Abstract
Chemical composition and antifungal activity of essential oils of Algerian Mentha species were studied. Chemical compositions of different Mentha species oils (Mentha rotundifolia, M. spicata, M. pulegium, and M. piperita) were investigated by capillary GC and GC/MS, and their antifungal activities were evaluated by means of paper disc diffusion method and minimum inhibitory concentration (MIC) assays. In total, 98 components from all Mentha species were identified. All oils were rich in monoterpene-oxygenated components. In addition, we reported fumigant antifungal activity of Algerian Mentha essential oils against four fungi: Botrytis cinerea, Penicillium expansum, Monilinia laxa, and M. fructigena. All oils demonstrated very good inhibition especially against B. cinerea, M. laxa, and M. fructigena. Both Monilinia fungi were extremely sensitive to all Algerian Mentha oils, which suggests that Mentha essential oils have the potential to be used as bio-pesticides to protect fruit trees, such as apple and pear trees, and provides an alternative to chemical pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic plants are increasingly used in agro-alimentary storage and bio-agriculture pest control. Essential oils and volatile constituents extracted from these plants are widely used as new bio-control alternative agents against microbial strains and insect pests, because of their specificity of action, biodegradable nature, and potential for commercial application (Kerdchoechuen et al. 2010; Park et al. 2003).
The Taxa of genus Mentha (Lamiaceae family) includes 25 species of herbaceous perennial (rarely annual) plants (Celenk et al. 2008; Fenwick and Ward 2001; Gobert et al. 2002; Khanuja et al. 2000; Krasnyanski et al. 1998; Shasany et al. 2005). Mints are distributed predominantly in the temperate region of the world and show substantial variation in terms of their natural habitats, growth characteristics, and aromas (Celenk et al. 2008; Shasany et al. 2005).
The genus Mentha consists of some of the most frequently cultivated spice plants in the world. Many species of Mentha are used in traditional folk medicine for many of its proprieties. Mentha EOs were known to be rich in oxygenated monoterpenes (Rösch et al. 2002). Depending on the nature of major components, they were used in several commercial and industrial fields. Recently, many works has shown strong activities of Mentha EOs as bio-control agents and possible substitute of traditional chemical fungicides, pesticides, and insecticides (Al-Bayati 2009; Al Yousef 2013; Mahboubi and Haghi 2008; Mohammadi et al. 2015; Odeyemi et al. 2008; Santana-Méridas et al. (2014, 2017); Soković et al. 2009).
In Algeria, the literature reported the occurrence of six species of Mentha genus: M. rotundifolia, M. spicata, M. pulegium, M. piperita, M. longifolia, and M. aquatica, and also three hybrids of these species: M. durandoana, M. niliaca, and M. schultzii (Quezel and Santa 1963). The same reference reported that M. longifolia and M. aquatica were very rare at that time and probably decimated now.
In the context of our characterization of Algerian aromatic plants, we investigated the chemical composition of four species of Mentha: M. rotundifolia, M. spicata, M. pulegium, and M. piperita from western Algeria, by analyzing their essential oils. A comparative analysis was performed between chemical compositions of all species. A chemical analysis was performed using a combination of capillary GC-RI and GC/MS after fractionation using column chromatography. Lastly, we report the bio-control effect for all essential oils against four fungal pathologies (M. fructigina, M. laxa, Penicillium expansum, and Botrytis cinerea) through the use of a fumigant antifungal assay.
Materials and methodologies
Plant material and oil isolation
The aerial parts of all Mentha from western Algeria were collected at the flowering stage (July 2014 to September 2014) from 11 location areas: 4 locations from M. rotundifulia (MRO1–MRO4), 2 locations from M. spicata (MSP1, MSP2), 3 locations from M. pulegium (MPU1-MPU3), and 2 locations from M. piperita (MPI1, MPI2) (Fig. 1). From each location, many samples were collected to poll collective oils from the same species. For all species, voucher specimens were deposited in the Herbarium of the University of Tlemcen. Essential oils were obtained from the fresh aerial parts of all stations by hydrodistillation for 4 h using a Clevenger-type apparatus according to the European Pharmacopoeia (Council of Europe 1997) and yielded (w/w) 0.78–0.96% from M. rotundifulia, 0.50–0.56% from M. spicata, 0.70–0.75% from M. pulegium, and 0.67–0.72% from M. piperita (see Fig. 1).
