follows: Hg2+ > As3+ > Cd2+ > Cu2+ > Zn2+ > Cr3+ > Ni2+. In conclusion, we found that PMOT of zebrafish sperm was an accurate and fast bioindicator of heavy metal load. Sperm analysis can be adopted to estimate the possible toxic effects of various chemicals in vitro. Future investigations should concentrate on the applicability of this assay to other contaminants (e.g., organic pollutants).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish species are among the most important model organisms in ecotoxicology. They are particularly vulnerable as they are exposed to pollutants throught multiple pathways including immersion where the entire surface of the body is in contact with the contaminant (Kime 1999). In aquatic toxicology, in vitro test systems are currently widely used due to their practical and ethical advantages over in vivo tests. For these experiments, mainly primary and permanent fish cell lines and tissues are used (Segner 1998; Baron et al. 2012; Abdul Majeed et al. 2013; Beitel et al. 2014; Grunow et al. 2015; Maunder et al. 2017; Mayer et al. 2017). Primary fish cells have one important advantage over permanent fish cell cultures: the cells can be collected freshly from the fish and maintenance of special cell lines is not necessary. Therefore, fish spermatozoa are suitable for in vitro tests as primary cells. In this case, we can disregard the fact that spermatozoa are gametes whose main function is to fertilize the egg. Sperm can be identified as a simple cell type that has measurable parameters such as motility or velocity of movement, which in turn can respond to the effects of toxicants in a dose-response manner (Chyb et al. 2000, 2001a, b; Hatef et al. 2010; Gazo et al. 2013). These parameters make fish sperm a suitable in vitro toxicological model.
Several studies have been published on the use of fish sperm as a tool for ecotoxicological assays; however, the described methods vary in many aspects, e.g., in the species of sperm donor fish. In most cases, large-bodied species had been used due to the larger volume of individually produced sperm, including sturgeon (Acipenser spp.; Li et al. 2010a; Dietrich et al. 2012), African catfish (Clarias gariepinus; Ebrahimi 2007, Kime et al. 1996), rainbow trout (Oncorhynchus mykiss; Dietrich et al. 2010), and common carp (Cyprinus carpio; Sarosiek et al. 2009; Li et al. 2010b). Zebrafish (Danio rerio) are specified in the Organisation for Economic Co-operation and Development (OECD) guidelines as a feasible model species in ecotoxicological studies; however, zebrafish sperm has not been reported as biological model to date. Zebrafish have many advantages compared to large-bodied species: easy maintenance in recirculation systems, year-round spawning, and sperm with constant quality can be collected without hormonal induction.
Another important difference among the published tests using fish sperm are the endpoints that were measured. Antioxidant responses (Li et al. 2010a, b) and enzyme activities (Ebrahimi 2007; Sarosiek et al. 2009; Li et al. 2010b) were studied in most cases. However, sperm motility has measurable parameters (progressive motility, velocity, linearity), which can be detected objectively and quickly and the measured parameters can provide direct information regarding the toxicity of the tested substance. Furthermore, these endpoints are biologically relevant as they are indicators of potentially reduced fertility. These variables had only been analyzed in a few studies under varying conditions; thus, their results are difficult to compare (Kime et al. 2001). Some studies have been carried out on cryopreserved fish sperm (Fabbrocini et al. 2012, 2016), while others were done on fresh sperm before cryopreservation (Dietrich et al. 2012). Cryopreservation itself can reduce the motility parameters of sperm; thus, not only the effect of pollutants is manifested in the results. In the case of zebrafish, sperm motility was measured in a few toxicological tests, but in these cases, the fish were treated in vivo (McAllister and Kime 2003; Thresher et al. 2011); therefore, the toxic effect was expressed in the sperm cells indirectly.
Sea urchin sperm cell toxicity tests have been widely used (Volpi Ghirardini and Arizzi Novelli 2001; Lera et al. 2006). This method has been standardized in all details, making the results comparable. Based on these studies, it is known that short exposure of sperm to heavy metals can cause damages to the developing embryos (Arizzi Novelli et al. 2003). During the fertilization process with exposed sperm, eggs also interact with toxic substances for approx. 20 min, and impairment in development can also be caused by the exposure of eggs. Information regarding the direct effects of exposure on sperm quality is available from some trials (Au et al. 2000a; Fabbrocini et al. 2010; D’Adamo et al. 2014). In these experiments, a strong correlation between sperm motility parameters and fertilization success was found. Because of this, changes in sperm motility measured by a CASA system could be used to predict reproductive success as an ecologically relevant effect. In addition, motility measurements are faster, easier to perform, and more objective than the evaluation of abnormalities in embryos. Furthermore, this endpoint does not depend on egg quality and availability (Fabbrocini et al. 2010).
