Abstract
Monitoring of pesticide residues in food commodities of plant origin is part of the regular controls on food to safeguard consumer’s health. This study reports for the first time in Ghana a 3-year (2010–2012) monitoring of pesticide contamination of fruits and vegetables and their health implications. A total of 3483 samples were purchased in notable markets within Accra Metropolis and analysed for pesticide residues, employing the modified quick, easy, cheap, effective, rugged and safe analytical procedure. The results indicated that almost all the fruits and vegetables studied had residues above maximum residue limits (MRLs). The commodities with the greatest concentrations exceeding the European Union (EU) MRLs were long green beans (60.6%) and lettuce (57.1%) with watermelon (10%) and green pepper (8.6%) having the least. The relative occurrence of the pesticides was fenvalerate 11.3%, fenitrothion 5.6%, lambda-cyhalothrin 3.6%, dimethoate 3.2%, permethrin 2.7% and deltamethrin 2.2%. These results will serve as a baseline on which annual or other long-term studies could be compared with, thus emphasizing the need for continuous monitoring programmes to regulate trends of pesticide residues in fruits and vegetables to safeguard the consumers’ health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing demand of food safety has resulted in accelerated studies as regards the risk associated with the consumption of foodstuffs contaminated with pesticides. Apparently, pesticides are well known for their key role in boosting agricultural crop production through crop pest eradication and also in the public health sector for disease vector control. Thus, in the course of applying these chemicals to crops, some may remain on the food as residues and/or may affect the terrestrial environment. Hence, it is not surprising that globally, public concern over pesticide residues in food is on the rise.
Pesticides enter human body by inhalation, ingestion and dermal contact. Food is the main exposure route for pesticide ingestion, and the residues in diet are far higher than any other exposure routes, for example air and drinking water. This could result in several ranges of human health problems, both acute (e.g. anaemia, flu, headaches, skin rashes, blurred vision) and chronic (e.g. cancer, infertility, endocrine disorders, reproductive abnormalities) diseases (Damalas 2009; Abhilash and Singh 2009). Moreover, foods like vegetables are rich sources of vitamins, minerals and fibre in a balanced diet. The World Health Organization (WHO) states that food consumption by man should contain at least 30% of fruits and vegetables depending on the weight of the individual because they contain some essential nutrients needed to fight against certain diseases. To this effect, in Ghana, the consumption of vegetables has increased (Osei-Fosu et al. 2014; Donkor et al. 2017). The percentage of household consuming fruits and vegetables is 99%, the quantity being 73.7 kg/person/year. This value is 50% of the Food and Agriculture Organization (FAO)/WHO minimum recommended level of 146 kg/person/year (Ruel et al. 2004). Nonetheless, because vegetables are consumed raw or semi-processed, there is a risk that they may contain elevated levels of pesticides. Consequently, the application of pesticides in agriculture is subjected to constant monitoring (Osei-Fosu et al. 2014; Donkor et al. 2017).
Nowadays, in many developed countries (e.g. the USA, European Union, Japan and Canada), consumers’ health is of priority and therefore have established legal directives in food safety and continuous monitoring of pesticide residues in food of plant origin. This is done to regulate the application of pesticides to permit the free circulation of pesticide-treated products as long as they comply with the set maximum residue limits (MRLs) (FAO/WHO 2004; Osman et al. 2010; Bošković and Pucarević 2012). Additionally, regular monitoring of pesticide residues in food assures conformity with the principles of good agricultural practice (GAP) and consumer risk assessment. However, the outcomes of numerous monitoring programmes demonstrate that American and European consumers are under lower risk associated with the toxic effects of pesticides which are as a consequence of the application of less toxic and persistent pesticides. Further, these chemicals are more effective at lower concentrations coupled with improved techniques of application than previous practices.
However, the situation in some developing countries in Asia and Sub-Saharan Africa remains poorly studied and the extent and seriousness of the problem are partly unknown. Many of these nations faced several challenges like lack of financial support for scientific research and inadequate environmental policy and regulations, and in countries where they exist, agencies responsible lack the required capacity to ensure compliance and enforcement of these regulations (Bempah and Donkor 2010; Bošković and Pucarević 2012). Moreover, reports from developed nations indicate that samples of crops from developing countries are always characterized by elevated levels of pesticide residues above MRLs, attesting the fact that there are no coordinated national monitoring programmes or regulatory control of these compounds (Bošković and Pucarević 2012). Accordingly, regulation and higher standards in food safety are presently being demanded by international bodies and/or developed nations who are the recipients of some of the food from the developing and transition world.
Ghana can boast of pesticide regulation and laws; these legal standards regulate all aspects related to pesticides, including registration, use, licensing, issuing of permit for exports and imports of chemicals, inspection and monitoring of industrial/consumer chemicals and agrochemicals, production, storage, transport and disposal. The Environmental Protection Agency Act (Act 490) is the pesticides regulation in Ghana and is administered by the Chemicals Control and Management Centre (CCMC) division of the Environmental Protection Agency since 1994. The pesticide registration in reality is supposed to evaluate the impact of pesticide application on human health. Nonetheless, Ghana has not established her own MRLs but depends on the limits set up by the Codex Alimentarius or European Union.
