Abstract
Aquatic environments are now recognized secondary habitat of potentially pathogenic Escherichia coli. In this study, PCR-based analyses were used to determine the phylogenetic composition and frequency of occurrence of eight clinically significant virulence genes (VGs) in E. coli isolates from sub-tropical Brisbane and cool temperate Tasmania freshwater in Australia. In Brisbane, non-commensal E. coli isolates belonging to the B2 and D phylogenetic group were dominant (72%). A significantly higher number (P < 0.05) of E. coli carrying VGs were detected in the sub-tropical freshwaters compared to the cool temperate water. Furthermore, diarrheagenic pathotype (EHEC) was also observed in the sub-tropical freshwater. The genes east1 and eaeA were significantly more common (P < 0.00001) than other VGs. The eaeA gene which codes for intimin protein along with toxin genes east1, stx 1 , stx 2 , and LT1 were mostly detected in phylogenetic groups B2 and D. The ANOVA results also suggested a statistically significant difference (P < 0.016) between the VGs carried by phylogenetic groups B2 and D. Class 1 integrase (intl1) and class 2 integrase (intl2) genes were detected in 38 (24.83%) and 23 (15.03%) of E. coli isolates, respectively. The Gretna site (Tasmania) with known fecal input from bovine and ovine sources had the highest number of E. coli carrying intl1 (29%) and intl2 (13%) genes. In addition, class 2 integron was more commonly detected in the phylogenetic group B2. The results of this study highlight the need to better understand sources and reasons for the high prevalence of E. coli carrying clinically significant VGs in a sub-tropical environment and its public health implications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Escherichia coli are generally viewed as part of the commensal microbial flora of the vertebrate gut, and therefore, traditionally used as indicators when testing water for fecal pollution in aquatic environments (Tenaillon et al. 2010). Land use factors such as wastewater treatment plant discharge, sewer overflow, storm water discharges, water runoff from pastures used for intensive animal farming, and animal manure-treated agricultural fields all provide source and pathways to deliver fecal pathogens to freshwater (Sidhu et al. 2013, 2014). This could lead to higher human health risks especially in areas where contact with such waters is likely such as swimming, boating, and areas where rural populations often access untreated freshwaters for various uses including drinking water. E. coli-mediated disease outbreaks related to exposure to contaminated freshwater are well documented (Olsen et al. 2002; Shelton et al. 2006). Despite the significant disease burden linked to pathogenic E. coli, factors underpinning the distribution, prevalence, survival, and environment transport of pathogenic E. coli remain poorly understood (Bridge et al. 2010).
Escherichia coli strains can be categorized in four main phylogenetic groups, A, B1, B2, and D on the basis of combination of the three genetic markers chuA, yjaA, and DNA fragment TspE4.C2 (Clermont et al. 2000), although some strains may also belong to additional groups C, E, and F (Clermont et al. 2013; Tenaillon et al. 2010). Extra-intestinal pathogenic E. coli (ExPEC) causing urinary tract infections, meningitis, and neonatal septicemia predominantly belong to group B2 and, to a lesser extent, to group D (Picard et al. 1999), whereas, intestinal diarrheagenic pathotypes (InPEC) and commensal strains belong to phylogenetic groups A and B1 (Johnson et al. 2001). E. coli strains belonging to B2 and D groups are reported to carry more virulence-associated genes compared to A and B1 group strains (Johnson et al. 2002).
Non-pathogenic bacteria can acquire antibiotic resistance and virulence genes (VGs) by horizontal or vertical gene transfer and through random DNA mutation (Barlow 2009). Gene transfer enables the exchange of genetic material located on mobile elements like plasmids and transposons among different strains or bacterial species (Frost et al. 2005). Integrons have been identified on these mobile elements which play an important role in the dissemination of antibiotic resistance genes (ARGs) as they carry determinants of site-specific recombination and an expression system, which integrates single or groups of mobile ARG cassettes (Hall and Collis 2006). Integrons have been associated with the presence of multiple ARGs in E. coli (Fluit and Schmitz 2004), and VGs could also be located in genetic mobile elements (Ochman et al. 2000); however, very little information is available on the relationship between the occurrence of VGs and the presence of integrons in E. coli isolates from aquatic environment.
To date, eight virulent E. coli pathotypes have been characterized and their unique mechanisms of pathogenesis were investigated (Croxen and Finlay 2010). These pathotypes are broadly classified by their ability to induce disease either within the gastrointestinal tract (diarrheagenic or InPEC) or in other niches of the body (extra-intestinal or ExPEC). Enterovirulent (or InPEC) strains can be classified as enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), Shiga-toxigenic E. coli (STEC), enterohemorrhagic E. coli (EHEC) which is sub-set of STEC, enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DEAC) (Nataro and Kaper 1998). These pathotypes possess different VG combinations for the attachment and elaboration of enterotoxins and hemolysins (Bertin et al. 2001). Although each pathogenic E. coli strain possesses a highly diverse and idiosyncratic repertoire of VGs, many share similar mechanisms of virulence strategies including attachment and effacement, the production of toxins, capsule and siderophore synthesis, and cell invasion (Kaper et al. 2004).
