Abstract
In this study, to examine the accumulated micropollutants in the spent carbon filter used in the water purifier, first, the method to desorb micropollutant from the activated carbon was developed and optimized. Then, using this optimized desorption conditions, we examined which micropollutants exist in spent carbon filters collected from houses in different regions in Korea where water purifiers were used. A total of 11 micropollutants (caffeine (CFF), acetaminophen (ACT), sulfamethazine (SMA), sulfamethoxazole (SMZ), metoprolol (MTP), carbamazepine (CBM), naproxen (NPX), bisphenol-A (BPA), ibuprofen (IBU), diclofenac (DCF), and triclocarban (TCB)) were analyzed using LC/MS-MS from the spent carbon filters. CFF, NPX, and DCF had the highest detection frequencies (>60%) in the carbon filters (n = 100), whereas SMA, SMZ, and MTP were only detected in the carbon filters, but not in the tap waters (n = 25), indicating that these micropollutants, which exist less than the detection limit in tap water, were accumulated in the carbon filters. The regional micropollutant detection patterns in the carbon filters showed higher levels of micropollutants, especially NPX, BPA, IBU, and DCF, in carbon filters collected in the Han River and Nakdong River basins where large cities exist. The levels of micropollutants in the carbon filter were generally lower in the regions where advanced oxidation processes (AOPs) were employed at nearby water treatment plants (WTPs), indicating that AOP process in WTP is quite effective in removing micropollutant. Our results suggest that desorption of micropollutant from the carbon filter used can be a tool to identify micropollutants present in tap water with trace amounts or below the detection limit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface water is commonly used as a source of drinking water in areas with growing urban populations (Stackelberg et al. 2007). Micropollutants such as pharmaceuticals and personal care products (PPCPs) can flow into the water environment from various sources, such as pharmaceutical factories, households, antibiotics from livestock, agricultural effluents, aquaculture, and landfill leachate (Focazio et al. 2008). As an another micropollutant category, endocrine-disrupting compounds such as bisphenol-A (BPA) and triclocarban (TCB) can cause dysfunctioning of the human endocrine system over time, even at very low levels (Duong et al. 2010). BPA has been widely utilized in the production of epoxy resins and polycarbonate plastics, such as food containers and baby bottles. TCB and triclosan are commonly found in personal care products, such as soaps, lotions, deodorants, toothpaste, and plastics (Li et al. 2010).
Micropollutants have been found in wastewater treatment plant (WWTP) effluent, surface water, and even tap water, in nanograms per liter up to micrograms per liter amounts (Vanderford et al. 2003; Kim et al. 2007; Kasprzyk-Hordern et al. 2008; Benotti et al. 2009; Rahman et al. 2009; Nam et al. 2014a). They cannot be completely removed using conventional water treatment processes (WTPs) such as coagulation and filtration (Ratola et al. 2012; Nam et al. 2014a). Previous studies reported that micropollutants such as acetaminophen (ACT), caffeine (CFF), naproxen (NPX), and ibuprofen (IBU) can be removed by greater than 80% in water treatment plants (WTPs), whereas other micropollutants such as carbamazepine (CBM), diclofenac (DCF), clofibric acid, and metoprolol (MTP) have shown much poorer removal efficiencies, implying that these micropollutants can be detected even in tap water (Yu et al. 2006; Gómez et al. 2007; Benotti et al. 2009; Nam et al. 2014a).
Activated carbon, such as granular activated carbon (GAC) or powder activated carbon (PAC), is known to effectively remove micropollutants such as taste and odor compounds, and PPCPs, by an adsorption process (Ternes et al. 2002; Westerhoff et al. 2005). Activated carbon is being widely used in water treatment plants (WTPs) and water purifiers, because it is cost-effective, simple, and has a high efficiency for micropollutant removal (Sotelo et al. 2012). However, adsorption efficiencies can vary by physicochemical properties such as the hydrophobicity of the compounds, pore size, amount of adsorbent, contact time, pH, and competition with natural organic matters (Yoon et al. 2003; Snyder et al. 2007; Nam et al. 2014b).
