Abstract
The widespread use of rare earth elements (REEs) in a number of technological applications raises unanswered questions related to REE-associated adverse effects. We have previously reported on the multiple impact of some REEs on the early life stages of the sea urchin Paracentrotus lividus. The present investigation was to evaluate REE toxicity to early life stages in two unrelated sea urchin species, Sphaerechinus granularis and Arbacia lixula. The comparative toxicities were tested of seven REEs, namely yttrium, lanthanum, cerium, neodymium, samarium, europium and gadolinium as chloride salts at concentrations ranging from 10−7 to 10−4 M. The evaluated endpoints included developmental defects and cytogenetic anomalies in REE-exposed embryos/larvae, and decreased fertilization success and offspring damage following sperm exposure. The results showed different toxicity patterns for individual REEs that varied according to test species and to treatment protocol, thus showing toxicity scaling for the different REEs. Further, the observed effects were compared with those reported for P. lividus either following embryo or sperm exposures. S. granularis showed a significantly higher sensitivity both compared to A. lixula and to P. lividus. This study provides clear-cut evidence for distinct toxicity patterns among a series of REEs. The differences in species sensitivity at micromolar REE levels may warrant investigations on species susceptibility to impacts along polluted coasts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is growing environmental concern raised by the widespread use of REEs in present-day life due to their presence in a huge number of technological applications as reported previously (US Environmental Protection Agency 2012; Gambogi and Cordier 2013; Pagano et al. 2015a,b). This concern is highlighted by the growing number of publications in recent years on REE-associated toxicity to a number of test organisms and cell systems. It should be noted, however, that the vast majority of publications on REE toxicity is confined to four elements (Gd, Y, Ce, La) out of 17 REEs (reviewed by Pagano et al. 2015a,b).
As a consequence, many questions arise on the associated toxicity of relatively poorly studied REEs. This is especially important considering that some of these elements, e.g., Nd, have established industrial applications and are increasingly used in various industrial processes, indicating the potential for environmental health impacts (Feyerabend et al. 2010; Bleiwas and Gambogi 2013; Gambogi and Cordier 2013; Rim 2016). Some reports have investigated comparative toxicities for selected groups of REEs, by suggesting either similar or distinct effects of individual elements (Nakamura et al. 1997; Oral et al. 2010; Cui et al. 2012; González et al. 2015).
Recently, we have reported the effect of some REEs (incl. Y, La, Ce, Nd, Sm, Eu and Gd) on the sea urchin Paracentrotus lividus (Pagano et al. 2016). The sea urchin toxicity bioassay model has been utilized for several decades in order to evaluate a number of adverse events related to the marine environment, e.g., ocean acidification (Cipollaro et al. 1986; Lewis et al. 2016), as well as impacting on other, non-marine environments. The observed biological effects on sea urchin early life stages include embryonic and cell differentiation, cell division, fertilization success, transmissible damage from gametes to offspring, and a number of molecular endpoints including, for example, redox metabolism or DNA damage. Indeed, we have found that REEs affect embryogenesis, fertilization, cytogenetic and redox endpoints in P. lividus (Pagano et al. 2016).
In this study, we evaluated the effects of these seven REEs on early life stages of two taxonomically unrelated sea urchin species (Sphaerechinus granularis and Arbacia lixula) using similar experimental protocols under the same conditions. Moreover, the results in terms of embryotoxicity and transmissible damage from sperm to offspring have been compared with those reported for P. lividus, in order to rank the susceptibility of the different species to REE-induced harm.
Materials and methods
Chemicals
Trichloride REE salts were from SIGMA-Aldrich (Italy). Solutions of Y(III), La(III), Ce(III), Nd(III), Sm(III), Eu(III) and Gd(III) were diluted from a 10−1 M stock solution stored at 4 °C at pH 3 (by HCl addition). In turn, stock solutions were diluted in HCl pH 3 up to the final test concentrations (10−4 to 10−7 M) by 10% scalar dilutions. The correspondence of nominal vs. analytical concentrations was determined by ICP-MS analysis using an Aurora M90 Brucker instrument as described in our previous report (Pagano et al. 2016), confirming the correspondence between nominal and analytically determined concentrations. This correspondence was further confirmed in a later study of heavy REEs (Gravina et al. submitted for publication).