Heavy metals are very important toxicants in the aquatic environment due to their continuous release through anthropogenic activities (processing industry, agriculture, manufacture, mining, smelting activities, etc.). Metals are not biologically degradable, and therefore, they can be accumulated in organs and tissues of living organisms. Aquatic species are particularly sensitive to the exposure to heavy metals and this sensitivity varies according to the type of the element. The 96-h LC50 values of different heavy metals to zebrafish are as follows (in order of the toxicity): 0.14 mg/L of Hg2+, 0.17 mg/L of Cu2+, 6.5 mg/L of Cd2+ (Wang et al. 2013), 6.75 mg/L of As3+ (Bhavani and Karuppasamy 2014), 9.07–18.92 mg/L of Ni2+ (Alsop and Wood 2011), 44.48 mg/L of Zn2+ (Wang et al. 2013), and 54.57 mg/L of Cr3+ (Nisha et al. 2016). Furthermore, there are several reports emphasizing the harmful effects of heavy metals also on the sperm quality of various fish species: lowest observed effect concentration (LOEC) of Cd was 50 mg/L for motility, VCL, and VSL at 24 and 48 h in common carp; it was 0.05 mg/L for velocity at 2 h in African catfish; LOEC of Hg was 0.1 mg/L for motility, VCL, and VSL at 5 min in European seabass (Abascal et al. 2007; Li et al. 2010a, Dietrich et al. 2011) as well as in the sea urchin (Young and Nelson 1974, Au et al. 2000a, b, Arizzi Novelli et al. 2003, Lera et al. 2006).
Consequently, due to the lack of fast, feasible standard procedures, the objective of our study was to develop an in vitro toxicological test system based on zebrafish sperm analysis. Progressive motility (PMOT), curvilinear velocity (VCL), and linearity (LIN) of sperm were assessed by a computer-assisted sperm analysis (CASA) system in order to determine the most suitable parameters for toxicological evaluations. Heavy metals were used as reference toxicants with known toxicity values.
Materials and methods
Animals
All experiments were carried out in the zebrafish holding facility of the Department of Aquaculture, Szent István University (Gödöllő, Hungary). Mature, wild-type zebrafish males of the AB line were used for the experiments. The fish were kept in 3-L polycarbonate tanks, in a Tecniplast ZebTec (Buguggiate, Italy) recirculating zebrafish housing system, under constant water quality parameters (25 ± 2 °C; pH 7.0 ± 0.2; conductivity 525 ± 50 μS; alkalinity < MDL, 0 mM CO32−, 0.4 mM HCO32−; hardness < 0.5° dH; DOC > 90%; from here onwards referred to as system water) and photoperiod (14-h light:10-h dark cycle). Zebrafish were fed two times a day with Zebrafeed by Sparos (Olhão, Portugal) supplemented with live Artemia salina nauplii every second day.
Sperm collection
Sperm was collected from the males by stripping. Before stripping, fish were anesthetized in 0.06% MS-222 buffered to pH 7.0 and rinsed in system water to prevent contamination of sperm with the anesthetic solution (Bromage 1992). Fish were fixed in a wet sponge with the abdomen facing upwards, then gentle pressure was applied to the abdominal wall by a forceps. The outflowing sperm was collected by a calibrated glass capillary to evaluate its quantity. The sperm was pooled directly into 50 μL of cyprinid immobilizing solution (200 mM KCl, 30 mM Tris, pH 8; Saad and Billard 1987) until a total of 10 μL of sperm was collected in each pool. This volume was collected from 10 ± 2 males containing 26 ± 8 × 106 spermatozoa/pool. The samples were stored on crushed ice until use. After stripping, fish were placed back into system water. Fish were stripped at a maximum of once a week.
Chemicals
Seven heavy metals were tested with the following: chromium (III) nitrate nonahydrate [Cr(NO3)3 × 9 H2O], zinc nitrate hexahydrate [Zn(NO3)2 × 6 H2O], copper nitrate trihydrate [Cu(NO3)2 × 3 H2O], nickel nitrate hexahydrate [Ni(NO3)2 × 6 H2O], cadmium nitrate tetrahydrate [Cd(NO3)2 × 4 H2O], mercury (II) nitrate monohydrate [Hg(NO3)2 × H2O], and arsenic trioxide [As2O3] (each obtained from Sigma Aldrich, St. Louis, USA). Each stock solution was prepared by dissolving the compounds in cyprinid immobilizing solution. In the case of arsenic trioxide, sonication was required to dissolve the compound completely (Branson Digital Sonifier® Models 250, 40% amplitude, 4 × 4 min). The stock solutions were stored at − 80 °C up to 7 days. The final solutions from stocks were prepared immediately prior to the experiments. Exposure concentrations refer to immediate concentrations of heavy metal ions.