There is a high percentage of water purifier usage among homes and offices in Korea due to the widespread reluctance to use tap water as drinking water. The reason for not drinking tap water is due to anxiety brought about by a distrust of water tanks and water pipes; therefore, most people in Korea drink purified waters (Yoo 2005). Most water purifiers use an activated carbon or membrane filter as a pre- or post-treatment process. Micropollutants which exist in the tap water at trace levels can accumulate in these carbon filters by the adsorption. If accumulated micropollutants can be desorbed from the spent carbon filters, we can identify which micropollutants exist in tap water even if these are not detected in tap water due to lower than the detection limit.
There are several techniques to regenerate by desorbing contaminants from used activated carbon, such as thermal desorption (Ania et al. 2004; Ledesma et al. 2014) and microwave-induced desorption (Ania et al. 2004, 2005). However, each technique has limitation such as low regeneration efficiencies, high energy consumption, high cost, generation of toxic products, and potential secondary pollution (Zhou et al. 2015). For the most effective desorption and regeneration technique, chemical extraction technique can be used (Lu et al. 2011). However, with our knowledge, there has been no attempt to desorb micropollutants especially from spent carbon filters used for the water purifiers.
In this study, to examine accumulated micropollutants in the spent carbon filter used in the water purifiers, first, the method to desorb micropollutant from spent carbon filters was developed and optimized. Then, using this optimized desorption conditions, we examined which micropollutants exist in spent carbon filters obtained collected from houses where water purifiers were used. A total of 11 micropollutants were selected based on the annual domestic productions, the occurrence in surface and tap waters in Korea, and rate constants with chlorination and ozonation which are typically used in the water treatment processes in Korea (Table 1), then the micropollutants desorbed from spent carbon filters obtained in different regions along the four major river basins in Korea were measured. Finally, the micropollutant detection patterns in spent carbon filters collected in different regions were compared.
Materials and methods
Materials
CFF, ACT, sulfamethazine (SMA), sulfamethoxazole (SMZ), MTP, CBM, NPX, BPA, IBU, DCF, and TCB were purchased from Sigma-Aldrich (Korea). The physical and chemical properties of these micropollutants are shown in the supplementary materials (Table S1).
One hundred milligrams of each micropollutant was dissolved in 100 mL methanol to make a stock solution, and then stored at 4 °C to prevent biodegradation. 13C6-sulfamethoxazole and 13C6-naproxen (Cambridge Isotope Laboratories, Cambridge, UK) were used as internal standards to compensate for the matrix effects. Methanol (LC-MS grade) and acetonitrile were obtained from Fisher Scientific (USA). Coconut shell-based powdered activated carbon (PAC, Coway Co. Korea, 80-325 mesh) was used as an adsorbent and prepared as a solution of 1 g/L hydrated for 24 h in distilled water to activate the surface of the PAC.
Saturation of micropollutants into the activated carbon
A series of 2-L beakers containing hydrated 1 g/L PAC with distilled water was installed in a jar tester. The initial concentration of 10 mg/L of each of the 11 micropollutants was then added to the beakers. Rapid mixing by agitation (140 rpm) with mixing time of 90 min was performed to ensure a thorough mixing solution state and to accelerate the saturation of the micropollutants into the PAC. Saturation experiments were conducted at neutral pH (6.5∼6.8). Since the preliminary test showed that all micropollutants reached an equilibrium concentration after 90 min, the optimal contact time during micropollutant saturation into PAC was set to 90 min (Table S2).
Then, the micropollutant laden PAC was separated by filtration using a 0.45-μm GF/C filter (Whatman, UK) and dried at room temperature (20 ± 1 °C) for 24 h. The liquid chromatography-tandem mass spectrometry (LC/MS-MS) analysis showed that all micropollutants were almost completely adsorbed in the PAC filters (94.0 to 99.9%) (Table S2). The resulting saturated activated carbons were used to optimize desorption of micropollutant from activated carbon.