Several factors have been linked to the environmental distribution of hazardous E. coli pathotypes including storm events, water type, land use (Masters et al. 2011), geography, and climate (Hamelin et al. 2006). While ETEC infections occur in high frequencies during all times of the year (Qadri et al. 2005), several studies have reported that ETEC infections from exposure to contaminated freshwater (both symptomatic and asymptomatic) are more prevalent during warm periods (Rao et al. 2003; Steinsland et al. 2002). In cold temperate climate, 70% of the E. coli strains from the river and freshwater around Munich, Germany, were commensal strains, classified as either phylogenetic B1 or A (Hoffmann et al. 2001). More recently, high prevalence of A and B1 phylogroups (up to ca. 70% together) has been reported in a temperate lagoon (Perini et al. 2015). Other studies from cold temperate climate Great Lakes (Ontario, Canada), Comox Lake (BC, Canada) and St. Clair River and Detroit River (Detroit, USA), and Rhine River (Germany) have also reported relatively low occurrence of E. coli carrying VGs and ARGs (Chandran and Mazumder 2015; Hamelin et al. 2006; Hamelin et al. 2007; Stange et al. 2016). Conversely, higher prevalence of VGs has been reported in E. coli isolates collected from the warmer water of Appalachia Bay (Florida, USA) (Parveen et al. 1997). A similar trend has been observed in the sub-tropical region of Brisbane, Australia, where InPEC VGs associated pathotypes (including ETEC) and ExPEC strains in various freshwaters have been observed in high frequencies (Anastasi et al. 2012; Sidhu et al. 2013). This suggests some influence of climatic conditions on the occurrence and survival of clinically significant E. coli pathotypes in the warmer environment.
Anthropogenic and animal sources are the major sources of E. coli release into the aquatic environment; therefore, the presence of virulent E. coli could be site specific. E. coli are generally considered as transient inhabitants of aquatic ecosystems (Edge and Hill 2005); however, they are also reported to survive and maintain populations in tropical ecosystems due to the availability of nutrients warm air, soil, and water temperatures (Jimenez et al. 1989; Winfield and Groisman 2003).
This study evaluated the profiles of E. coli strains isolated from freshwater in sub-tropical and cool temperate climate of two selected areas in Australia. Freshwater from three sites each in Brisbane (a sub-tropical climate) and Tasmania (a cool temperate climate) was examined for (i) the presence of E. coli possessing VGs associated with human pathology, (ii) distribution of E. coli phylogenetic groups, and (iii) presence of class 1 and 2 integrons and relationship between the occurrence of VGs and integrons.
Materials and methods
Sampling sites and sample collection
Freshwater samples were collected from three sites in Tasmania and three sites in Brisbane. A brief site description along with GPS coordinates is provided in Table 1. Grab samples were collected 1 m from the shore and at a depth of about 0.5 m using a telescopic sampler. On each sampling occasion, two 500-mL water samples were collected in sterile glass bottles. In Brisbane, samples (n = 12) were collected from three sites (Milton, Enoggera Creek, and Mount Ommaney) between April and June 2013. Collected samples were transported to the laboratory in an esky on ice packs and processed within 6 h of collection. The water temperatures during the sampling period varied from 22 to 24 °C in Brisbane, and in Tasmania, it was between 15 and 20 °C. In Tasmania, water samples (n = 12) were collected at three sites (Gretna, Merton, and Tunbridge) between April and June 2013. Collected samples were shipped to Brisbane laboratory in an esky with ice packs and were processed within ~16 h after collection. The ambient water temperatures during the sampling period varied from 10 to 11 °C.
Isolation of E. coli from water samples
Membrane filtration method with ChromoCult coliform as selective agar (Merck, Germany) was used to capture and culture E. coli from the water samples (Sidhu et al. 2013). Briefly, 100 μL, 1 mL, and 10 mL water samples from Brisbane sites, 10 and 100 mL samples from Tasmanian sites, were filtered through 0.45-μm nitrocellulose (Millipore) filters (47 mm) in triplicate, placed on the plates, and incubated over a 24-h period at 37 °C to allow colonies to grow. Typical colonies showing dark blue to violet color were counted as E. coli. Two to three E. coli colonies were picked from the membranes and streaked onto the fresh ChromoCult medium. After this purification step, single colonies were picked from the plates and inoculated into 2-mL centrifuge tubes containing 1.5 mL nutrient broth (Oxoid) and incubated for 24 h at 37 °C in a shaking platform incubator at 100 rpm. All E. coli isolates were stored at −80 °C in nutrient broth and 15% (vol/vol) glycerol.
DNA extraction
To extract DNA, presumptive E. coli isolates were resuscitated overnight in 5 mL of nutrient broth at 37 °C. One milliliter of the cell culture from each isolate was centrifuged at 6000×g for 3 min. The resulting supernatants were removed, and the cell pellets were re-suspended by vortexing in 200 μL of sterile water. DNA was extracted from the cell pellets using the InstaGene matrix according to manufacturer’s instructions (Bio-Rad Laboratories). E. coli isolates were confirmed by PCR amplification of the uidA gene as described previously (Sidhu et al. 2013).
PCR positive controls
E. coli ATCC 9637 was used as a positive control (uidA gene) in PCR assays to confirm presumptive E .coli strains. E. coli O157:H7 (ATCC 35150) was used as a positive control for eaeA, stx 1 , and stx 2 genes. The E. coli strain belonging to serotype O138 of porcine origin was used as a positive control for heat-stable (ST) and heat-labile (LT) toxin genes. For the remaining target genes, pure cultures of clinical E. coli isolates containing target genes were used as positive controls.
Phylogenetic classification and screening for virulence genes and integrons
All confirmed E. coli isolates (n = 153) were assigned to four phylogenetic groups (A, B1, B2, or D) using a triplex PCR based on the presence or absence of three DNA fragments: chuA, yjaA, and TspE4C2, as previously described (Clermont et al. 2000). All E. coli isolates were screened for the presence of six diarrheagenic E. coli VGs by using previously published primer sets (Sidhu et al. 2013), Shiga toxin I and II producing genes stx 1 and stx 2 (López-Saucedo et al. 2003), intimin protein coating gene eaeA (Wang et al. 2002), type IV bundle-forming pili gene bfp and heat-labile toxin 1 gene bfp (Vidal et al. 2004), heat-stable toxin 1 producing gene ST (Guion et al. 2008) and heat-stable enterotoxin producing gene east1 (Yamamoto and Nakazawa 1997), and transcriptional regulator gene aggR (Toma et al. 2003). The presence of class 1 and 2 integrons carrying the integrase genes intl1 (Kerrn et al. 2002) and intl2 (Orman et al. 2002), respectively, was also determined using previously published primers sets.