Sperm dilution and exposure
Of the sperm from the pooled samples, 10 μL was exposed to 10 μL of double-concentrated heavy metal solution in order to reach the final concentration of the substance following dilution. Consequently, each test solution contained 4 ± 1 × 106 sperm cells which were sufficient for an exact analysis of motility parameters. Four concentrations of each heavy metal were tested in duplicates on sperm from the same pool. The applied concentrations were defined based on preliminary range finding tests. These were as follows: 0.5, 1, 2.5, and 5 mg/L in the case of Hg2+; 1, 5, 25, and 50 mg/L in the cases of Cu2+ and Cd2+; 50, 100, 150, and 200 mg/L in the cases of Cr3+, Zn2+, and As3+; and 600, 800, 1000, and 1200 mg/L in the case of Ni2+. In parallel, a control group from the same pool was exposed only to cyprinid immobilizing solution at the same dilution ratio. All experiments were carried out using five independently collected pools of sperm; thus, for statistical analysis, the number of independent samples was N = 5. Exposure duration was 4 h. Samples were stored on crushed ice during the exposure.
Assessment of sperm concentration and motility parameters
Sperm concentration was determined using a Bürker-Türk-type hemocytometer. Sperm quality was evaluated using a microscope (Olympus BX 41) with a negative-phase contrast objective (× 20 magnification) and connected to a computer-assisted sperm analysis (CASA) system (Sperm VisionTM v. 3.7.4., Minitube of America, Venture Court Verona, USA). Progressive sperm motility (PMOT, the percentage of cells performing forward movement, %), curvilinear velocity (VCL, time-averaged velocity of a sperm head along its actual curvilinear path, μm/s), and linearity (LIN, the linearity of a curvilinear path, %) were measured immediately after stripping to evaluate the fresh sperm quality and every 30 min during the exposure. Definitions of PMOT, VCL, and LIN were used in accordance with the WHO laboratory manual for the examination and processing of human semen (WHO 2010).
CASA was used with the following settings: (1) threshold limit of the PMOT: straight-line distance between the initial and the end point of moving DSL > 5 μm; (2) pixel/μm ratio: 151/100; (3) size of head area 1–100 μm2. Spermatozoa were activated in a Makler chamber (SEFI Medical Instruments, Haifa, Israel). System water was used for the activation at a ratio of 5:1 (v/v) in the case of fresh-pooled sperm and at 2.5:1 in the case of further diluted, exposed sperm and its controls. The system water was supplemented with bovine serum albumin (BSA) to prevent cells from sticking to the Makler chamber (Harrison et al. 1982). At least two activations were carried out in the case of each sample at each measuring point (thus, each measurement was conducted in duplicate).
Statistical analysis
The number of tested pools was regarded as the number of independent samples (N = 5) at each measurement point. The results were evaluated by GraphPad Prism 5.0 and R statistical softwares. Two-way ANOVA with Bonferroni’s post hoc test was used to determine which concentrations had a major effect on the PMOT, VCL, and LIN values of the sperm at different measuring points in time. The significance level was P = 0.05 in each case. Homogeneity of variances and normality of distribution was verified prior to the test. At those points of time, when the values of measured variables reduced beyond the half of the control’s, median effective concentrations (EC50 values) with standard deviation were calculated by fitting dose-response curves (Ritz et al. 2015). A two-sample t test was used to compare the mean PMOT, VCL, and LIN values in the control between the 30th and 240th minute of exposure.
Results
Progressive motility
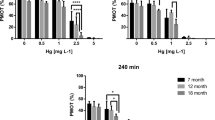
The average PMOT value of all fresh-pooled samples of sperm was 76 ± 6% (n = 35). PMOT of the control samples decreased significantly between the 30th and the 240th minute of exposure in all pools (P = 0.0001–0.0357 depending on the pool, n = 5 in each pool). In general, a significant main effect of both the time of exposure and the concentration of heavy metals was observed (P < 0.0001 for all seven heavy metals). In addition, a significant interaction (P < 0.0001) was also observed among the main effects for all tested toxicants. Dose-response effects were observed following each treatment, although the sensitivity of zebrafish sperm to various toxicants varied with the period of exposure (Fig. 1). In the case of Zn2+, Cu2+, and Cd2+, there was no effect on PMOT after 30 min of exposure when compared to the control; however, a significant reduction of PMOT was observed at 240 min, which was particularly apparent at the two highest concentrations of Cu2+ and Cd2+. In the case of Hg2+ and As3+, a significant decrease was already observed after 30 min of exposure in comparison with the control, with the reduction becoming even more significant (P < 0.001) after 240 min. Concerning Cr3+ and Ni2+, toxic effects were also observed immediately already after 30 min of exposure; however, the measured PMOT values decreased only slightly until the 240th minute and the dose-dependent pattern of PMOT reduction was preserved during the entire exposure period.