Optimization of micropollutant desorption from activated carbon
Table S3 shows the experimental conditions for desorption optimization of the micropollutants. To maximize micropollutant desorption from saturated activated carbon (10 mg of 11 selected micropollutant saturated in 1 mg PAC), four desorption parameters were investigated: stirrer, shaker, and sonicator; mixing time; extraction solvent (pH-adjusted distilled water, 10 mM CaCl2 in distilled water, methanol, and acetonitrile); and solvent volume.
For mixing tool experiment, batch type desorption experiments were carried out in the stirrer, shaker, or sonicator. Rapid mixing at 300 rpm was provided by a shaker and a 400-W sonication power (40 kHz) to ensure micropollutant desorption. Methanol and acetonitrile were used as organic extraction solvents. 10 mM CaCl2 in water and pH-adjusted deionized water were also compared with organic extraction solvent. After determining the extraction solvent as acetonitrile, different volumes of acetonitrile solvent (200, 400, and 800 mL) were applied to optimize the solvent volume. Finally, sonication time (30, 60, and 90 min) was also optimized (Table S3).
After the desorption, a 10-mL aliquot was withdrawn using a syringe and filtered through a 0.45-μm membrane filter, concentrated for 24 h using a centrifugal concentrator (CVE-3100, EVELA, Japan) and then eluted using 1.0 mL methanol. The desorbed micropollutants were then analyzed by LC/MS-MS.
Collecting spent carbon water filters
Figure 1 shows the four major rivers in Korea: the Han River, the Keum River, the Youngsan River, and the Nakdong River. Major cities such as Seoul, Busan, and Daegu are located near the Han and Nakdong river basins. The population of Seoul, in the Han River basin, is approximately 10 million in an area of 605.18 km2 (population density is 16,659 people/km2), and the Nakdong River basin has two large cities [“Daegu” city with a population of 2,518,467 in an area of 883.63 km2 (2857 capita/km2) and “Busan” city with a population more than 3 million in an area of 769.82 km2 (4628 capita/km2)], and several industrial complexes.
To investigate the detection pattern of micropollutants, a total of 100 spent carbon filters and 25 tap water (40 filters and 10 tap waters in the Han River basin, 12 filters and 3 tap waters in the Keum River basin, 16 filters and 4 tap waters in the Yeongsan River basin, 32 filters and 8 tap waters from Nakdong River basin) were collected from the houses where water purifiers were used in each river basin (Fig. 1). Water purifiers use tap water as raw water, and all tap waters are provided by the local WTPs, which collect source water mainly from the four major river basins.
We have collected two types of spent (post-use) carbon filters used in commercial water purifiers (C Company) with the same number amounts in the four major river basins. Type A filter has 10 μm in pore size, 16 cm in length, and contains approximately 130 g (dry weight (dw)) of carbon; type B filter has 1 μm in pore size, 8 cm in length, and contains approximately 60 g (dw). Type A filter is usually replaced every 6 months, and type B filter is usually replaced every 4 months. Preliminary experiment showed that there were no differences in the adsorption efficiencies of micropollutants from two types of spent carbon filters.
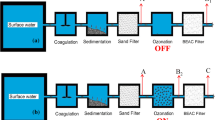
The collected carbon filters were grouped according to the presence of advanced oxidation process (AOP) facilities in nearby WTPs; 7 out of 10 have AOP in the Nakdong River basin and 5 out of 8 WTPs in the Nakdong River Basin. Table 2 shows the treatment processes in the nearby WTPs along the four major river basins where spent carbon filters are collected. The most widely used AOP process in WTP in Korea is ozonation followed by GAC. There was no WTP adopting the AOP process in the Keum River, or Youngsan River basin (Table 2).