Gas chromatography
GC analyses were carried out using a Perkin Elmer Clarus 600 GC apparatus (Walhton, MA, USA) equipped with a single injector and two flame ionization detectors (FIDs). The apparatus was used for simultaneous sampling of two fused-silica capillary columns (60 m × 0.22 mm, film thickness 0.25 μm) with different stationary phases: Rtx-1 (polydimethylsiloxane) and Rtx-Wax (polyethylene glycol). Temperature program 60 to 230 °C at 2 °C min−1 and then held isothermal 230 °C (30 min). Carrier gas was hydrogen (0.7 mL min−1). Injector and detector temperatures were held at 280 °C. Split injection was conducted with a split ratio of 1:80. Injected volume was 0.1 μL.
Gas chromatography-mass spectrometry
The oils and the fractions obtained by CC were investigated using a Perkin Elmer TurboMass quadrupole analyzer, directly coupled with a Perkin Elmer Autosystem XL equipped with two fused-silica capillary columns (60 m × 0.22 mm, film thickness 0.25 μm), Rtx-1 (polydimethylsiloxane) and Rtx-Wax (polyethylene glycol). Other GC conditions were the same as described above. Ion source temperature was 150 °C and energy ionization 70 eV; electron ionization mass spectra were acquired with a mass range of 35–350 Da and scan mass of 1 s. Oil injected volume was 0.1 μL and fraction injected volume of 0.2 μL.
Component identification
Identification of the components was based (i) on the comparison of their GC retention indices (RI) on non-polar and polar columns, determined to the retention time of a series of n-alkanes with linear interpolation, with those of authentic compounds for our laboratory library from University of Corsica and literatures data (Jennings and Shibamoto 1980; Konig et al. 2001), and (ii) on computer matching with mass spectral of our laboratory library and commercial mass spectral libraries (McLafferty and Stauffer 1988).
Component quantification
The quantification of the essential oil components was performed using the methodology reported by Bicchi et al. (2008) and adapted in laboratory of UMR CNRS 6134 from University of Corsica (Djabou et al. 2013). Component quantification was carried out using peak normalization, including FID response factors (RFs) relative to tridecane (0.7 g/100 g), used as an internal standard and expressed as normalized % abundance.
Statistical analysis
Data analyses were performed using principal component analysis (PCA) (Brereton 2003). This method aims to reduce the multivariate space in which objects (oil samples) are distributed but are complementary in their ability to present results (Massart 1998). Indeed, PCA provides the data in which both objects (oil samples) and variables (oil major components and/or antibacterial inhibition) are plotted while canonical analysis informs a classification tree in which objects (sample locations) are gathered. PCA was carried out using function “PCA” from the statistical R software (factominR package). The variables (major components and/or antibacterial inhibition) have been selected using function from the statistical software (compounds > 1% were selected for the analysis).
Microbial strains and growth conditions
B. cinerea and P. expansum strains were obtained from Institut Pasteur, Paris, France (IP 1854.89, batch 185489; IP 1405.82, batch 050291 and IP 1350.82, batch 230992). M. laxa and M. fructigena strains were obtained from Centraalbureau voor Schimmelcultures, Utrecht, Netherlands (CBS 127258, batch 127258 and CBS 101499, batch 101499). They were grown on Sabouraud Glucose Agar (Sigma-Aldrich, Saint-Louis, Missouri, USA) and incubated at 23 °C in darkness.
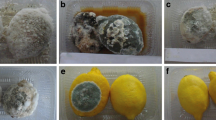
Fumigant antifungal activity
Essential oils were evaluated for fumigant antifungal activity based on their ability to inhibit mycelial growth. Six-millimeter-diameter mycelial plugs of each fungal strain from a 7-day-old culture were placed into a Petri dish. Essential oils were introduced onto a 6-mm cellulose disc, placed on the agar-free lid of the Petri dish. A negative control (cellulose disc without essential oil) was performed in the same way. Petri dishes were then sealed with parafilm and incubated at 23 °C in the dark.
Mycelial radial growth was measured after 3 to 7 days of incubation, and the antifungal index was calculated with the formula as follows:
where Dex is the diameter of growth zone in the experimental plate (mm) and Dc is the diameter of growth zone in the control plate (mm).