Average progressive motility (PMOT) with SD (in percentage, n = 5) of zebrafish sperm exposed to various heavy metal concentrations recorded at every 30th minute of exposure. Significant differences compared to the control value are labeled with an asterisk (*) at *p < 0.05, **p < 0.01, and ***p < 0.001
Curvilinear velocity
Average VCL of all fresh-pooled samples was 77 ± 9 μm/s (calculated from all pools involved in all experiments in this study (n = 35)). VCL changed significantly in the control between 30 and 240 min of exposure only in three experiments (those involving As3+, Cd2+, Cu2+; P = 0.0066–0.0398, n = 5) while no significant change was observed in the tests of the other four heavy metals. A significant main effect of exposure time (P = 0.0019 for Ni2+, P < 0.0001 for all other heavy metals) and toxicant concentration (P = 0.0055 for Zn2+ and P < 0.0001 for all other tested heavy metals) was observed on the VCL of zebrafish sperm. Also, a significant interaction was found (P < 0.0001) in the case of all heavy metals except Cr3+ and Ni2+. Thus, a dose-response in VCL was observed for all tested heavy metals (Fig. 2). In the case of Zn2+ and Cd2, similarly to the PMOT values, the toxic effect did not appear after 30 min of exposure; however, after 240 min, a significant decrease was observed compared to the control. In contrast to these two, a significant reduction in VCL already had manifested after 30 min in all other heavy metal treatment groups and the 4-h exposure showed a dose-dependent reduction of VCL regardless of exposure time. A notable exception was the case of Ni2+ where the dose-dependent initial effect had been leveled by the end of the exposure period with the highest concentrations showing the lowest reduction of VCL values after 240 min in comparison to those at 30 min.
Linearity of spermatozoa
Average LIN of all fresh-pooled samples was 78 ± 4% (calculated from all pools involved in all experiments in this study (n = 35)). No significant change was observed in the LIN of the controls between 30 and 240 min of exposure in any of the conducted experiments. A significant main effect of exposure time was observed in the case of As3+ (P = 0.0163), Cr3+ (P = 0.0209), Zn2+ (P = 0.0085), Cu2+, and Hg2+ (P < 0.0001 for both). The concentration of heavy metals As3+ (P = 0.0192), Cr3+, Cu2+, and Hg2+ (P < 0.0001 for all three) also had a significant main effect on LIN values. In addition, significant interaction of the tested variables was observed for Cr3+ (P = 0.0054) Cd2+, Cu2+, and Hg2+ (P < 0.0001 for all three). Regardless of the observed significant main effects, LIN was not adequate for the estimation of toxic effects of the tested substances: a clear dose-response was found only in the case of Hg2+ starting from the 90th minute of exposure (Fig. 3).
In the case of Cu2+, As3+, Zn2+, and Cr3+, changes in LIN values were observed only at the highest concentrations. Regarding Cu2+, Zn2+, and As3+, these changes were apparent only later during the exposure occurring only at 240 min for Cu2+ and Zn2+ and at 210 min for As3+. Cr3+ was a notable exception as at the highest concentration of 200 mg/L, LIN has decreased significantly in comparison to the control at 30 min of exposure and then in the absence of progressively moving spermatozoa, it reached zero at 60 min. Additionally, no effects of Cd2+ and Ni2+ on LIN were observed.
Comparison of median effective concentrations on progressive motility and curvilinear velocity
Effective concentrations of each heavy metal for PMOT (Table 1) displayed a clear decrease with exposure duration as shown by EC50 values. In the case of Cr3+, Ni2+, and Hg2+, PMOT values were reduced to half of those of the control already after 30 min of exposure, and in the case of As3+, this degree of reduction was also observed after 60 min. In the case of Cu2+ and Cd2+, a 90-min exposure was needed to reduce PMOT to 50% of the control values. In the case of Zn2+, EC50 values were calculated only from data obtained after 180 min. Due to the lowest variation, EC50 values at 240-min exposure were used to compare among the measured parameters. The EC50 values ranged from 0.9 ± 0.1 mg/L (Hg2+) to 592 ± 116 mg/L (Ni2+) for the PMOT values; the order of toxicity expressed as PMOT was Hg2+ > As3+ > Cd2+ > Cu2+ > Zn2+ > Cr3+ > Ni2+.
In the case of VCL, too, effective concentrations of each tested heavy metal could be calculated, which showed an evident reduction parallel to the exposure duration (Table 2). However, higher concentrations of heavy metals did cause a 50% reduction in VCL compared to the PMOT control. Furthermore, in the case of Zn2+ and Ni2+, there was only one measuring point of time where EC50 value could be calculated (240 and 120 min of exposures, respectively). The EC50 values ranged from 1.3 ± 0.2 mg/L (Hg2+) to around 500 mg/L (Ni2+); the order of toxicity expressed as VCL was Hg2+ > Cd2+ > Cu2+ > As3+ > Cr3+ > Zn2+ > Ni2+.
This order could not be determined for LIN due to the lack of dose-response in any of the investigated cases (except for Hg2+).