Desorption of micropollutants from spent carbon filters
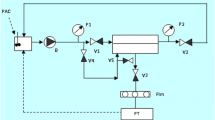
The collected spent carbon filters were evenly cut into slices with a thickness of less than 3 mm. The cut filters were then dried at 20 ± 1 °C for 24 h to remove any residual water. After drying, they were ground using a blender to obtain fine particle powders. From the preliminary desorption experiment with each part from spent carbon filter, we found that there was no difference in the micropollutant desorption levels depending on the depth of the spent filter. Therefore, the desorption experiment was performed by grinding the entire filter (both type A and type B). These ground carbon filters were then used for the desorption experiment, as shown in Fig. 2.
Desorption of micropollutant from carbon filter was performed using the optimized condition for desorption. After adding the carbon filters into acetonitrile solvent, they were sonicated for 90 min with 400 W sonication intensity, and then a 50-mL sample was withdrawn and filtered through a 0.45-μm filter. The samples were concentrated for 24 h using a centrifugal concentrator (CVE-3100, EVELA, Japan) and then eluted with 1 mL methanol. The desorbed micropollutants were then analyzed by LC/MS-MS.
Analytical methods
The selected micropollutants were analyzed by UPLC (Nexera, Shimadzu, Kyoto, Japan) connected to a triple quadrupole mass spectrometer (API-4000, Forster City, Canada) with an electron ion spray source working in both positive and negative modes. The selected micropollutants were separated with a reverse phase C18 column (Luna 3 μm; 150 × 20 mm, Phenomenex, Torrance, Canada). The flow rate was 0.2 mL/min, and the injection volume was 10 μL. The isocratic mobile phase was 98:2 (v/v, %) of methanol and water containing 10 mM ammonium formate and 0.3% formic acid (v/v) in the positive mode, and 98:2 (v/v, %) of methanol and water containing 5 mM ammonium acetate (v/v) in the negative mode (Nam et al. 2014a). The micropollutants were separated by ionization into negative (ACT, BPA, DCF, IBU, NPX, and TCB) or positive (CBM, CFF, MTP, SMA, and SMZ) ion groups. The optimized ion pairs of the 11 micropollutants and MS collision conditions are shown in Table S4. Also, the quality assurance and quality control (QA/QC) data is summarized in Table S5.
Results and discussion
Optimization of micropollutant desorption
In order to maximize desorption of micropollutant from the spent carbon filter, the desorption was optimized using micropollutant saturated activated carbon (10 mg of 11 micropollutants in 1 mg of PAC). Four parameters such as mixing tool, mixing time, extraction solvent, and solvent amount were investigated. First, the effects of the mixing tool (stirrer, shaker, and ultrasonic mill) were examined and the results are shown in Table 3. The sonicator showed the best micropollutant desorption efficiency followed by shaker and stirrer. The sonicator showed 2 to 10 times better desorption efficiencies than the shaker in almost all micropollutants (Table 3). The improvement effect of the sonication can be due to the cavitation effect between PAC particles generated by ultrasonic irradiation. Cavitation during sonication can generate rapid whirlpool action due to high-pressure oscillation, resulting in the weakening of the binding energy between the micropollutant and the PAC particles (Zhou et al. 2015).
The solubility of the compounds in the solvent and the affinity for the activate carbon surface have a significant influence on the regeneration efficiency (Karanfil and Dastgheib 2004). Therefore, the desorption efficiencies of different extraction solvents (distilled water, 10 mM CaCl2 in distilled water as water-based solvents, methanol and acetonitrile as organic-based solvents) were then compared (Table 3). The result showed that the micropollutants were hardly desorbed in both pH 12 and 10-mM CaCl2 distilled waters. In contrast, desorption of the micropollutants using organic solvents was significantly improved. In particular, acetonitrile solvent showed 2–10 times higher desorption performance than when using methanol (Table 3). This result may be due to the organic-organic interaction between organic micropollutant and organic solvent, and higher polarity of acetonitrile in organic solvents, resulting in a better chemical attraction towards the polar micropollutants (Martinez and Iverson 2012). However, DCF (log K ow = 3.91) and TCB (log K ow = 4.2–4.6) with higher log K ow values (see Table S1) showed lower desorption amounts; even methanol and acetonitrile solvents were used. This result indicates that micropollutants with higher log K ow values cannot easily be desorbed from activated carbon even with organic solvents. From the results, acetonitrile was chosen as a desorption solvent from spent carbon filter since the acetonitrile solvent generally exhibits better desorption of micropollutants.