After a preliminary assay to determine antifungal index, Minimal Inhibitory Concentration (MIC) was determined for more active essential oils. In each treatment, the MIC was determined, with two replicates for preliminary assays and three replicates for the determination of MIC.
Results and discussion
Composition of the essential oils
Preliminary analysis of 11 Algerian oil sample areas showed that the GC chromatograms of all samples from the same species were qualitatively similar but differed by abundances of their major components from the same species of Mentha studied, except for M. rotundifulia species where we found qualitative differences between major components in different areas of the same species (see Table 1). In the 11 oils from four Mentha species, 98 compounds were identified, (14 monoterpene hydrocarbons, 17 sesquiterpene hydrocarbons, 45 oxygenated monoterpenes, 8 oxygenated sesquiterpenes, and 14 non-tearpenic oxygenated compounds). In Table 1, the compositions from all samples of each species are presented. In total, we identified 80 compounds in M. rotundifulia samples (60 compounds in MRO1 simple representing 98.2% of the total number of compounds identified, 64 compounds in MRO2 sample representing 99.2% of total, 62 compounds in MRO3 simple representing 98.6% of total, and 59 compounds in MRO4 simple representing 98.9% of total), among them MRO1 (14 monoterpene hydrocarbons, 13 sesquiterpene hydrocarbons, 15 oxygenated monoterpenes, 7 oxygenated sesquiterpenes, and 11 non-terpenic oxygenated compounds), MRO2 (15 monoterpene hydrocarbons, 13 sesquiterpene hydrocarbons, 18 oxygenated monoterpenes, 7 oxygenated sesquiterpenes, and 11 non-terpenic oxygenated compounds), MRO3 (14 monoterpene hydrocarbons, 14 sesquiterpene hydrocarbons, 25 oxygenated monoterpenes, and 9 non-terpenic oxygenated compounds), and MRO4 (14 monoterpene hydrocarbons, 14 sesquiterpene hydrocarbons, 22 oxygenated monoterpenes, and 9 non-terpenic oxygenated compounds) (see Table 2). Regarding M. spicata, we identified 32 compounds (in both MSP1 and MSP2 samples representing 98.5 and 98.1% of total respectively from MSP1 and MSP2), among them 11 monoterpene hydrocarbons, 7 sesquiterpene hydrocarbons, 13 oxygenated monoterpenes, and 1 non-terpenic oxygenated compound (see Table 2). About M. pulegium, we reported 28 compounds in different stations (MPU1, MPU2, and MPU3 samples representing 98.5, 98.7, and 98.4% of total respectively from MPU1, MPU2, and MPU3), among them 6 monoterpene hydrocarbons, 5 sesquiterpene hydrocarbons, 14 oxygenated monoterpenes, and 3 non-terpenic oxygenated compounds (see Table 2). Finely, we reported 38 compounds in both M. piperita essential oil samples (MPI1 and MPI2 representing 97.9 and 98.8% of total respectively from MPI1 and MPI2), among them 8 monoterpene hydrocarbons, 10 sesquiterpene hydrocarbons, 15 oxygenated monoterpenes, 2 oxygenated sesquiterpenes, and 3 non-terpenic oxygenated compounds) (see Table 2).
From all species analyzed, 92 components were verified by comparing their EI-MS and retention indices with those in our laboratory library (UMR CNRS 6134 from University of Corsica). Six components were identified by comparing their EI-MS and apolar retention indices with those of literature libraries under the same analytical conditions as ours (see Table 1),
In M. rotundifulia (MRO) essential oils, four different types of oils were shown to be different both qualitatively and quantitatively in their compositions. MRO1 was characterized by the abundance of piperitenone oxide (34%), terpinene-4-ol (10.4%), E-β-caryophyllene (7.5%), and 1-oct-3-enyl acetate (6.5%). The oil was dominated by oxygenated compounds (70.8%), especially oxygenated monoterpenes (55.3%). MRO2 was characterized by the abundance of piperitenone oxide (36.1%), Z-piperitone oxide (11.7%), and E-piperitone oxide (10.6%). The oil was dominated by oxygenated compounds (82.5%), especially oxygenated monoterpenes (73.3%). MRO3 was characterized by the abundance of pulegone (56.3%), menthone (16.8%), and iso-menthone (5.5%). The oil was dominated by oxygenated compounds (90.5%), especially oxygenated monoterpenes (87%). Finely MRO4 was characterized by the abundance of menthone (28.5%), iso-menthone (19%), neo-menthol (10.4%), pulegone (5.6%), and neo-menthyl acetate (5%). The oil was dominated by oxygenated compounds (92.4%), especially oxygenated monoterpenes (91.3%) (see Table 1).