PCR reactions were performed on a Bio-Rad iQ5 thermocycler system (Bio-Rad Laboratories, California, USA), using SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories, CA, USA). PCR amplification for phylogenetic classification, of VGs, intl1, and intl2, was performed in 20-μL reaction mixtures. Each 20-μL PCR reaction mixture contained 10 μL of EvaGreen® Supermix, 100–200 nM of each primer, Milli-Q water, and 3 μL of template DNA. Thermal cycling conditions for the detection of VGs were replicated from our previous study (Sidhu et al. 2013). Thermocycling parameters for the amplification of chuA, yjaA, and TspE4C2 were initial denaturing for 10 min at 95 °C, followed by 40 cycles of 30 s at 94 °C, annealing at 55 °C for 30 s, and extension for 30 s depending on the product length at 72 °C followed by a final extension for 5 min at 72 °C. For the detection of intl1 and intl2, annealing temperatures were 58 and 48 °C, respectively. For each PCR run, corresponding positive (target DNA) and negative (sterile water) controls were included. A melt curve analysis was performed after each PCR run to differentiate between actual products and primer dimers and to eliminate the possibility of false positive results. The melt curve was generated using 80 cycles of 10 s each starting at 55 °C and increasing at 0.5 °C intervals to a final temperature of 95 °C. The midpoint temperate (T m) for each amplicon was determined using the iQ5 software (Bio-Rad).
Statistical analysis
Association between the prevalence of VGs and integrase genes (intl1 and intl2) among phylogenetic groups and association between VGs and the presence of class 1 and 2 integrase genes were determined using χ 2or Fisher’s exact test. Associations among genes were considered statistically significant when P values were <0.05. The Student’s t test was performed to compare the significance of the difference between E. coli carrying VGs across sites; all tests were considered significant if the P value was <0.05. The difference in VG distribution among four phylogenetic groups across the six sites was determined by analysis of variance (ANOVA) on the pooled E. coli data from Brisbane and Tasmanian isolates with significance defined as P < 0.05. Statistical calculations were performed with Microsoft Excel and Statistica 10 (StatSoft).
Results
Identification and phylogenetic classification of E. coli
The overall occurrence of culturable E. coli in the freshwater samples from Brisbane was relatively high (100–2000 cfu 100 mL−1 for each site). E. coli numbers in the water samples collected from two Tasmania sites (Merton and Tunbridge) were low (<10 cfu 100 mL−1), whereas, the numbers in water samples collected from Gretna were higher (10–50 cfu 100 mL−1). All 84 presumptive E. coli isolates, from the Brisbane samples, were confirmed as E. coli based on the presence of the uidA gene. Out of 70 E. coli isolates from Tasmania, 69 were confirmed as E. coli.
In this study, we were able to classify all confirmed E. coli isolates into A, B1, B2, and D phylogenetic groups (Fig. 1). In Brisbane isolates, the phylogenetic group B2 was the most prevalent (47%), followed by group D (25%), group A (17%), and group B1 (11%). Overall, potentially ExPEC strains B2 and D (72%) dominated over the commensal A (24%) strains. In Tasmanian isolates, the phylogenetic group B1 was the most prevalent (42.1%), followed by group B2 (23.1%), whereas, groups A and D had a similar frequency (17.4%). Overall, the commensal strains A and B1 (59.5%) dominated Tasmanian isolate population over the potentially ExPEC strains B2 and D (40.5%).
Distribution of virulence genes among E. coli isolates
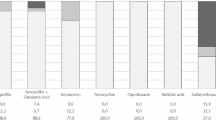
A significantly higher proportion (P < 0.05) of E. coli isolates from Brisbane (83%) carried VGs compared to Tasmania isolates (52%). The main EHEC virulence determinant gene, intimin gene (eaeA), was detected in 39 (25.49%) isolates followed by stx 1 in 11 (7.18%) and stx 2 in 9 (5.58%) isolates (Fig. 2 and Table 2). The main virulence determinants of ETEC, heat-stable toxin gene (ST1), were found to be more common (16.33%) than heat-labile toxin gene (LT1) which was detected in only three isolates (1.96%). The EAEC transcriptional regulator gene (aggR) and EaggEC heat-stable enterotoxin gene (east1) were detected in 10.45 and 36.60%, respectively.
Fifty percent of the E. coli isolates from Brisbane carried (40 out of 84) two or more VGs (Fig. 3). The most prevalent combination of genes was eaeA and east1 (11 isolates), with both these genes observed to be in combination with two or three other clinically relevant VGs. Shiga toxin genes (stx 1 and 2 ) were also detected in combination with eaeA and/or east1. This combination of genes which is typically carried by the EHEC pathotype was observed in at least 10 isolates. The main virulence determinant gene of EAEC pathotype (east1 and aggR) was carried by five isolates. Three isolates were detected with a combination of east1 and bfp genes which put them in the EPEC pathotype category. A number of isolates also had combinations of genes from both EHEC and EAEC pathotypes.
In comparison E. coli isolates from Tasmania, only three of the eight VGs tested were detected (Table 2). The east1 gene was the most commonly detected in 28 isolates (40.6%) followed by the eaeA gene which was detected in four isolates (5.8%). Multiple VGs were detected only in three isolates with two isolates that carried east1 and eaeA and one isolate with east1 and bfp gene combination. Two isolates carrying LT1 gene (heat-labile toxin 1) usually present in the ETEC pathotypes were also detected.