Discussion
During the experiments, our hypothesis that exposure of heavy metals trigger a dose-response in motility parameters of zebrafish sperm and the effective concentrations reduce in parallel with exposure duration was found to be correct. Damages to spermatozoa as a result of in vitro exposure to heavy metals had previously been described in some fish species. Heavy metals have been shown to affect the physiology of spermatozoa and, therefore, caused decreases in fertilization success in a variety of ways: (1) injuring mitochondria (Au et al. 2000b); (2) affecting water channels of plasma membrane (Abascal et al. 2007); (3) displacing Ca2+-ions (Dietrich et al. 2011); and (4) inducing oxidative stress (Li et al. 2010a).
Considering the experimental conditions, the exposure of samples on melting ice was reasonable, which had also been described by different authors; higher temperature increased the intensity of sperm motility, however, decreased its duration in various fish species (Kime et al. 2001; Wilson-Leedy and Ingermann 2007; Jing et al. 2009). Consequently, fish sperm preserves its motility for a longer time at lower temperatures; thus, exposure of sperm on melting ice is recommended. Accordingly, during the in vitro examination of toxicants on fish sperm motility parameters, sperm had been incubated at low temperature in most cases (Kime et al. 1996; Dietrich et al. 2010; Li et al. 2010a, b). Also, sea urchin sperm had been stored and chilled after the collection in some studies (Young and Nelson 1974; Fabbrocini et al. 2010). The temperature of exposure played a minor effect in the toxicity of the tested heavy metals as clear dose-response on PMOT values was observed in all cases. Another experimental condition to consider was the duration of sperm storage prior to exposure. In our experiments, motility parameters of the control decreased significantly during the 4-h exposure in most cases; thus, it is advisable to initiate the experiment after the stripping as soon as possible (within 30 min).
During the experiments, experimental variation (expressed as SD) was reduced parallel to exposure time, and consequently, more reliable results have been observed at the end of the exposure. Thus, a minimum of 4-h exposure is recommended for an exact analysis to evaluate the effects of toxicants on sperm motility parameters. Another factor that supports a minimum of 4-h exposure is that for most chemicals, clear dose-response was not observed at shorter exposure times. While there was a significant interaction of the tested variables on PMOT values in all experiments and on VCL values on most of them, this interaction can be considered trivial. Prolonged exposure and increasing concentrations of heavy metals had a joint effect on motility parameters and they strengthened each other. Of the three investigated motility parameters, PMOT was the most sensitive: low concentrations were required to generate changes in this value. VCL was less sensitive for each of the given test substances than PMOT: higher concentrations were needed to observe changes. LIN was the least sensitive parameter in comparison to the changes in PMOT and VCL values: changes were not found in each of the tested heavy metals. Our results are in contrast with those of the cryopreserved sea bream sperm test (Fabbrocini et al. 2016), where the total motile percentage of the cells was the least susceptible variable to the toxic effects of the tested contaminants (in this case, a dumpsite leachate sample). Moreover, our results are also in contrast with those of Dietrich et al. (2010), where LIN of rainbow trout sperm was more sensitive to exposure to mercury, than motility or VCL values. Exposure to 1 mg/L Hg2+ did not cause any changes in motility and VCL, and LIN decreased to half of the control value immediately after exposure; however, this toxic effect was reduced after the 4-h exposure (Dietrich et al. 2010). In our study, 1 mg/L of Hg2+ caused significant decrease in motility and also in VCL during the 4-h exposure; yet, there were no changes in LIN values at this concentration. In the case of cadmium, our observations match the results of the abovementioned authors: motility was the most sensitive of the three parameters and 10 mg/L of Cd2+ reduced the PMOT value significantly during the 4-h exposure, with effective concentrations in the same range, as in our study. Exposure of fresh Siberian sturgeon sperm to 1 mg/L of Hg2+ significantly decreased its motility after cryopreservation and thawing (Dietrich et al. 2012). In the case of cadmium, 10 mg/L was needed to induce the same effect. This also means that exposed sperm has lower tolerance to the stress caused by cryopreservation and thawing. The authors found motility to be more a sensitive indicator than VCL for both heavy metals. In the case of catfish sperm, 100 mg/L cadmium and 2000 mg/L zinc had a significant effect on motility parameters after a 24-h exposure (Kime et al. 1996); yet, no information was provided regarding dose-response and the effect of exposure duration. Contrary to our results, 0.05 mg/L of Cd2+ and 5 mg/L of Cr3+ already led to a significant decrease in velocity of sterlet sperm after a 2-h exposure, with no changes in motility (Li et al. 2010a). In our experiments, 5 mg/L of cadmium and 100 mg/L of chromium were needed to show a reduction in the PMOT values significantly after 2 h and even higher concentrations were required to induce changes in VCL. According to these observations and the LC50 values of the investigated heavy metals in zebrafish, it can be concluded that with the exception of sterlet, zebrafish and its sperm display similar sensitivity to the exposure to heavy metals as that of other species.