Sea urchins
Sea urchins (S. granularis) were collected along the Rovinj coast (north Adriatic Sea, Croatia) by the staff of the Center for Marine Research - Ruđer Bošković Institute, and A. lixula were collected by R. Oral along the Çeşme coast (Aegean Sea, Turkey). Gametes were obtained and embryos were reared as reported previously in Pagano et al. 2001. Embryo exposures to REE trichloride salts at concentrations in the order of 10−7 to 10−5 M started from zygote (10 min post-fertilization) up to the pluteus larval stage (72 h post-fertilization). Embryos were incubated in REE solutions or FSW at 18 ± 1 °C in Corning™ Falcon™ Polystyrene Microplates (6 wells, 10 ml/well, code # 353046). Embryotoxicity assays were run with at least 3-replicate embryo cultures from different male and female lots. Control cultures were run in triplicate, of which one was “open”, tagged as “1” in order to check the suitability of the whole culture. The other two controls, as well as each treated culture, were tagged by random numbers, in order achieve blind readings. The same criteria applied to the other treatment schedules, for cytogenetic analysis and for sperm pretreatment.
Embryological analysis
Embryological analysis was performed on living plutei that were immobilized in 10−4 M chromium sulfate for 10 min prior to observation, approx. 72 h after fertilization (Pagano et al. 2001). In each treatment schedule, the first 100 plutei were scored for the percentages of (1) normal larvae (N); (2) retarded larvae (size <1/2 N); (3) malformed larvae (P1), mostly observed through damaged skeletal differentiation; (4) embryos/larvae unable to attain the pluteus stage—i.e., abnormal blastulae or gastrulae (P2), and (5) dead (D) embryos or larvae. Total developmental defects (DD) were scored as (P1 + P2).
Cytogenetic analysis
Cytogenetic analysis was carried out on 30 cleaving embryos from triplicate cultures of REE-exposed S. granularis embryos, and triplicate controls (each in triplicate cultures) amounting to a total of 9 control cultures. A novel pilot test was also performed on cleaving embryos generated by sperm exposed to three REEs [La(III), Sm(III) and Gd(III)].
S. granularis embryos were fixed in Carnoy’s fluid (60% ethanol, 30% chloroform and 10% glacial acetic acid) 5 h after fertilization, and stained by 0.5% acetic carmine. Mitotoxicity endpoints included mean number of mitoses per embryo (MPE), and percent interphase embryos (IE). The induction of mitotic aberrations was evaluated by measuring the mean number of mitoses per embryo and percent embryos displaying ≥ mitotic aberrations.
Cytogenetic analysis was not performed in A. lixula embryos due to poor transparence of blastomeres preventing proper observation of chromosomes.
Sperm bioassays
A series of experiments was performed on both S. granularis and A. lixula sperm. A 50-μl sperm pellet was suspended for 10 min (S. granularis) or 1 h (A. lixula) in 30 ml FSW containing REE salts at concentrations of 10−5 to 10−4 M. Thereafter, 50 μl of REE-containing sperm suspension was used to inseminate 10 ml of untreated eggs (~50 eggs/ml). Thus, by a 200× dilution of REE-containing sperm suspension, offspring embryos were reared at REE concentrations of 5 × 10−8 to 5 × 10−7 M.
Fertilization success was measured as percent fertilized eggs and also expressed as fertilization rate (FR) (out of 100 fertilized + unfertilized eggs) on live cleaving embryos within an interval of 1 to 3 h post-fertilization. Thereafter, the embryos generated by REE-treated sperm were cultured up to pluteus stage and scored for developmental defects (72 h post-fertilization) as described above in order to evaluate the effects, if any, of sperm exposure on offspring development.
Statistical analysis
Statistical analysis was carried out by using a GraphPad Prism software (GraphPad, San Diego, CA, USA). Results are given as mean ± standard error, or with 95% confidence interval (CI). The half maximal effective concentrations (EC50s) were calculated by using a non-linear regression analysis with CIs. Experimental data were fitted to a log-logistic model with variable slope. Statistical assumptions were verified at the onset of each analysis, and a square root data transform was applied when underlying statistical assumptions were violated. Differences between control and treatment groups were determined through an unpaired two-tailed Student’s t test or with one-way Anova with Dunnett’s multiple comparison test as a post hoc analysis. The variables that were unsuitable for a parametric approach (cytogenetic analysis) were evaluated with nonparametric tests: χ 2 test and Mann–Whitney U test. Differences were considered significant when p < 0.05. To carry out several simultaneous comparisons, Tukey’s and Bonferroni’s methods were used.