Distribution of virulence genes among four phylogenetic groups
The distribution of VGs among four E. coli phylogenetic groups is presented in Fig. 4. In general, isolates belonging to the phylogenetic groups B2 carried the highest number of VGs which was followed by the group D isolates. The eaeA gene which codes for intimin protein along with toxin genes east1, stx 1 , stx 2 , and LT1 were mostly detected in phylogenetic groups B2 and D. The ANOVA results also suggested a statistically significant difference (P < 0.0016) between the VGs carried by the phylogenetic groups B2 and D E. coli. Both east1 and eaeA genes were significantly (P < 0.00001) more common as compared to other VGs in all phylogenetic groups.
Occurrence of integrons
The presence of class 1 integron was observed in 12 (14.29%) isolates from Brisbane and 26 (37.68%) of isolates from Tasmania (Table 2). Overall, the difference in intI1 (37.28 vs 14.29%) and intI2 (23.18 vs 8.33%) carriage between Tasmania and Brisbane E. coli isolates sites was statistically significant (P = 0.001 and 0.013, respectively). The class 2 integron was observed in low frequencies in seven isolates (8.33%) from Brisbane and 16 (23.18%) of isolates from Tasmania. The intl1 carrying E. coli mostly belonged to phylogenetic groups B1 17 (39%) and B2 14 (32%) phylogenetic groups, whereas, intl2 was more common in the B2 15 (65%) phylogenetic group (Fig. 4). One site in Tasmania (Gretna) had the highest prevalence of E. coli carrying intl1 (20) and intl2 (9). The Merton site also had the second highest number of E. coli carrying intl1 (three) and intl2 gene (seven). In comparison, the Brisbane site, Milton, had the most E. coli carrying intl1 (seven) and intl2 gene (five). The frequencies of presence or absence of isolates carrying the intl1 gene in four major phylogenetic groups were similar, with no statistically significant differences (ANOVA P > 0.05). However, the intl2 gene was significantly more likely to be absent in phylogenetic groups A and B1 (P < 0.001 and <0.0001, respectively). A comparison of total VGs carried by integron (intl1 and/or intl2 genes) positive and negative strains was carried out to determine if certain VGs were linked to the presence of integrons. The χ 2 or Fisher’s exact test results revealed the statistically significant negative relationship between the presence of Int1 and VGs eaeA, east1, and ST1 (Table S1). Similarly, negative relationship between the presence of Int2 and eaeA gene was revealed.
Discussion
Escherichia coli with VGs in clinically significant combinations have been associated with significant intestinal and extra-intestinal human pathology (Kaper et al. 2004; Turner et al. 2006). In this study, significantly higher (P < 0.05) prevalence of single InPEC, VGs (eaeA, stx 1 , stx 2 , LT, ST, and east1) was observed in E. coli isolates from the Brisbane compared to Tasmania (Table 2). In particular, VGs eaeA (41.67%), ST1 (29.76%), stx 1 (13.10%), and stx 2 (10.70%) were highly prevalent in E. coli isolated from all Brisbane sites (Enoggera, Milton, and Mt Ommaney), whereas, east1 (40.6%) eaeA gene (5.8%) was detected in the isolates from Tasmania.
The overall higher prevalence of E. coli carrying VGs from Brisbane locations compared to Tasmania locations could potentially be due to the urbanized nature of the former and predominantly rural nature of the later or alternatively prolonged survival of E. coli carrying VGs in warmer waters of Brisbane. Aquatic environments in highly urbanized environments tend to have more diverse sources of fecal input into freshwater; E. coli strains carrying VGs have been reported to be highly prevalent in polluted urban streams (Higgins et al. 2005). In a previous study from Brisbane, Australia, a similar pattern of occurrence of these three genes in E. coli isolates eaeA (56%), stx 1 (6%), and stx 2 (10%) was reported (Sidhu et al. 2013). In comparison, a much higher frequency of eaeA (93.3%), stx 1 (46.7%), and stx 2 (13.3%) genes has been reported in the total DNA extracted from water samples collected from Michigan and Indiana River passing through highly urbanized areas (Duris et al. 2009). This suggests that anthropogenic activities may be the sources of E. coli carrying clinically relevant VGs into freshwater.
In addition to the detection of four typical EHEC strains carrying eaeA gene along with stx 1 , stx 2 , or both (Boerlin et al. 1999) in Brisbane water, the high prevalence of stx 1 /stx 2 /eaeA genes in other isolates was also observed. This suggests a high prevalence of atypical EHEC in freshwater which is in concurrence with the previously reported occurrence of a typical EHEC strains in surface water (Shelton et al. 2006). In contrast, in Tasmanian isolates, the prevalence of eaeA gene (6%) was low and none of the isolates carried stx 1 or stx 2 gene. High prevalence of EHEC pathotypes in sub-tropical climate including Brisbane (Sidhu et al. 2013), Bangladesh (Qadri et al. 2005), India (Khan et al. 2002), and China (Chen et al. 2011) has also been previously reported.
In this study, E. coli strains belonging to phylogenetic groups B2 and D were predominant in the freshwater from Brisbane (72%) but less common among Tasmanian isolates (40.5%). Although we have not tested for the prevalence of VGs defining ExPEC, the clones responsible for human extra-intestinal infections typically belong to the B2 and to a lesser extent the D phylogenetic group (Lecointre et al. 1998). Therefore, it is likely that a high proportion of our B2 and D group isolates from Brisbane may belong to ExPEC pathotype. In the previous studies, the high prevalence of ExPEC strains in sub-tropical freshwater has been reported (Hamelin et al. 2007; Masters et al. 2011). Conversely, a high prevalence of commensal strains (groups A and B1) in Tasmanian freshwater (59.5%) suggests a low prevalence of the ExPEC pathotype. A high prevalence (70%) of commensal strains E. coli belonging to the phylogenetic group B1 and A has been reported in the temperate river and freshwater around Munich, Germany (Hoffmann et al. 2001). Since both Brisbane (Australia) and Munich (Germany) are urbanized, the reasons for the high prevalence of non-commensal strains in sub-tropical water of the former are unknown. One possible explanation could be the difference in the adaptability of phylogenetic groups. E. coli belonging to the phylogenetic group B1 tend to be more environmentally adaptive (Berthe et al. 2013); therefore, it is possible that commensal E. coli strains are more adaptive and hence abundant in colder climate. The results of this study suggest a link between warmer climate and high prevalence of non-commensal E. coli carrying clinically important VGs which needs further investigation.