Comparing our results to the sea urchin sperm cell test (Arizzi Novelli et al. 2003), where the EC50 values ranged from 0.017 to 8.4 mg/L and the order of toxicity expressed in development of embryos was Hg2+ > Cu2+ > Zn2+ > Cr3+ > As3+ > Ni2+ > Cd2+, in our experiments, higher concentrations of heavy metals were needed to affect sperm motility parameters. A possible reason for this is that only sperm cells were in contact with the exposure media in our study, unlike in the sea urchin sperm cell tests, where beside sperm, embryos were also exposed to the toxic substances during fertilization. However, fertilization tests with exposed sperm are required to validate this assumption in the future. Moreover, regardless of the differences in the order of toxicity, mercury was found to be the most toxic substance in both studies.
In this study, EC50 values of heavy metals on PMOT were higher than the LC50 values reported for these toxicants in zebrafish; however, the comparison of these two values will lead to inaccurate conclusions. LC50 is normally calculated from the results of a 96-h exposure test according to the OECD guideline 203, whereas sperm requires a significantly shorter exposure time to provide dose-response. In addition, measurement of sperm motility as an in vitro test has several advantages over the in vivo tests that result in LC50 values. In vivo tests need to be conducted on live fish that ultimately result in their death—indeed, LC50 can only be calculated if mortality is observed. Sperm, on the other hand, can be collected from live individuals.
Arguably, the exposure times used in this study are unrealistic as normally fish sperm is not exposed to the effects of toxicants in vitro prior to fertilization. The same, however, is true for the sperm of sea urchin which is used in a standard test. In addition, our results show that in vitro exposure of fish sperm results in a dose-related decrease of motility parameters which in turn can have an effect on their fertilizing capacity. The use of motility measurements allows the replacement of fertilization tests without compromising the sensitivity of the given test.
The relevance of our observations is that a clear dose-response was observed when zebrafish sperm was exposed to various heavy metals which increased with the duration of exposure. The findings are generally in line with those observed in other fish species (Dietrich et al. 2010, 2012; Kime et al. 1996), even if differences were observed in the measured parameters and experimental design. The time-dependent dose-response observed in this study allows further studies to concentrate on the effect of other toxic substances (such as organic contaminants) on the sperm of zebrafish, a common laboratory model.
Conclusions
Zebrafish is an appropriate donor for the spermatotoxicological experiments. The progressive motility of zebrafish sperm has proved to be a rapid and accurate bioindicator of aquatic pollution compared to curvilinear velocity and linearity; dose-response was observed on this parameter affected by each of the tested heavy metals. Thus, a zebrafish sperm test can be developed to estimate the possible toxic effects of chemicals in vitro. Thereby, it is not necessary to carry out preliminary tests on living animals; furthermore, it can reduce the release of dangerous waste, which fits into the replacement, reduction, and refinement (3R) principles of the EU.
In this work, a simple in vitro toxicology test system was developed as follows: (1) stripping of 10 μL of sperm pool from different males in 50 μL cyprinid immobilizing solution, (2) dilution 10 μL of sperm form the pool with toxicant included immobilizing solution at a dilution ratio of 1:1 to reach the final toxic concentration, (3) storage of the samples on melting ice during the exposure, and (4) measurement of progressive motility with a CASA system after a 4-h exposure to determine the possible toxic effect of the tested solution.
References
Abascal FJ, Cosson J, Fauvel C (2007) Characterization of sperm motility in sea bass: the effect of heavy metals and physicochemical variables on sperm motility. J Fish Biol 70:509A–522A
Abdul Majeed S, Nambi KS, Taju G, Sundar Raj N, Madan N, Sahul Hameed AS (2013) Establishment and characterization of permanent cell line from gill tissue of Labeo rohita (Hamilton) and its application in gene expression and toxicology. Cell Biol Toxicol 29(1):59–73
Alsop D, Wood CM (2011) Metal uptake and acute toxicity in zebrafish: common mechanisms across multiple metals. Aquat Toxicol 105(3–4):385–393