Next, by increasing the acetonitrile extraction volume from 200 to 800 mL, the desorbed amount of microorganisms increased significantly (Table 3), indicating that desorption can be improved by increasing the ratio of extraction solvent to carbon. The desorption amount also increased by increasing the mixing time from 30 to 90 min. However, there was a slight difference between 90 and 120 min (date not shown). Therefore, the optimal condition for desorption was set to 800 mL of acetonitrile and 90 min sonication for the desorption of micropollutant from activated carbon.
Detection of micropollutants from carbon filters using the optimized desorption conditions
Using optimized desorption conditions, desorption of micropollutants from 100 spent carbon filters used at home for 4–6 months was performed. Table 4 shows the detection frequency and desorption amount of 11 micropollutants from the spent carbon filter with the optimized desorption conditions. The result showed that NPX, DCF, and CFF were detected in more than 80% of the 100 spent carbon filters, but SMA, SMZ, and TCB were detected at rates of less than 10% (Table 4). Among the micropollutants, NPX was the most frequently detected and showed the highest level of desorption from the carbon filters (Table 4, Fig. S1). Similar result was reported in Korea previously. Kim et al. (2007) showed that NPX and CFF are the most frequently observed micropollutants in the surface water and WWTP effluents in Korea. They also reported that SMZ and CBM could be detected at significant concentrations in the surface water in Korea.
Recent studies in other countries reported that NPX were detected frequently tap water samples but at low concentration (∼52 ng/L) in Spain (Carmona et al. 2014). They also reported that the concentration of DCF and IBU ranged from 1 to 39 ng/L. Another study showed that CFF can be detected at concentrations of 2.5–225 ng/L in untreated and treated drinking water treatment systems in the USA (Wang et al. 2011). They also showed that SMZ can be removed efficiently by a chlorination treatment in conventional WTPs, but CBM and CFF are resistant to chlorination process (Wang et al. 2011). In fact, in this study, SMZ was actually not detected, and CBM was detected only at low levels in spent carbon filters by desorption (Table 4). Benotti et al. (2009) also reported that CBM and SMZ were present in tap water in the USA in quantities of 6.8 and 0.32 ng/L, respectively. This result implies that NPX, DCF, CBM, and CFF can survive in tap water after chlorination of WTP even at low levels.
Next, to compare the detection pattern and the levels of micropollutants in between spent carbon filters and tap waters which are used as raw waters in the water purifiers, we obtained 25 tap water samples from the houses where the carbon filters were collected (Fig. 1), and measured the levels of micropollutants in tap waters. Table 5 showed that while most micropollutants were detected in higher frequencies except that SMA, SMZ, and MTP were not detected in tap water. In contrast, MTP were detected in the spent carbon filters with 67% detection frequency (compared to SMA with only 2% detection frequency), respectively (Table 4). This result indicates that trace amounts of MTP may be present in amounts below the detection limit of tap water. Our results indicate that the measurement of micropollutants desorbed from the carbon filter used can be a tool to identify the micropollutants present below the detection limit in tap water.