Results
Embryotoxicity tests
By rearing S. granularis embryos in REE chloride salts at levels ranging from 10−7 to 10−5 M, concentration-related increases in developmental defects (% DD) were observed, as shown in Fig. 1a. The highest DD values were significantly increased in S. granularis exposed to La(III), Y(III), Nd(III) and Sm(III), whereas Gd(III) resulted in the lowest DD values that were close to controls at Gd(III) levels up to 10−6 M, with a non-statistically significant increase at the 10−5 M Gd(III) level. No relevant mortality was detected in S. granularis larvae up to 10−5 M REEs (mostly <1%), except for Y(III), La(III) and Ce(III) showing sporadic excess mortality in individual cultures (data not shown).
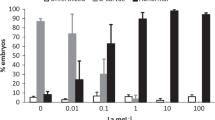
a Percent developmental defects (DD) in S. granularis embryos/larvae reared in REEs at concentrations ranging 10−7 to 10−5 M. *p < 0.05. b DD and % mortality in A. lixula embryos/larvae reared in REEs at concentrations ranging 10−7 to 10−5 M. This highest concentration showed, for Y(III) and Gd(III), 100% DD + mortality. *p < 0.05; **p < 0.01; ***p < 0.001
When A. lixula embryos were exposed to REEs in the same concentration range (10−7 to 10−5 M), the DD values were significantly increased (p < 0.05 to p < 0.01) even at 10−6 M REE concentrations, and resulted in 100% acute toxicity [DD ( ) + mortality (■)] in larvae exposed to 10−5 M Y(III) and Gd(III) (Fig. 1b).
) + mortality (■)] in larvae exposed to 10−5 M Y(III) and Gd(III) (Fig. 1b).
The inter-species findings of sub-acute REE exposures (10−7 and 10−6 M) of embryos from S. granularis and A. lixula were compared, together with the previously published results of P. lividus embryotoxicity assays at the same REE concentrations (Pagano et al. 2016). As shown in Fig. 2, S. granularis larvae almost invariably resulted in the highest DD rates, with the exception of 10−6 M Gd(III), which showed the highest developmental toxicity in P. lividus larvae. A significantly higher toxicity in embryos of the three species was exerted by 10−7 M La(III) and Y(III) and, in particular, S. granularis embryos displayed highest sensitivity vs. P. lividus and A. lixula embryos. P. lividus embryos exposed to 10−6 M Gd(III) displayed highest sensitivity in contrasts with the relatively low sensitivity to the other tested REEs when compared to S. granularis embryos (Fig. 2 and Supplement # 1).
Comparisons among DD in embryos/larvae of S. granularis, A. lixula and P. lividus reared in 10−7 and 10−6 M REEs. Data from P. lividus are from Pagano et al. 2016, showing significantly higher toxicity for Gd(III)
As shown in Table 1, EC50 data showed the highest sensitivity of S. granularis embryos/larvae for most of tested REEs, with the exception of Gd(III) resulting in most severe effects in P. lividus embryos/larvae.
Cytogenetic analysis
S. granularis embryos were exposed to REEs at concentrations ranging from 10−6 to 10−4 M and were evaluated for changes in mitotic activity and for frequencies of mitotic aberrations. Embryo exposure to REEs failed to increase mitotic aberrations (data not shown). However, a mitotoxic effect was found, in terms of % interphase embryos (i.e., failing to show any mitotic figures), as shown in Fig. 3, with concentration-related trends for 5 out of 7 tested REEs [La(III), Ce(III), Nd(III), Sm(III) and Gd(III)].
Sperm pretreatment
Both exposures of S. granularis and of A. lixula sperm to REEs failed to result in any significant decrease of sperm fertilization success (data not shown). The offspring of REE-exposed sperm resulted in increased malformations in both species, as shown in Fig. 4. The offspring of REE-exposed (10−5 and 10−4 M) S. granularis sperm was most severely affected by 10−4 M La(III), Nd(III), Gd(III) and Eu(III) (t values = 5.9, 8.3 and 6.4 respectively with df = 22) (p < 0.001), whereas a non-significant increase in developmental defects (DD) was observed in the offspring of Ce(III)-exposed sperm (Fig. 4a). The offspring of REE-exposed (10−5 and 10−4 M) A. lixula sperm resulted in significantly increased DD following sperm exposures to La(III), Sm(III) and Eu(III), whereas lesser or non-significant offspring damage was observed following sperm exposures to Y(III), Ce(III), Nd(III) and Gd(III) (Fig. 4b).