In M. spicata (MSP) essential oils, two oils were identified, with only qualitative differences in the abundance of their major components. MSP1 and MSP2 were characterized respectively by the abundance of carvone (79.3 and 54.1%), limonene (8.4 and 21.9%), and myrcene (0.7 and 3.3%). Both oils were dominated by oxygenated monoterpene (86.8 and 67.9%) and monoterpene hydrocarbons (10.3 and 28.7%) respectively from MSP1 and MSP2 (see Table 1).
In M. pulegium (MPU) essential oils, tree oils were characterized and differenced only quantitatively by the abundance of their major components. MPU1, MPU2, and MPU3 were characterized respectively by the abundance of pulegone (77.3, 40.7, and 9.2%), neo-menthol (1.6, 9.1, and 42.3%), and menthone (10.8, 38.3, and 40.5%). All oils were dominated respectively by oxygenated monoterpene (94.2, 96.3, and 96.1%) (see Table 1).
In M. piperita (MPI) essential oils, both oils were qualitatively similar but differed by the abundance of their major components. MPI1 and MPI2 were respectively dominated by linalool (47.6 and 40.4%), linalyl acetate (12 and 32.6%), α-Terpineol (10.4 and 6.4%), 1,8-Cineole (5.4 and 3.8%), geranyl acetate (5.3 and 2.5%), and geraniol (5 and 2.4%). Both oils were dominated by oxygenated monoterpene (91.1 and 92.5%) respectively in MP1 and MP2 (see Table 1).
As presented in Table 2, chemical composition of essential oils of Mentha species were characterized by the high amount of oxygenated compounds (70.8 to 96.3%) in all samples, especially monoterpene-oxygenated compounds (55.3 to 95.3%), in accordance with literature. Only some samples of M. rotundifulia and M. spicata exhibited a significant level of hydrocarbon compounds. Finally, M. spicata, M. piperita, and M. rotundifulia (MRO3 and MRO4) were characterized by high amounts of aldehydes and ketones (52.1 to 91.8%). M. piperita were characterized by the presence of alcohols (50.8 to 65.3%) and esters (20.7 to 38.7%), and finally, MRO1 and MRO2 from M. rotundifulia were dominated by oxides (35.7 to 57.1%) and alcohols (17.6 to 25%) (see Table 2).
To identify possible correlation between the chemical oil compositions of Algerian Mentha species, principal component analysis (PCA) was applied to matrix linking essential oil components to species identities. PCA (Figs. 2 and 3) confirmed our interpretation that there are similarities between M. pulegium (MPU1, MPU2, and MPU3) and MRO3 and MRO4 from M. rotundifulia especially MRO3. Both simples (MSP1 and MSP2) of M. spicata were also close to M. pulegium. The most important differences were found in M. piperita (MPI1 and MPI2) and in the MRO1 and MRO2 from M. rotundifulia, which were quite different from the others.
Variable factor map (principal component analysis). X8 = α-Pinene; X10 = Oct-1-en-3-ol; X13 = myrcene; X16 = α-terpinene; X17 = p-cymene; X18 = limonene; X19 = 1,8-cineole; X20 = (Z)-α-ocimene; X22 = α-terpinene; X23 = trans-hydrate sabinene; X25 = linalool; X27 = cis-sabinene hydrate; X28 = 1-oct-3-enyl acetate; X33 = menthone; X34 = p-menth-3-en-8-ol; X35 = iso-menthone; X36 = borneol; X39 = neo-menthol; X41 = terpinene-4-ol; X42 = menthol; X43 = iso-menthol; X44 = Z-dihydro carvone; X46 = α-terpineol; X47 = E-dihydro carvone; X49 = Nerol; X50 = pulegone; X51 = carvone; X52 = Z-piperitone oxide; X53 = E-piperitone oxide; X54 = piperitone; X55 = geraniol; X56 = linalyl acetate; X62 = bornyl acetate; X64 = menthyl acetate; X65 = iso-menthyl acetate; X66 = dihydro carvyl acetate; X67 = piperitenone; X68 = piperitenone oxide; X69 = α-terpenyl acetate; X70 = neryl acetate; X71 = geranyl acetate; X75 = E-α-caryophyllen; X81 = germacrene D; X87 = α -cadinene; X92 = caryophyllene oxide; X94 = 1,10-di-epi-cubenol; X98 = cadinol
Fumigant antifungal activity
The antifungal activity of essential oils of Algerian Mentha species was tested against for fungi. Results of fumigant antifungal activity are shown in Tables 3 (inhibition rate) and 4 (MIC against more sensitive fungal strains). P. expensum is less sensitive than are other strains and for this reason, MICs were only identified for Monilinia sp. and B. cinerea strains.