Arizzi Novelli A, Losso C, Ghetti PF, Volpi Ghirardini A (2003) Toxicity of heavy metals using sperm cell and embryo toxicity bioassays with Paracentrotus lividus (Echinodermata: Echinoidea): comparisons with exposure concentrations in the lagoon of Venice, Italy. EnvironToxicol Chem 22(6):1295–1301
Au DWT, Chiang MWL, Tang JYM, Yuen BBH, Wang YL, Wu RSS (2000a) Impairment of sea urchin sperm quality by UV-B radiation: predicting fertilization success from sperm motility. Mar Poll Bull 44:583–589
Au DWT, Chiang MWL, Wu RSS (2000b) Effects of cadmium and phenol on motility and ultrastructure of sea urchin and mussel spermatozoa. Arch Environ Contam Toxicol 38:455–463
Baron MG, Purcell WM, Jackson SK, Owen SF, Jha AN (2012) Towards a more representative in vitro method for fish ecotoxicology: morphological and biochemical characterisation of three-dimensional spheroidal hepatocytes. Ecotoxicology 21(8):2419–2429
Beitel SC, Doering JA, Patterson SE, Hecker M (2014) Assessment of the sensitivity of three North American fish species to disruptors of steroidogenesis using in vitro tissue explants. Aquatic Toxicol 152:273–283
Bhavani K, Karuppasamy R (2014) Acute toxicity bioassay and behavioural changes on zebra fish, Danio rerio (Hamilton) under arsenic trioxide. Int J Mod Res Rev 2(1):40–46
Bromage N (1992) Propagation and stock improvement. In: Shepherd J, Bromage N (eds) Intensive fish farming. Balckwell Scientific Publications, Oxford, pp 103–153
Chyb J, Kime DE, Mikolajczyk T, Szczerbik P, Epler P (2000) Influence of zinc on sperm motility of common carp—a computer assisted studies. Arch Pol Fisheries 8:5–14
Chyb J, Kime DE, Szczerbik P, Mikolajczyk T, Epler P (2001a) Computer assisted analysis (CASA) of common carp Cyprinus carpio L. spermatozoa motility in the presence of cadmium. Arch Pol Fisheries 9:173–181
Chyb J, Sokolowska-Mikolajczyk M, Kime DE, Socha M, Epler P (2001b) Influence of mercury on computer analysed sperm motility of common carp Cyprinus carpio L., in vitro. Arch Pol Fisheries 9:51–60
D’Adamo R, Specchiulli A, Cassin D, Botter M, Zonta R, Fabbrocini A (2014) The effect of floods on sediment contamination in a microtidal coastal lagoon: the lagoon of Lesina, Italy. Arch Environm Contam Toxicol 67:297–309
Dietrich GJ, Dietrich M, Kowalski RK, Dobosz HK, Demianowicz W, Glogowski J (2010) Exposure of rainbow trout milt to mercury and cadmium alters sperm motility parameters and reproductive success. Aquat Toxicol 97:277–284
Dietrich M, Dietrich GJ, Hliwa P, Ciereszko A (2011) Carp transferrin can protect spermatozoa against toxic effects of cadmium ions. Comp Biochem Physiol C 153:422–429
Dietrich GJ, Ciereszko A, Kowalski RK, Rzemieniecki A, Bogdan E, Demianowicz W, Dietrich M, Kujawa R, Glogowski J (2012) Motiliy and fertilizing capacity of frozen/thawed sperm of Siberian sturgeon after a short-time exposure of fresh semen to mercury and cadmium. J Appl Ichthyol 28:973–977
Ebrahimi M (2007) Effects of in vivo and in vitro zinc and cadmium treatment on sperm steroidogenesis of the African catfish Clarias gairepinus. Pak J Biol Sci 10(17):2862–2867
Fabbrocini A, Di Stasio M, D’Adamo R (2010) Computerized sperm motility analysis in toxicity bioassays: a new approach to pore water quality assessment. Ecotoxicol Environ Saf 73:1588–1595
Fabbrocini A, D’Adamo R, Del Prete F, Langellotti AL, Rinna F, Silvestri F, Sorrenti G, Vitiello V, Sansone G (2012) Cryopreserved semen in ecotoxicological bioassays: sensitivity and reliability of cryopreserved Sparus aurata spermatozoa. Ecotoxicol Environ Saf 84:293–298
Fabbrocini A, D’Adamo R, Del Prete F, Maurizio D, Specchiulli A, Oliveira LFJ, Silvestri F, Sansone G (2016) The sperm motility pattern in ecotoxicological tests. The CRYO-Ecotest as a case study. Ecotoxicol Environ Saf 123:53–59
Gazo I, Linhartova P, Shaliutina A, Hulak M (2013) Influence of environmentally relevant concentrations of vinclozolin on quality, DNA integrity, and antioxidant responses of sterlet Acipenser ruthenus spermatozoa. Chem Biol Interact 203:377–385
Grunow B, Mohamet L, Shiels HA (2015) Generating an in vitro 3D cell culture model from zebrafish larvae for heart research. J Exp Biol 218(Pt 8):1116–1121
Harrison RA, Dott HM, Foster GC (1982) Bovine serum albumin, sperm motility, and the “dilution effect”. J Exp Zool 222(1):81–88
Hatef A, Alavi SMH, Linhartova Z, Rodina M, Policar T, Linhart O (2010) In vitro effects of bisphenol a on sperm motility characteristics in Perca fluviatilis L. (Percidae; Teleostei). J Appl Ichthyol 26:696–701
Jing R, Huang C, Bai C, Tanguay R, Dong Q (2009) Optimization of activation, collection, dilution and storage methods for zebrafish sperm. Aquaculture 209:165–171