A pilot test was carried out by measuring cytogenetic abnormalities in embryos generated by sperm exposed to three REEs [La(III), Sm(III) and Gd(III)] at concentrations ranging from 10−6 to 10−4 M. Again, no increase in mitotic aberrations was detected, while a significant mitotoxic effect was observed as percent Interphase Embryos (lacking active mitoses),, as shown in Supplement # 2.
By comparing the offspring damage observed in the three sea urchin species, again S. granularis showed significantly higher sensitivity both compared to A. lixula and to P. lividus (Pagano et al. 2016). The offspring of A. lixula sperm showed the lowest sensitivity, with the exception for the offspring of La(III)-exposed sperm that showed overlapping—and higher—sensitivity for A. lixula and S. granularis compared to P. lividus (Fig. 5).
Comparisons among DD in offspring embryos/larvae of the three sea urchin species, following sperm exposure to REEs, 10−5 M. Data from P. lividus are from Pagano et al. 2016
By comparing the different offspring damage effects ranking of the seven tested REEs across the three sea urchin species, highest sensitivity of S. granularis to almost all of tested REEs was observed at the 10−5 M concentration, except for La(III) which resulted in comparable effects in S. granularis and A. lixula (see Supplement # 3).
By summarizing the findings of embryotoxicity and offspring damage following REE embryo or sperm exposures, Table 2 shows the highest sensitivity of S. granularis embryos and sperm compared to the other two sea urchin species.
Discussion
The available literature is focused on the toxicity of four REEs (Gd, Y, La and Ce), while the relative scarcity of reports on the other REEs represents a challenge to the investigations on REE-associated toxicity. By considering that several REEs are not actually “rare” in terms of geological occurrence, mining and subsequent technological applications, the task of acquiring suitable toxicological information becomes a mandatory challenge. This must be accomplished by appropriate comparative toxicity investigations across several test models, environmental health assessments and broadly unexplored epidemiological investigations (Snow et al. 2014; Liu et al. 2015; Rim 2016; Li et al. 2016; Wang et al. 2016).
In the framework of toxicity testing systems, sea urchin bioassays have a unique role in characterizing a number of endpoints that are relevant across various taxa, involving a number of key biological events such as cell division and differentiation, genetic damage and redox endpoints. Thus, sea urchin bioassays may provide useful hints and contributions in the overall evaluations of potential modes of action of exposures to various xenobiotics across more than just marine species. This statement relies on an extensive database of literature on the use of sea urchin bioassays gathered over several decades and focused on inorganics, organics, pharmaceuticals and complex mixtures (reviewed by Pagano et al. 2017a,b). Thus, the present study provides a dataset which should not be regarded as limited to sea urchins and marine organisms, as far as it provides information on a set of key events such as cell division and differentiation, and transmissible (genetic) effects.
This study attempted to evaluate the effects on sea urchin early life stages of seven REEs in terms of developmental defects in REE-exposed pluteus larvae or in the offspring of REE-exposed sperm. Previous studies reported on the effects of several REEs in plants or in cell systems, in terms of bioaccumulation (Carpenter et al. 2015; Huang et al. 2011), yet without clear-cut distinctions in-between the behaviors of different REEs and their modes of action. In this context, suitable procedures to obtain reliable and comparable data for individual REEs were recommended by González et al. (2014,2015), and ad hoc studies are warranted both in toxicity bioassays and in mammalian studies.
We found that some of the tested REEs affected embryogenesis in embryos exposed to 10−6 M (Fig. 2). Micromolar REE concentrations may be regarded as realistic in REE-polluted marine sediment (Bustamante and Miramand 2005), or in other REE-containing complex mixtures as, e.g., bauxite residues (Karadağ et al. 2009; Wang et al. 2010) or fly ash from coal combustion (Franus et al. 2015; Taggart et al. 2016). Thus, ad hoc investigations on REE toxicity in other test models may be timely. Our findings showed that individual REEs give rise to different toxicity patterns displaying a clear toxicity gradient with, e.g., La(III) displaying the highest toxicity to sea urchin developing embryos among the tested REEs.