Comparison of micropollutant desorption quantities among major river basins in Korea
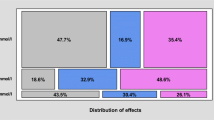
Because the carbon filters used were collected in different regions along the four major river basins in Korea, we can compare the regional patterns of the detection of the micropollutant in the carbon filters. Figure 3 showed that the carbon filters obtained in the Han and Nakdong River basins generally showed higher levels of micropollutants in the carbon filter. This result may be due to the close proximity of the highly populated cities, such as Seoul, Busan, and Daegu metropolitan cities, to these river basins. In these cities, the usage of micropollutants among the people would be much higher than for other less populated areas. Especially, NPX, BPA, IBU, and DCF showed higher levels (Fig. 3). Table 1 showed that NPX and DCF are previously reported with higher levels in surface waters, and BPA are detected in higher levels in tap water in Korea.
Next, the desorption of the micropollutant from the carbon filter was compared to the regions according to the presence of the AOP in nearby WTPs where the carbon filter used was collected. The most widely adopted AOP process in WTPs in Korea is the ozone process and biologically active carbon (Table 2). According to the results of comparison, the level of micropollutants in the carbon filter used was generally lower in the regions where AOPs were employed in nearby WTPs (Fig. 4). In particular, CFF, NPX, BPA, and DCF were statistically significantly lower at sites where AOP was used in WTP (p < 0.05) (Fig. 4). The results indicate that the AOP process is effective at removing micropollutants in WTP. This result is consistent with previous studies that biological activated carbon filtration after ozonation is effective in removing micropollutants, natural organic materials, taste and odor compounds, and disinfection by-product pre-cursors (Simpson 2008; Reungoat et al. 2010; Reungoat et al. 2012).
Conclusions
In order to investigate the micropollutants adsorbed in the carbon filter used in the water purifier, this study first optimized the method of desorbing particulates from the spent carbon filter. Then, we examined which micropollutants exist in spent carbon filters used in water purifier by using optimized desorption conditions. As a result, CFF, NPX, and DCF showed high detection frequency (>80%) in the spent carbon filter, but SMA, SMZ, and MTP were detected only in the carbon filter, not in tap water, indicating that these micropollutants were present at below the detection limit in tap water, and thus accumulated in the carbon filters. Higher concentrations of micropollutants, especially NPX, BPA, IBU, and DCF, were observed in the spent carbon filters collected in the Han River and Nakdong River basins of the metropolitan area. The results also show that the advanced oxidation process of WTPs is effective in removing micropollutants. In future studies, micropollutant desorption strategies can be used to detect as-yet unknown micropollutants from spent carbon filters and further compare the local characteristics of tap water.
References
Acero JL, Benitez FJ, Francisco JR, Fernando T (2016) Micropollutants removal from retentates generated in ultrafiltration and nanofiltration treatments of municipal secondary effluents by means of coagulation, oxidation, and adsorption processes. Chem Eng J 289:48–58. doi:10.1016/j.cej.2015.12.082
Ania CO, Menendez JA, Parra JB, Pis JJ (2004) Microwave-induced regeneration of activated carbons polluted with phenol. A comparison with conventional thermal regeneration. Carbon 42(7):1383–1387. doi:10.1016/j.carbon.2004.01.010