Heat-stable enterotoxin gene east1 is associated with EAEC-mediated diarrhea in adults and children (Caprioli et al. 2005). The east1 gene was the second most prevalent in the E. coli isolates from Brisbane (33.33%) and Tasmania (40.6%). The specific implications of the presence of east1 in E. coli isolates, however, are not straightforward. While some studies have reported that E. coli carrying east1 alone could cause diarrhea in subjects from Japan and Spain (Itoh et al. 1997; Viljanen et al. 1990), this gene has also been detected in commensal E. coli isolates (Ménard and Dubreuil 2002). For this reason, it was difficult to draw conclusions regarding the pathogenicity of east1 carrying E. coli in this study. In Brisbane, 41.67% of the E. coli isolates carried either only the eaeA gene or in combination with the heat-stable enterotoxin gene east1. The intimin gene (eaeA) is reported to be the primary virulence factor of EPEC strains and known to cause infantile diarrhea even in the absence of other virulence or toxin genes (DeVinney et al. 1999). The results suggest that potential EPEC strains are more frequently detected in warm tropical water of Brisbane compared to colder Tasmanian water.
The proportion of E. coli isolates classified as the phylogenetic group A was relatively similar between Brisbane and Tasmania at 17 and 18%, respectively. In principle, strains belonging to the phylogenetic group A lack InPEC VG combinations given their commensal nature (Bonacorsi et al. 2000). This held true for all Tasmanian commensal phylogenetic group A isolates, with none of the isolate carrying any InPEC VGs. However, all Brisbane commensal phylogenetic group A isolates possessed Shiga toxin VGs (stx 1 or stx 2 ), with one isolate classified as an EHEC pathotype as this strain synchronously possessed eaeA and stx 2 VGs. Phylogenetic groups A and B1 predominate in the gut flora of vertebrates, and these strains must acquire virulence factors to become pathogenic (Duriez et al. 2001). In view of this, the current study suggests that Brisbane commensal phylogenetic group A strains have acquired VGs potentially via horizontal gene transfer (HGT).
Site-specific point and non-point sources of fecal pollution are expected to contribute towards the presence of E. coli carrying clinically relevant VGs and integrons. A high proportion of InPEC pathotype isolates was detected at the Milton site, located in the heavily urbanized area of Brisbane with close proximity to a storm water drain. This was potentially due to mobilization and transport of fecal contaminants from land to Brisbane River after the storm events (Sidhu et al. 2013). In comparison, the Merton site in Mt Wellington provides a significant proportion (30%) of Hobart’s drinking water. The catchment area is well protected from point and non-point of sources of pollution and had a very low prevalence of E. coli isolates carrying VGs and integrons.
The E. coli from Gretna (Tasmania) carried more VGs and integrons compared to all other sites including Brisbane sites (Fig. 2). The most likely sources of E. coli isolates carrying VGs at this site are livestock (bovine and ovine) in the pastures surrounding Derwent River. This observation is supported by the high prevalence of the B1 phylogenetic group E. coli (45%) in the Gretna isolates which are more likely to be carried by the vertebrate animals (Gordon and Cowling 2003). It is interesting to note that out of five E. coli isolates carrying both integrons, four came from this site. In a recent study from Poland, an association between integron carriage in coliform bacteria and colder water temperature has been reported (Koczura et al. 2015). Therefore, the site-specific source of fecal pollution along with potentially higher persistence of integron-positive bacteria in colder water may increase the prevalence of potentially pathogenic E. coli. This suggests that E. coli of animal origin may pose health risks to humans as animals are recognized sources of ExPEC strains (Belanger et al. 2011).
Antibiotic resistance gene cassettes are carried on integrons (Hall and Collis 2006) that are a highly efficient mechanism for the dissemination of ARGs among bacterial pathogens (Fluit and Schmitz 2004). The prevalence of class 1 integron-integrase gene (intl1) has been suggested as a good proxy for the presence of pollution from anthropogenic sources (Gillings et al. 2014). In this study, class 1 integron was twice as commonly detected (24.83%) compared to class 2 integron (15.03%). The observed high prevalence of int1 gene is most likely due to its wide distribution in E. coli and other gram-negative bacteria and its presence on plasmids that can be transferred to other bacteria (Hall and Collis 2006). In comparison, class 1 integrons were reported to be highly prevalent in the E. coli isolates from sediments (69%) and freshwater (71%) from Santa Ana River (Southern California, USA), while class 2 integrons were detected in low frequency in both sediments (15%) and freshwater (7%) (Ibekwe et al. 2011). Similarly, the high prevalence of class 1 integrons (41%) in E. coli isolates has also been reported from Minjiang River (China) (Chen et al. 2011).
Class 1 and 2 integrons are considered markers of multidrug resistance (MDR), and their presence in the bacterial genome, even regardless of the genetic content of the variable region, is associated with the MDR phenotype (Deng et al. 2015). High prevalence of E. coli carrying integrons along with the multiple VGs in Brisbane isolates shows high pathogenicity and antibiotic resistance potential. As a variable region, class 1 and 2 integrons may contain up to nine resistance genes in gene cassettes (Partridge et al. 2009). In this study, we have only focused on the E. coli; the presence of class 1, 2, 3, and 4 integrons in bacteria such as Aeromonas spp., Pseudomonas spp., and Salmonella spp. often detected in water remains unknown. The role of such integrons in the dissemination of antimicrobial resistance in the aquatic environment, require further investigation.