Sperm exposure to REEs, though compatible with fertilization success, resulted in offspring damage expressed through increased developmental defects and embryonic/larval mortality. Again, distinct effects were noted among the tested REEs, and distinct effects in the three species (Fig. 5). Altogether, these results support the notion that a generalization of REE-induced adverse effects would not be appropriate, and should prompt ad hoc investigations on distinct REE toxicities in other bioassay models.
A noteworthy finding of the present study relates to the different REE sensitivities of the three sea urchin species. S. granularis displayed significantly higher sensitivity compared to A. lixula and P. lividus, both following embryo and sperm exposures. Burić et al. (2015) tested the effects of silver nanoparticles on fertilization and early development of the same sea urchin species, and found A. lixula to be the most sensitive species to silver nanoparticles. Other studies reported on different echinoid species sensitivities, by testing salinity as a confounding factor, or antibiotics and disinfectants associated with fish farming (Carballeira et al. 2011,2012). A recent report by Martino et al. (2016) found different species sensitivities to Gd(III) in four unrelated sea urchin species. Our results corroborate their study as we have shown variable sensitivities of exposure to 10−6 M Gd(III) across our three tested species. The greatest Gd(III)-induced effect was noted for P. lividus embryo exposure (Fig. 2), while displaying minor, non-significant effects on S. granularis and A. lixula embryos. This result was in contrast with the findings of the other REEs, where S. granularis and A. lixula appeared more sensitive than P. lividus. This paradoxical finding deserves further investigation.
Beyond the present REE-focused study, it may be noted that the observed enhanced sensitivity of S. granularis to REEs might be an indication of a more general sensitivity of this species to environmental pollution. Indeed, since 2015 and to date, a scanty occurrence of S. granularis has been unexpectedly observed in the Bay of Naples as this species had been present—and utilized in a number of our studies—for several decades (from Pagano et al. 1983 to De Nicola et al. 2007). This observation might warrant studies at the community level.
Conclusion
The tested REEs induced multiple damage to sea urchin early life stages, including developmental defects in REE-exposed embryos and in the offspring of REE-exposed sperm, moreover by inhibiting cell division in cleaving embryos. The effects pointed to different toxicities of individual tested REEs, and to different sensitivities of three sea urchin species.
This study corroborates previous evidence for distinct toxicity patterns of seven light REEs in sea urchin early development, by prompting studies in other bioassay models.
Differing species sensitivity to REE-induced effects was found confirming previous reports, and pointing to the need to extrapolate these findings to ecosystem health and integrity while considering species fitness in areas with apparently minor, yet effective environmental pollution, warranting ad hoc investigations.
References
Bleiwas DI, Gambogi J (2013) Preliminary estimates of the quantities of rare-earth elements contained in selected products and in imports of semimanufactured products to the United States, 2010. Open-File Report 2013–1072. U.S. Department of the Interior; U.S. Geological Survey
Burić P, Jakšić Ž, Štajner L, Dutour Sikirić M, Jurašin D, Cascio C, Calzolai L, Lyons DM (2015) Effect of silver nanoparticles on Mediterranean sea urchin embryonal development is species specific and depends on moment of first exposure. Mar Environ Res 111:50–59. doi:10.1016/j.marenvres.2015.06.015
Bustamante P, Miramand P (2005) Subcellular and body distributions of 17 trace elements in the variegated scallop Chlamys varia from the French coast of the Bay of Biscay. Sci Total Environ 337:59–73. doi:10.1016/j.scitotenv.2004.07.004