Ania CO, Parra JB, Menendez JA, Pis JJ (2005) Effect of microwave and conventional regeneration on the microporous and mesoporous network and on the adsorptive capacity of activated carbons. Micropor Mesopor Mater 85:7–15. doi:10.1016/j.micromeso.2005.06.013
Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA (2009) Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ Sci Technol 43:597–603. doi:10.1021/es801845a
Carmona E, Andreub V, Picóa Y (2014) Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci Total Environ 484:53–63. doi:10.1016/j.scitotenv.2014.02.085
Duong CN, Ra JS, Cho J, Kim SD, Choi HK, Park JH, Kim KW, Inam E, Kim SD (2010) Estrogenic chemicals and estrogenicity in river waters of South Korea and seven Asian countries. Chemosphere 78:286–293. doi:10.1016/j.chemosphere.2009.10.048
Focazio MJ, Kolpin DW, Barnes KK, Furlong ET, Meyer MT, Zaugg SD, Barber LB, Thurman ME (2008) A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States—II untreated drinking water sources. Sci Total Environ 402:201–216. doi:10.1016/j.scitotenv.2008.02.021
Gómez MJ, Bueno MJM, Lacorte S, Fernandez-Alba AR, Aguera A (2007) Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 66:993–1002. doi:10.1016/j.chemosphere.2006.07.051
Karanfil T, Dastgheib SA (2004) Trichloroethylene adsorption by fibrous and granular activated carbons: aqueous phase, gas phase, and water vapor adsorption studies. Environ Sci Technol 38:5834–5841. doi:10.1021/es0497936
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water res 42(13):3498–3518. doi:10.1016/j.watres.2008.04.026
Korea Ministry of Environment (2007) The 3rd investigation of the amount of chemical usage. Res Rep 138–199
Korea Pharmaceutical Manufacturers Association (2003) Statistics of Korea Pharmaceutical Manufacturers Association, 6
Kim SD, Cho J, Kim IS, Vanderford BJ, Snyder SA (2007) Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water res 41:1013–1021. doi:10.1016/j.watres.2006.06.034
Ledesma B, Roman S, Sabio E, Álvarez-Murillo A (2014) Aqueous thermal desorption as an effective way to regenerate spent activated carbons. J Supercrit Fluids 85:24–30. doi:10.1016/j.supflu.2013.10.017
Lee Y, von Gunten U (2010) Oxidative transformation of micropollutants during municipal wastewater treatment: comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water res 44:555–566. doi:10.1016/j.watres.2009.11.045
Lee Y, von Gunten U (2012) Quantitative structure—activity relationships (QSARs) for the transformation of organic micropollutants during oxidative water treatment. Water res 46:6177–6195. doi:10.1016/j.watres.2012.06.006
Li X, Ying GG, Su HC, Yang XB, Wang L (2010) Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ Int 36:557–562. doi:10.1016/j.envint.2010.04.009
Lu PJ, Lin HC, Yu WT, Chern JM (2011) Chemical regeneration of activated carbon used for dye adsorption. J Taiwan Inst Chem Eng 42:305–311. doi:10.1016/j.jtice.2010.06.001
Martinez CR, Iverson BL (2012) Rethinking the term “pi-stacking”. Chem Sci 3:2191–2201. doi:10.1039/C2SC20045G
Nam SW, Jo BI, Yoon Y, Zoh KD (2014a) Occurrence and removal of selected micropollutants in a water treatment plant. Chemosphere 95:156–165. doi:10.1016/j.chemosphere.2013.08.055
Nam SW, Choi DJ, Kim SK, Her N, Zoh KD (2014b) Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J Hazard Mater 270:144–152. doi:10.1016/j.jhazmat.2014.01.037
National Institute of Environmental Research (2007) Development of analytical method and study of exposure of pharmaceuticals and personal care products in environment (II). Res Rep 1–393
National Institute of Environmental Research (2011) Development of analytical method and study of residual pharmaceuticals and personal care products. Res Rep 1–358.