In this study, we also explored the association between the presence of intl1 and intl2 genes, VGs, and phylogenetic group affiliation. The frequencies of presence or absence of intl1 gene in all four phylogenetic groups were statistically non-significant (χ 2 or Fisher’s test, P > 0.05) in all four phylogenetic groups in warmer and cold waters. The results are in agreement with the previously reported non-association with the presence of intl1 gene and phylogenetic groups in E. coli isolates from Warta River in Poland (Koczura et al. 2013). In contrast, class 1 integrons were reported to be significantly less prevalent in group A, E. coli isolated from freshwater and wastewater (Figueira et al. 2011). In this study, class 2 integron (intl2) was more commonly detected in the phylogenetic group B2 and was significantly less likely to be present in phylogenetic groups A and B1 isolates (P < 0.01). In contrast, class 2 integrons were reported to be more common in phylogenetic groups A1 and D2 E. coli isolates collected from freshwater and wastewater in northern Portugal (Figueira et al. 2011). This discrepancy is most likely due to the high mobility of transposons (Frost et al. 2005).
The results of this study suggested that warmer sub-tropical water harbors E. coli in high numbers which may carry clinically significant VGs. It is possible that favorable conditions for growth and proliferation (nutrients and warmer temperatures) may facilitate HGT, thereby increasing the presence of pathogenic E. coli in such environments. In a laboratory-scale study focused on the HGT in E. coli, transfer of stx 1/2 genes from pathogenic to commensal strains was found to be strongly influenced by high water temperature (37 °C) and strong UV irradiation (0.5 kJ/m2) (Yue et al. 2012). Since water temperature and UV exposure are characteristically higher in tropical climates, this study potentially offers an explanation as to why E. coli carrying VGs may be more prevalent in the warmer waters. An alternative explanation could be the growth and proliferation of E. coli carrying VGs in the sub-tropical water due to the prevalence of nutrients and ideal growing temperature (Jimenez et al. 1989; Winfield and Groisman 2003). Further investigation is required to elucidate reasons for high prevalence of E. coli carrying multiple VGs in warmer sub-tropical climates.
Conclusions
The results of this study suggest that VGs and integron—bearing E. coli responsible for waterborne disease outbreaks (both InPEC and ExPEC pathotypes)—are comparatively more prevalent in the warmer climate which might pose a threat to public health. However, the reasons for this are not clear and need further investigation. In terms of public health significance, site-specific anthropogenic sources of pollution are a major source of pathogenic E. coli into the aquatic environment. Therefore, there is a need for proper management of land use activities within the catchments that are used for the production of drinking water and recreational to limit human health risks.
References
Anastasi E, Matthews B, Stratton H, Katouli M (2012) Pathogenic Escherichia coli found in sewage treatment plants and environmental waters. Appl Environ Microbiol 78:5536–5541
Barlow M (2009) What antimicrobial resistance has taught us about horizontal gene transfer. Methods Mol Biol 532:397–411
Belanger L, Garenaux A, Harel J, Boulianne M, Nadeau E, Dozois CM (2011) Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol Med Microbiol 62:1–10
Berthe T, Ratajczak M, Clermont O, Denamur E, Petit F (2013) Evidence for coexistence of distinct Escherichia coli populations in various aquatic environments and their survival in estuary water. Appl Environ Microbiol 79:4684–4693
Bertin Y, Boukhors K, Pradel N, Livrelli V, Martin C (2001) Stx2 subtyping of Shiga toxin-producing Escherichia coli isolated from cattle in France detection of a new Stx2 subtype and correlation with additional virulence factors. J Clin Microbiol 39:3060–3065
Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL (1999) Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol 37:497–503
Bonacorsi SPP, Clermont O, Tinsley C, Le Gall I, Beaudoin J-C, Elion J, Nassif X, Bingen E (2000) Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect Immun 68:2096–2101
Bridge JW, Oliver DM, Chadwick D, Godfray HCJ, Heathwaite AL, Kay D, Maheswaran R, McGonigle DF, Nichols G, Pickup R (2010) Engaging with the water sector for public health benefits waterborne pathogens and diseases in developed countries. Bull World Health Organ 88:873–875
Caprioli A, Morabito S, Brugere H, Oswald E (2005) Enterohaemorrhagic Escherichia coli emerging issues on virulence and modes of transmission. Vet Res 36:289–311
Chandran A, Mazumder A (2015) Pathogenic potential, genetic diversity, and population structure of Escherichia coli strains isolated from a forest-dominated watershed (Comox Lake) in British Columbia, Canada. Appl Environ Microbiol 81:1788–1798
Chen B, Zheng W, Yu Y, Huang W, Zheng S, Zhang Y, Guan X, Zhuang Y, Chen N, Topp E (2011) Class 1 integrons, selected virulence genes, and antibiotic resistance in Escherichia coli isolates from the Minjiang River, Fujian Province, China. Appl Environ Microbiol 77:148–155
Clermont O, Bonacorsi S, Bingen E (2000) Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66:4555–4558
Clermont O, Christenson JK, Denamur E, Gordon DM (2013) The Clermont Escherichia coli phylo-typing method revisited improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 5:58–65
Croxen MA, Finlay BB (2010) Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol 8:26–38