Carballeira C, Martín-Díaz L, Delvalls TA (2011) Influence of salinity on fertilization and larval development toxicity tests with two species of sea urchin. Mar Environ Res 72:196–203. doi:10.1016/j.marenvres.2011.08.008
Carballeira C, De Orte MR, Viana IG, Delvalls TA, Carballeira A (2012) Assessing the toxicity of chemical compounds associated with land-based marine fish farms: the sea urchin embryo bioassay with Paracentrotus lividus and Arbacia lixula. Arch Environ Contam Toxicol 63:249–261. doi:10.1007/s00244-012-9769-0
Carpenter D, Boutin C, Allison JE, Parsons JL, Ellis DM (2015) Uptake and effects of six rare earth elements (REEs) on selected native and crop species growing in contaminated soils. PLoS One 10(6):e0129936. doi:10.1371/journal.pone.0129936
Cipollaro M, Corsale G, Esposito A, Ragucci E, Staiano N, Giordano GG, Pagano G (1986) Sub-lethal pH decrease may cause genetic damage to eukaryotic cell. A study on sea urchins and Salmonella typhimurium. Teratog Carcinog Mutagen 6:275–288. doi:10.1002/tcm.1770060404
Cui J, Zhang Z, Bai W, Zhang L, He X, Ma Y, Liu Y, Chai Z (2012) Effects of rare earth elements La and Yb on the morphological and functional development of zebrafish embryos. J Environ Sci 24:209–213. doi:10.1016/S1001-0742(11)60755-9
De Nicola E, Meriç S, Gallo M, Iaccarino M, Della Rocca C, Lofrano G, Russo T, Pagano G (2007) Vegetable and synthetic tannins induce hormesis/toxicity effects in sea urchin early development and in algal growth. Environ Pollut 146:46–54. doi:10.1016/j.envpol.2006.06.018
Feyerabend F, Fischer J, Holtz J, Witte F, Willumeit R, Drücker H, Vogt C, Hort N (2010) Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater 6:1834–1842. doi:10.1016/j.actbio.2009.09.024
Franus W, Wiatros-Motyka MM, Wdowin M (2015) Coal fly ash as a resource for rare earth elements. Environ Sci Pollut Res Int 22:9464–9474. doi:10.1007/s11356-015-4111-9
Gambogi J, Cordier DJ (2013) Rare earths, in Metals and Minerals. U.S. Geological Survey. pubs.usgs.gov/of/2013/1072/OFR2013-1072
González V, Vignati DAL, Leyval C, Giamberini L (2014) Environmental fate and ecotoxicity of lanthanides: are they a uniform group beyond chemistry? Environ Int 71:148–157. doi:10.1016/j.envint.2014.06.019
González V, Vignati DA, Pons MN, Montarges-Pelletier E, Bojic C, Giamberini L (2015) Lanthanide ecotoxicity: first attempt to measure environmental risk for aquatic organisms. Environ Pollut 199:139–147. doi:10.1016/j.envpol.2015.01.020
Huang P, Li J, Zhang S, Chen C, Han Y, Liu N, Xiao Y, Wang H, Zhang M, Yu Q, Liu Y, Wang W (2011) Effects of lanthanum, cerium, and neodymium on the nuclei and mitochondria of hepatocytes: accumulation and oxidative damage. Environ Toxicol Pharmacol 31:25–32. doi:10.1016/j.etap.2010.09.001
Karadağ MM, Küpeli Ş, Arýk F, Ayhan A, Zedef V, Döyen A (2009) Rare earth element (REE) geochemistry and genetic implications of the Mortaş bauxite deposit (Seydişehir/Konya –Southern Turkey). Chem Erde - Geochem 69:143–159. doi:10.1016/j.chemer.2008.04.005Lewis
Lewis C, Ellis RP, Vernon E, Elliot K, Newbatt S, Wilson RW (2016) Ocean acidification increases copper toxicity differentially in two key marine invertebrates with distinct acid-base responses. Sci Rep 6:21554. doi:10.1038/srep21554
Li Y, Yu H, Zheng S, Miao Y, Yin S, Peng Li P, Bian Y (2016) Direct quantification of rare earth elements concentrations in urine of workers manufacturing cerium, lanthanum oxide ultrafine and nanoparticles by a developed and validated ICP-MS. Int J Environ Res Public Health 13:350. doi:10.3390/ijerph13030350
Liu H, Wang J, Yang Z, Wang K (2015) Serum proteomic analysis based on iTRAQ in miners exposed to soil containing rare earth elements. Biol Trace Elem Res 167:200–208. doi:10.1007/s12011-015-0312-9
Martino C, Bonaventura R, Byrne M, Roccheri M, Matranga V (2016) Effects of exposure to gadolinium on the development of geographically and phylogenetically distant sea urchins species. Mar Environ Res S0141-1136(16):30098–30098. doi:10.1016/j.marenvres.2016.06.001