Essential oils of M. rotundifulia are less active than the essential oils from other species of Mentha, except for MRO3, which has an MIC of 36 × 103 μl/ml air against B. cinerea. We observed an MIC between 36 and 142 × 103 μl/ml air for M. spicata, M. pulegium, and M. piperita. These results can be correlated with both their chemical compositions and statistical results. Oils dominated by alcohols (MPI1 and MPI2 and MPU3), aldehydes and ketones (MRO3, MSP1, MSP2, MPU1, MPU2, and MPU3) exhibited an interesting inhibition again the fungi tested, in contrast to MRO1 and MRO2 which were rich in oxide compounds and were less active. On the basis of these results, we suggest that the most interesting activity of Mentha essential oils was due to alcohol, aldehyde, and ketone compounds, like linalool in M. piperita; carvone in M. spicata; and pulegone, menthone, and neo-menthol in both M. pulegium and M. rotundifulia. Those results were in accordance with the results published recently and demonstrated strong activities of molecules like iso-menthose, pulegone, carvone, piperitone, piperitone oxide, and piperitenone oxide present in essential oils of M. spicata and M. pulegium, as good activities against insect pests (Leptinotarsa decemlineata, Spodoptera littoralis, and Myzus persicae), root-knot nematodes (Meloydogine javanica), and plants (Lactuca sativa, Lolium perenne, Solanum lycopersicum) (Santana-Méridas et al. 2017). In addition, we suggest that these molecules can significantly inhibit the growth of fungi, especially Molilinia sp. and B. cinerea, and to a lesser degree P. expansum.
Conclusions
The aerial parts of the Algerian Mentha species produce one type of essential oil dominated by oxygenated compounds; however, the oils can be classed in three different groups. The most important group is characterized by ketone compounds like carvone, menthone, and pulegone and the second by alcohol compounds like linalool and neo-menthol. Finally, the third group was characterized by oxide compounds like piperitenone oxide and piperitone oxide.
The Mentha essential oil activity was very interesting and almost all the oils tested demonstrated a strong inhibition against the fungi studied except for P. expensum where the activity was moderate. Thus, the Mentha essential oils could be considered as a good bio-control agent to protect fruit trees, such as apple and pear trees, which are known targets for fungi like Molilinia sp. and B. cinerea, and as an alternative to chemical pesticides, to treat trees infected with these fungi.
References
Al Yousef S (2013) Antifungal activity of volatiles from lemongrass (Cymbopogon citratus) and peppermint (Mentha piperita) oils against some respiratory pathogenic species of Aspergillus. Int J Curr Microbiol App Sci 2:261–272
Al-Bayati FA (2009) Isolation and identification of antimicrobial compound from Mentha longifolia L. leaves grown wild in Iraq. Ann Clin Microbiol Antimicrob 8:20
Bicchi C, Liberto E, Matteodo M, Sgorbini B, Mondello L, Zellner BA, Costa R, Rubiolo P (2008) Quantitative analysis of essential oils: a complex task. Flavour Frag J 23:382–391
Brereton RG (2003) Chemometrics: data analysis for the laboratory and chemical plant, first edn. Wiley Interscience, New-York
Celenk S, Tarimcilar G, Bicakci A, Kaynak G, Malyer H (2008) A palynological study of the genus Mentha L. (Lamiaceae). Bot J Linn Soc 157:141–154
Council of Europe (1997) European Pharmacopoeia, First edn. Council of Europe, Strasbourg
Djabou N, Andreani S, Varesi L, Tomi F, Costa J, Muselli A (2013) Analysis of volatile fraction of Teucrium marum L. Flavour Frag J 28:14–24
Fenwick AL, Ward SM (2001) Use of random amplified polymorphic DNA markers for cultivar identification in mint. Hort Sci 36:761–764