Deng Y, Bao X, Ji L, Chen L, Liu J, Miao J, Chen D, Bian H, Li Y, Yu G (2015) Resistance integrons: class 1, 2 and 3 integrons. Ann Clin Microbiol Antimicrob 14:45
DeVinney R, Gauthier A, Abe A, Finlay BB (1999) Enteropathogenic Escherichia coli a pathogen that inserts its own receptor into host cells. Cell Mol Life Sci 55:961–976
Duriez P, Clermont O, Bonacorsi S, Bingen E, Chaventre A, Elion J, Picard B, Denamur E (2001) Commensal Escherichia coli isolates are phylogenetically distributed among geographically distinct human populations. Microbiology 147:1671–1676
Duris JW, Haack SK, Fogarty LR (2009) Gene and antigen markers of shiga-toxin producing E. coli from Michigan and Indiana river water occurrence and relation to recreational water quality criteria. J Environ Qual 38:1878–1886
Edge TA, Hill S (2005) Occurrence of antibiotic resistance in Escherichia coli from surface waters and fecal pollution sources near Hamilton, Ontario. Can J Microbiol 51:501–505
Figueira V, Serra E, Manaia CM (2011) Differential patterns of antimicrobial resistance in population subsets of Escherichia coli isolated from waste- and surface waters. Sci Total Environ 409:1017–1023
Fluit AC, Schmitz FJ (2004) Resistance integrons and super-integrons. Clin Microbiol Infect 10:272–288
Frost LS, Leplae R, Summers AO, Toussaint A (2005) Mobile genetic elements the agents of open source evolution. Nat Rev Microbiol 3:722–732
Gillings MR, Gaze WH, Pruden A, Smalla K, Tiedje JM, Zhu Y-G (2014) Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J 9:1269–1279
Gordon DM, Cowling A (2003) The distribution and genetic structure of Escherichia coli in Australian vertebrates host and geographic effects. Microbiology 149:3575–3586
Guion CE, Ochoa TJ, Walker CM, Barletta F, Cleary TG (2008) Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J Clin Microbiol 46:1752–1757
Hall RM, Collis CM (2006) Mobile gene cassettes and integrons capture and spread of genes by site-specific recombination. Mol Microbiol 15:593–600
Hamelin K, Bruant G, El-Shaarawi A, Hill S, Edge TA, Bekal S, Fairbrother JM, Harel J, Maynard C, Masson L, Brousseau R (2006) A virulence and antimicrobial resistance DNA microarray detects a high frequency of virulence genes in Escherichia coli isolates from Great Lakes recreational waters. Appl Environ Microbiol 72:4200–4206
Hamelin K, Bruant G, El-Shaarawi A, Hill S, Edge TA, Fairbrother J, Harel J, Maynard C, Masson L, Brousseau R (2007) Occurrence of virulence and antimicrobial resistance genes in Escherichia coli isolates from different aquatic ecosystems within the St. Clair River and Detroit River areas. Appl Environ Microbiol 73:477–484
Higgins JA, Belt KT, Karns JS, Russell-Anelli J, Shelton DR (2005) tir- and stx-positive Escherichia coli in stream waters in a metropolitan area. Appl Environ Microbiol 71:2511–2519
Hoffmann H, Hornef MW, Schubert S, Roggenkamp A (2001) Distribution of the outer membrane haem receptor protein ChuA in environmental and human isolates of Escherichia coli. Int J Med Microbiol 291:227–230
Ibekwe AM, Murinda SE, Graves AK (2011) Genetic diversity and antimicrobial resistance of Escherichia coli from human and animal sources uncovers multiple resistances from human sources. PLoS One 6:e20819
Itoh Y, Nagano I, Kunishima M, Ezaki T (1997) Laboratory investigation of enteroaggregative Escherichia coli O untypeable H10 associated with a massive outbreak of gastrointestinal illness. J Clin Microbiol 35:2546–2550
Jimenez L, Muniz I, Toranzos GA, Hazen TC (1989) Survival and activity of Salmonella typhimurium and Escherichia coli in tropical freshwater. J Appl Bacteriol 67:61–69
Johnson JR, O'Bryan TT, Kuskowski M, Maslow JN (2001) Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect Immun 69:5363–5374
Johnson JR, Oswald E, O'Bryan TT, Kuskowski MA, Spanjaard L (2002) Phylogenetic distribution of virulence-associated genes among Escherichia coli isolates associated with neonatal bacterial meningitis in the Netherlands. J Infect Dis 185:774–784
Kaper JB, Nataro JP, Mobley HL (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140
Kerrn MB, Klemmensen T, Frimodt-Moller N, Espersen F (2002) Susceptibility of Danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of sul genes conferring sulphonamide resistance. J Antimicrob Chemother 50:513–516
Khan A, Das SC, Ramamurthy T, Sikdar A, Khanam J, Yamasaki S, Takeda Y, Nair GB (2002) Antibiotic resistance, virulence gene, and molecular profiles of Shiga toxin-producing Escherichia coli isolates from diverse sources in Calcutta, India. J Clin Microbiol 40:2009–2015
Koczura R, Mokracka J, Barczak A, Krysiak N, Kaznowski A (2013) Association between the presence of class 1 integrons, virulence genes, and phylogenetic groups of Escherichia coli isolates from river water. Microb Ecol 65:84–90
Koczura R, Krysiak N, Taraszewska A, Mokracka J (2015) Coliform bacteria isolated from recreational lakes carry class 1 and class 2 integrons and virulence-associated genes. J Appl Microbiol 119:594–603
Lecointre G, Rachdi L, Darlu P, Denamur E (1998) Escherichia coli molecular phylogeny using the incongruence length difference test. Mol Biol Evol 15:1685–1695
López-Saucedo C, Cerna JF, Villegas-Sepulveda N, Thompson R, Velazquez FR, Torres J, Tarr PI, Estrada-García T (2003) Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli. Emerg Infect Dis 9:127–131
Masters N, Wiegand A, Ahmed W, Katouli M (2011) Escherichia coli virulence genes profile of surface waters as an indicator of water quality. Water Res 45:6321–6333