Nakamura Y, Tsumura Y, Tonogai Y, Shibata T, Ito Y (1997) Differences in behavior among the chlorides of seven rare earth elements administered intravenously to rats. Fundam Appl Toxicol 37:106–116. doi:10.1006/faat.1997.2322
Oral R, Bustamante P, Warnau M, D’Ambra A, Guida M, Pagano G (2010) Cytogenetic and developmental toxicity of cerium and lanthanum to sea urchin embryos. Chemosphere 81:194–198. doi:10.1016/j.chemosphere.2010.06.057
Pagano G, Esposito A, Bove P, de Angelis M, Rota A, Giordano GG (1983) The effects of hexavalent and trivalent chromium on fertilization and development in sea urchins. Environ Res 30: 442–452
Pagano G, Korkina LG, Iaccarino M, De Biase A, Deeva IB, Doronin YK, Guida M, Melluso, G, Meriç S, Oral R, Trieff NM, Warnau M (2001) Developmental, cytogenetic and biochemical effects of spiked or environmentally polluted sediments in sea urchin bioassays. In: Garrigues P, Walker CH, Barth H (eds) Biomarkers in Marine Ecosystems: A Practical Approach. Elsevier, pp. 85–129
Pagano G, Guida M, Tommasi F, Oral R (2015a) Health effects and toxicity mechanisms of rare earth elements—knowledge gaps and research prospects. Ecotoxicol Environ Saf 115C:40–48. doi:10.1016/j.ecoenv.2015.01.030
Pagano G, Aliberti F, Guida M, Oral R, Siciliano A, Trifuoggi M, Tommasi F (2015b) Human exposures to rare earth elements: state of art and research priorities. Environ Res 142:215–220. doi:10.1016/j.envres.2015.06.039
Pagano G, Guida M, Siciliano A, Oral R, Koçbaş F, Palumbo A, Castellano I, Migliaccio O, Thomas PJ, Trifuoggi M (2016) Comparative toxicities of selected rare earth elements: sea urchin embryogenesis and fertilization damage with redox and cytogenetic effects. Environ Res 147:453–460. doi:10.1016/j.envres.2016.02.031
Pagano G, Guida M, Trifuoggi M, Thomas P, Palumbo A, Romano G, Oral R (2017a) Sea urchin bioassays in toxicity testing: I. Inorganics, organics, complex mixtures and natural products. Expert Opin Environ Biol 6:1. doi: 10.4172/2325-9655.1000142
Pagano G, Thomas J, Guida M, Palumbo A, Romano G, Oral R, Trifuoggi M (2017b) Sea urchin bioassays in toxicity testing: II. Sediment evaluation Expert Opin Environ Biol 6:1. doi:10.4172/2325-9655.1000141
Rim KT (2016) Trends in occupational toxicology of rare earth elements. In: Pagano G (ed) Rare earth elements in human and environmental health: at crossroads between toxicity and safety. Pan Stanford, Singapore, pp 11–46. isbn:978-981-4745-00-0
Snow SJ, McGee J, Miller DB, Bass V, Schladweiler MC, Thomas RF, Krantz T, King C, Ledbetter AD, Richards J, Weinstein JP, Conner T, Willis R, Linak WP, Nash D, Wood CE, Elmore SA, Morrison JP, Johnson CL, Gilmour MI, Kodavanti UP (2014) Inhaled diesel emissions generated with cerium oxide nanoparticle fuel additive induce adverse pulmonary and systemic effects. Toxicol Sci 142:403–417. doi:10.1093/toxsci/kfu187
Taggart RK, Hower JC, Dwyer GS, Hsu-Kim H (2016) Trends in the rare earth element content of U.S.-based coal combustion fly ashes. Environ Sci Technol 50:5919–5926. doi:10.1021/acs.est.6b00085
US Environmental Protection Agency (2012) Rare earth elements: a review of production, processing, recycling, and associated environmental issues. EPA 600-R-12-572. http://www.epa.gov/ord
Wang Q, Jun Deng J, Liu X, Zhang Q, Sun S, Jiang C, Zhou F (2010) Discovery of the REE minerals and its geological significance in the Quyang bauxite deposit, West Guangxi, China. J Asian Earth Sci 39:701–712. doi:10.1016/j.jseaes.2010.05.005
Wang B, Yan L, Huo W, Lu Q, Cheng Z, Zhang J, Li Z (2016) Rare earth elements and hypertension risk among housewives: a pilot study in Shanxi Province, China. Environ Pollut S0269-7491(16):31455–31455. doi:10.1016/j.envpol. 2016.10.066
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Trifuoggi, M., Pagano, G., Guida, M. et al. Comparative toxicity of seven rare earth elements in sea urchin early life stages. Environ Sci Pollut Res 24, 20803–20810 (2017). https://doi.org/10.1007/s11356-017-9658-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9658-1