National Institute of Environmental Research (2014) A study on management of unregulated trace hazardous compounds in drinking water. Res Rep 1–606
Rahman MF, Yanful EK, Jasim SY (2009) Endocrine disrupting compounds (EDCs) and pharmaceuticals and personal care products (PPCPs) in the aquatic environment: implications for the drinking water industry and global environmental health. J Water Health 7:224–243. doi:10.2166/wh.2009.021
Ratola N, Cincinelli A, Alves A, Katsoyiannis A (2012) Occurrence of organic microcontaminants in the wastewater treatment process. A mini review. J Hazard Mater 239:1–18. doi:10.1016/j.jhazmat.2012.05.040
Reungoat J, Escher BI, Macova M, Argaud FX, Gernjak W, Keller J (2012) Ozonation and biological activated carbon filtration of wastewater treatment plant effluents. Water res 46:863–872. doi:10.1016/j.watres.2011.11.064
Reungoat J, Macova M, Escher BI, Carswell S, Mueller JF, Keller J (2010) Removal of micropollutants and reduction of biological activity in a full scale reclamation plant using ozonation and activated carbon filtration. Water res 44:625–637. doi:10.1016/j.watres.2009.09.048
Simpson DR (2008) Biofilm processes in biologically active carbon water purification. Water res 42:2839–2848. doi:10.1016/j.watres.2008.02.025
Snyder SA, Adham S, Redding AM, Cannon FS, DeCarolis J, Oppenheimer J, Wert EC, Yoon Y (2007) Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 202:156–181. doi:10.1016/j.desal.2005.12.052
Sotelo JL, Rodriguez A, Alvarez S, Garcia J (2012) Removal of caffeine and diclofenac on activated carbon in fixed bed column. Chem Eng res des 90:967–974. doi:10.1016/j.cherd.2011.10.012
Stackelberg PE, Gibs J, Furlong ET, Meyer MT, Zaugg SD, Lippincott RL (2007) Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci Total Environ 377:255–272. doi:10.1016/j.scitotenv.2007.01.095
Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, Gulde BH, Preuss G, Wilme U, Seibert NZ (2002) Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol 36:3855–3863. doi:10.1021/es015757k
Vanderford BJ, Pearson RA, Rexing DJ, Snyder SA (2003) Analysis of endocrine disruptors, pharmaceuticals, and personal care products in water using liquid chromatography/tandem mass spectrometry. Anal Chem 75:6265–6274. doi:10.1021/ac034210g
Wang C, Shi H, Adams CD, Gamagedara S, Stayton I, Timmons T, Ma Y (2011) Investigation of pharmaceuticals in Missouri natural and drinking water using high performance liquid chromatography-tandem mass spectrometry. Water res 45:1818–1828. doi:10.1016/j.watres.2010.11.043
Westerhoff P, Yoon Y, Snyder S, Wert E (2005) Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ Sci Technol 39:6649–6663. doi:10.1021/es0484799
Yoo SH (2005) Analyzing household bottled water and water purifier expenditures: simultaneous equation bivariate Tobit model. Appl Econ Lett 12:297–301. doi:10.1080/1350485042000293121
Yoon Y, Westerhoff P, Snyder SA, Esparza M (2003) HPLC-fluorescence detection and adsorption of bisphenol A, 17 beta-estradiol, and 17 alpha-ethynyl estradiol on powdered activated carbon. Water res 37:3530–3537. doi:10.1016/S0043-1354(03)00239-2
Yu JT, Bouwer EJ, Coelhan M (2006) Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agric Water Manag 86:72–80. doi:10.1016/j.agwat.2006.06.015
Zhou C, Gao NY, Li RY, Deng Y (2015) Desorption of bisphenol-A (BPA) and regeneration of BPA-spent granular activated carbon using ultrasonic irradiation and organic solvent extraction. Desalin Water Treat 54:3106–3113. doi:10.1080/19443994.2014.912961
Acknowledgements
This work was supported by Ministry of Environment (MOE) as “Technologies for the Water Supply & Sewerage Policy of Public Technology Program based on Environmental Policy (2016000700005)”. We also acknowledge the financial support from Coway Co., Ltd. with supplying the filter samples (900-20140091).
Author information
Authors and Affiliations
Corresponding author
Additional information
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see http://www.textcheck.com/certificate/r9l1mj.
Electronic supplementary material
ESM 1
(DOCX 69 kb)
Rights and permissions
About this article
Cite this article
Kwon, DS., Tak, SY., Lee, JE. et al. Desorption of micropollutant from spent carbon filters used for water purifier. Environ Sci Pollut Res 24, 17606–17615 (2017). https://doi.org/10.1007/s11356-017-9311-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9311-z