Ménard L-P, Dubreuil JD (2002) Enteroaggregative Escherichia coli heat-stable enterotoxin 1 (EAST1) a new toxin with an old twist. Crit Rev Microbiol 28:43–60
Nataro JP, Kaper JB (1998) Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201
Ochman H, Lawrence JG, Groisman EA (2000) Lateral gene transfer and the nature of bacterial innovation. Nature 405:299–304
Olsen SJ, Miller G, Breuer T, Kennedy M, Higgins C, Walford J, McKee G, Fox K, Bibb W, Mead P (2002) A waterborne outbreak of Escherichia coli O157:H7 infections and hemolytic uremic syndrome implications for rural water systems. Emerg Infect Dis 8:370–375
Orman BE, Pineiro SA, Arduino S, Galas M, Melano R, Caffer MI, Sordelli DO, Centron D (2002) Evolution of multiresistance in nontyphoid Salmonella serovars from 1984 to 1998 in Argentina. Antimicrob Agents Chemother 46:3963–3970
Partridge SR, Tsafnat G, Coiera E, Iredell JR (2009) Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev 33:757–784
Parveen S, Murphree RL, Edmiston L, Kaspar CW, Portier KM, Tamplin ML (1997) Association of multiple-antibiotic-resistance profiles with point and nonpoint sources of Escherichia coli in Apalachicola Bay. Appl Environ Microbiol 63:2607–2612
Perini L, Quero GM, Garcia ES, Luna GM (2015) Distribution of Escherichia coli in a coastal lagoon (Venice, Italy): temporal patterns, genetic diversity and the role of tidal forcing. Water Res 87:155–165
Picard B, Garcia JS, Gouriou S, Duriez P, Brahimi N, Bingen E, Elion J, Denamur E (1999) The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun 67:546–553
Qadri F, Svennerholm AM, Faruque AS, Sack RB (2005) Enterotoxigenic Escherichia coli in developing countries epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18:465–483
Rao MR, Abu-Elyazeed R, Savarino SJ, Naficy AB, Wierzba TF, Abdel-Messih I, Shaheen H, Frenck RW, Svennerholm A-M, Clemens JD (2003) High disease burden of diarrhea due to enterotoxigenic Escherichia coli among rural Egyptian infants and young children. J Clin Microbiol 41:4862–4864
Shelton DR, Karns JS, Higgins JA, Van Kessel JA, Perdue ML, Belt KT, Russell-Anelli J, Debroy C (2006) Impact of microbial diversity on rapid detection of enterohemorrhagic Escherichia coli in surface waters. FEMS Microbiol Lett 261:95–101
Sidhu JP, Ahmed W, Hodgers L, Toze S (2013) Occurrence of virulence genes associated with diarrheagenic pathotypes in Escherichia coli isolates from surface water. Appl Environ Microbiol 79:328–335
Sidhu JP, Skelly E, Hodgers L, Ahmed W, Li Y, Toze S (2014) Prevalence of enterococcus species and their virulence genes in fresh water prior to and after storm events. Environ Sci Technol 48:2979–2988
Stange C, Sidhu JP, Tiehm A, Toze S (2016) Antibiotic resistance and virulence genes in coliform water isolates. Int J Hyg Environ Health 219:823–831
Steinsland H, Valentiner-Branth P, Perch M, Dias F, Fischer TK, Aaby P, Mølbak K, Sommerfelt H (2002) Enterotoxigenic Escherichia coli infections and diarrhea in a cohort of young children in Guinea-Bissau. J Infect Dis 186:1740–1747
Tenaillon O, Skurnik D, Picard B, Denamur E (2010) The population genetics of commensal Escherichia coli. Nat Rev Microbiol 8:207–217
Toma C, Lu Y, Higa N, Nakasone N, Chinen I, Baschkier A, Rivas M, Iwanaga M (2003) Multiplex PCR assay for identification of human diarrheagenic Escherichia coli. J Clin Microbiol 41:2669–2671
Turner SM, Scott-Tucker A, Cooper LM, Henderson IR (2006) Weapons of mass destruction virulence factors of the global killer enterotoxigenic Escherichia coli. FEMS Microbiol Lett 263:10–20
Vidal R, Vidal M, Lagos R, Levine M, Prado V (2004) Multiplex PCR for diagnosis of enteric infections associated with diarrheagenic Escherichia coli. J Clin Microbiol 42:1787–1789
Viljanen MK, Peltola T, Junnila SY, Olkkonen L, Jarvinen H, Juistila M, Huovinen P (1990) Outbreak of diarrhoea due to Escherichia coli O111:B4 in schoolchildren and adults association of Vi antigen-like-reactivity. Lancet 336:831–834
Wang G, Clark CG, Rodgers FG (2002) Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157 H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J Clin Microbiol 40:3613–3619
Winfield MD, Groisman EA (2003) Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl Environ Microbiol 69:3687–3694
Yamamoto T, Nakazawa M (1997) Detection and sequences of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene in enterotoxigenic E. coli strains isolated from piglets and calves with diarrhea. J Clin Microbiol 35:223–227
Yue WF, Du M, Zhu MJ (2012) High temperature in combination with UV irradiation enhances horizontal transfer of stx2 gene from E. coli O157H7 to non-pathogenic E. coli. PLoS One 7:e31308
Acknowledgements
This research was undertaken and funded as part of the Urban Water Security Research Alliance, a scientific collaboration in South East Queensland, Australia, between the Queensland Government, CSIRO Water for a Healthy Country Flagship Program, The University of Queensland, and Griffith University. The authors also wish to acknowledge the support of TasWater staff in the collection of water samples from Tasmania.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Diane Purchase
Electronic supplementary material
Table S1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Sidhu, J.P.S., Jagals, P., Smith, A. et al. Comparative prevalence of Escherichia coli carrying virulence genes and class 1 and 2 integrons in sub-tropical and cool temperate freshwater. Environ Sci Pollut Res 24, 18263–18272 (2017). https://doi.org/10.1007/s11356-017-9497-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9497-0