Gobert V, Moja S, Colson M, Taberlet P (2002) Hybridization in the section Mentha (Lamiaceae) inferred from AFLP markers. Am J Bot 89:2017–2023
Jennings W, Shibamoto T (1980) In: Jovanovich HB (ed) Qualitative analysis of flavour and fragrance volatiles by glass-capillary gas chromatography, Academic Press, New York
Kerdchoechuen O, Laohakunjit N, Singkornard S, Matta FB (2010) Essential oils from six herbal plants for bicontrol of the maize weevil. Hortscience 45(4):592–598
Khanuja SPS, Shasany AK, Srivastava A, Kumar S (2000) Assessment of genetic relationships in Mentha species. Euphytica 111:121–125
Konig WA, Hochmuth DH, Joulain D (2001) Terpenoids and related constituents of essential oils library of mass finder 2.1. Ed. Institute of Organic Chemistry, Hamburg
Krasnyanski S, Ball TM, Sink KC (1998) Somatic hybridization in mint: identification and characterization of Mentha piperita M. spicata hybrid plants. Theor Appl Genet 96:683–687
Mahboubi M, Haghi G (2008) Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J Ethnopharmacol 119:325–327
Massart DL (1998) Chemometrics: a textbook. Elsevier Science Ltd, New-York
McLafferty FW, Stauffer DB (1988) The Wiley/NBS registry of mass spectra data. Wiley-Interscience. Ed, New-York
Mohammadi A, Hashemi M, Masoud Hosseini S (2015) Comparison of antifungal activities of various essential oils on the Phytophthora drechsleri, the causal agent of fruits decay. Iran J Microbiol 7(1):31–37
Odeyemi OO, Masika P, Afolayan AJ (2008) Insecticidal activities of essential oil from the leaves of Mentha longifolia L. subsp. capensis against Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae). Afr Entomol 16:220–225
Park IK, Lee SG, Choi DH, Park JD, Ahn YJ (2003) Insecticidal activities of constituents identified in the essential oil from leaves of Chamaecyparis obtusa against Callosobruchus chinesis (L.) and Sitophilus oryzea (L.) J. Stored Prod Res 39:375–384
Quezel P, Santa S (1963) Nouvelle Flore de l’Algérie. Centre National de la Recherche Scientifique, Paris
Rösch P, Kiefer W, Popp J (2002) Chemotaxonomy of mints of genus Mentha by applying Raman spectroscopy. Biopolymers 67(6):358–361
Santana-Méridas O, Fe Andrés M, Sanz J, Errahmani N, Abdeslam L, González-Coloma A (2014) Valorization of essential oils from Moroccan aromatic plants. Nat Prod Commun 9(8):1109–1114
Santana-Méridas O, González-Coloma A, Fe Andrés M, Vidali VP, Polissiou MG, Kimbaris AC (2017) Biocidal compounds from Mentha sp essential oils and their structure-activity relationships. Chem Biodivers. https://doi.org/10.1002/cbdv.201600270
Shasany AK, Darokar MP, Dhawan S, Gupta AK, Gupta S, Shukla AK, Patra NK, Khanuja SPS (2005) Use RAPD and AFLP markers to identify inter- and intraspecific hybrids of Mentha. J Hered 96(5):542–549
Soković MD, Vukojević J, Marin PD, Brkić DD, Vajs V, Van Griensven LJ (2009) Chemical composition of essential oils of thymus and Mentha species and their antifungal activities. Molecules 14:238–249
Acknowledgements
The authors are indebted to Fayçal HASSANI and Tawfik FEROUANI from University of Tlemcen, Laboratory of Ecology and Management of Natural Ecosystems, to botanical identification of all Mentha species.
The complement spectroscopic experiments have been performed using the “Biodiversité et Biotechnologies Marines” (Bio2Mar) facilities at the University of Perpignan.
The authors gratefully thank Jeanine ALMANY for providing the English language editing (as well as constructing comments) which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Benomari, F.Z., Andreu, V., Kotarba, J. et al. Essential oils from Algerian species of Mentha as new bio-control agents against phytopathogen strains. Environ Sci Pollut Res 25, 29889–29900 (2018). https://doi.org/10.1007/s11356-017-9991-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9991-4