Kime DE (1999) A strategy for assessing the effects of xenobiotics on fish reproduction. Sci Total Environ 225:3–11
Kime DE, Ebrahimi M, Nysten K, Roelants I, Rurangwa E, Moore HDM, Ollevier F (1996) Use of computer assisted sperm analysis (CASA) for monitoring the effects of pollution of sperm quality of fish; application to the effects of heavy metals. Aquat Toxicol 36:223–237
Kime DE, Van Look KJW, McAllister BG, Huyskens G, Rurangwa E, Ollevier F (2001) Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp Biochem Physiol Part C 130:425–433
Lera S, Macchia S, Pellegrini D (2006) Standardizing the methodology of sperm cell test with Paracentrotus lividus. Environ Monit Assess 122:101–109
Li ZH, Li P, Dzyuba B, Randak T (2010a) Influence of environmental related concentrations of heavy metals on motility parameters and antioxidant responses in sturgeon sperm. Chem Biol Interact 188:473–477
Li ZH, Li P, Rodina M, Randak T (2010b) Effect of human pharmaceutical carbamazepine on the quality parameters and oxidative stress in common carp (Cyprinus carpio L.) spermatozoa. Chemosphere 80:530–534
Maunder RJ, Baron MG, Owen SF, Jha AN (2017) Investigations to extend viability of a rainbow trout primary gill cell culture. Ecotoxicology 26(10):1314–1326
Mayer E, Novak E, Springler A, Schwartz-Zimmermann HE, Nagl V, Reisinger N, Hessenberger S, Schatzmayr G (2017) Effects of deoxynivalenol (DON) and its microbial biotransformation product deepoxy-deoxynivalenol (DOM-1) on a trout, pig, mouse, and human cell line. Micotoxin Res 33(4):297–308
McAllister BG, Kime DE (2003) Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio). Aquat Toxicol 65:309–316
Nisha JC, Raja Jeya Sekar R, Chandran R (2016) Acute effect of chromium toxicity on the behavioral response of zebra fish Danio rerio. Int J Plant Anim Env 6(2):6–14
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS One 10(12):e0146021
Saad A, Billard R (1987) Spermatozoa production and volume of semen collected after hormonal stimulation in the carp, Cyprinus carpio. Aquaculture 65:67–77
Sarosiek B, Pietrusewicz M, Radziwoniuk J, Glogowski J (2009) The effect of copper, zinc, mercury and cadmium on some enzyme activities in the common carp (Cyprinus carpio L.) Reprod Biol 9(3):295–301
Segner H (1998) Fish cell lines as a tool in aquatic toxicology. In: Braunbeck T, Hinton DE, Streit B (eds) Fish Ecotoxicology. Birkhäuser Verlag, Switzerland, pp 1–38
Thresher R, Gurney R, Canning M (2011) Effects of lifetime chemical inhibition of aromatase on the sexual differentiation, sperm characteristics and fertility of medaka (Oryzias latipes) and zebrafish (Danio rerio). Aquat Toxicol 105:355–360
Volpi Ghirardini A, Arizzi Novelli A (2001) A sperm cell toxicity test procedure for the Mediterranean species Paracentrotus Lividus (Echinodermata: Echinoidea). Environ Technol 22(4):439–445
Wang H, Liang Y, Li S, Chang J (2013) Acute toxicity, respiratory reaction, and sensitivity of three cyprinid fish species caused by exposure to four heavy metals. PLoS One 8(6):e65282
WHO (2010) Computer-aided sperm analysis. In: WHO laboratory manual for the examination and processing of human semen—5th ed., World Health Organization 2010, Geneva, pp. 136–141
Wilson-Leedy JG, Ingermann RL (2007) Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 67:661–672
Young LG, Nelson L (1974) The effects of heavy metal ions on the motility of sea urchin spermatozoa. Biol Bull 147:236–246
Funding
This work was supported by the Hungarian Government (Research Centre of Excellence—11476-3/2016/FEKUT) and by the NVKP_16-2016-1-0003: “Risks of some endocrine disruptors and developments for risk mitigation in Budapest Metropolitan Region” project, financed by the NRDI Fund. Árpád Ferincz was supported by the Bolyai János Postdoctoral Fellowship provided by the Hungarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Markus Hecker
Highlights
• Zebrafish sperm is a suitable model for in vitro ecotoxicological tests because of its measurable parameters
• Median effective concentrations (EC50) were calculated for progressive motility (PMOT) and curvilinear velocity (VCL)
• Dose-response was observed in the case of PMOT and VCL for each tested heavy metal
• PMOT is the most sensitive of the investigated parameters
• Linearity (LIN) is the least sensitive parameter: dose-response was observed only in the case of mercury
Rights and permissions
About this article
Cite this article
Kollár, T., Kása, E., Ferincz, Á. et al. Development of an in vitro toxicological test system based on zebrafish (Danio rerio) sperm analysis. Environ Sci Pollut Res 25, 14426–14436 (2018). https://doi.org/10.1007/s11356-018-1613-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1613-2