In Ghana, organochlorine pesticides (OCPs) were prohibited in farming practices because of its persistency, bioaccumulative properties and human health implications. It was enforced in May 2004 by the Stockholm Convention. Accordingly, organophosphorous (OP) and synthetic pyrethroid (SP) pesticides are the only registered and most commonly applied pesticides for pest and disease vector eradication. Thus, in fruits and vegetables, these pesticides are used for better quality and yield. Some are applied to crops during the entire period of growth and, sometimes, at the fruiting stage; others are used to protect produce after harvesting. These are absorbed by the crops which turned out to be noxious when consumed by humans (Donkor et al. 2016). Many farmers applied combinations of pesticides (cocktail of synthetic insecticides such as lambda-cyhalothrin (Karate), acetellic and dimethoate) indiscriminately on their crops. However, procedural spraying is hard to be found. Farmers have the belief that the more pesticides sprayed, the better, forgetting that it rather affects yield and the end user of the product. In few cases, the application rates have been noted; for example, Karate 2.5 EC used on vegetables like cabbage, tomato and garden egg is sprayed at the rate of 200–800 ml/ha, whereas chlorpyrifos (Dursban 4E) is at 24 g AI/ha (Ntow et al. 2006; Afari-Safa et al. 2015).
In recent years, there has been a rapid increase in the quantity and use of pesticides (insecticides, fungicides, herbicides, bactericides, rodenticides and plant growth regulators) in agriculture, ranging from 957,474.2 t in 1992 to 1,912,994 t in 2007 in Ghana (Donkor et al. 2016 and references therein) and this is expected to rise in the next decades. Agricultural pesticides are used in cocoa, coffee and cotton farming; in vegetable and fruit production; and for other mixed crop farming systems involving cereals (mostly maize), tuber crops (e.g. yam, cassava), legumes (e.g. cowpeas), sugarcane, rice, etc. The majority of these pesticides are employed in the forest areas or farming regions noted for the production of these crops located in Ashanti, Brong-Ahafo, Eastern and Western regions of Ghana (Ntow 2005; Amoah et al. 2006). Ntow et al. (2006) gave the proportions of pesticides used extensively on vegetable farms, small or large by farmers, as herbicides (44%), fungicides (23%) and insecticides (33%). Herbicides are the predominant pesticide type use in vegetable production in Ghana probably due to the farmers’ perception of weed control.
More significantly, in Ghana, there are limited studies on pesticide residue analysis data on fruits, vegetables and other food commodities. (e.g. Amoah et al. 2006; Bempah and Donkor 2010; Bempah et al. 2011, 2012). All these studies monitored pesticide residues in fruits and vegetables for a very limited period, usually between 3 and 11 months. All the same applications of pesticides by farmers in their pest control are still on the increase without compliance to good agricultural management practices. Likewise, there are no monitoring programmes for pesticide residues in foods currently in place in Ghana aimed at evaluating compliance with international MRLs, as may be the case in many of the Sub-Saharan nations. From a public health point of view, assessing the actual exposure to these chemicals and their evolving trends over time is very pertinent even if concentrations found are below the accepted limits. Additionally, it provides information on individual pesticide control, the levels and sources of contamination in foods and the amounts of contaminants ingested by humans which could impact their health negatively. Further, it ensures that pesticides are applied as safely as possible, addresses the problem of dietary risk assessment among consumers and also assesses the risk of different chemicals. It also gives valuable information for law makers who determine the agricultural and environmental policies of the country (Dogheim et al. 2002; Fontcuberta et al. 2008; Osman et al. 2010; Bošković and Pucarević 2012).
In this regard, this paper reports for the first time results obtained from the monitoring and analysis of pesticide residues in vegetable and fruit samples from different parts of Ghana and beyond arriving at well-known markets in Accra Metropolis between 2010 and 2012. The possible health risk associated with pesticides having violations is also discussed. The results from this study hope to be the baseline data upon which annual or other long-term monitoring studies could be compared with as well as utilizing it in estimating the potential health risks associated with the consumption of fruits and vegetables. More so, with such data, the Ministry of Food and Agriculture can be assured of compliance of the principles of good agricultural practices and consumer risk assessment. Finally, it can be used when drafting future environmental policy and control programmes for Ghana and taking preventive actions to minimize human health risks.
Materials and methods
Chemicals and reagents
Sodium chloride (NaCl) (high purity), disodium hydrogen citrate sesquihydrate, trisodium citrate dehydrate, anhydrous magnesium sulphate (MgSO4) (all analytical grade) and formic acid (pesticide grade) were obtained from the BDH Laboratory Supplies, England. The anhydrous MgSO4 (≥98% purity) was baked at 130 °C for 12 h in an oven to remove phthalates. Bondesil—primary-secondary amine sorbent of 40 μm particle size—was obtained from Varian (Harbor City, CA, USA). All organic solvents (ethyl acetate, acetone and acetonitrile) used in the study were pesticide grade.
Thirty-six pesticides with reference standards of >98% purity were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The pesticides were 13 organophosphorous (dimethoate, parathion, profenofos, fonofos, diazinon, fenitrothion, phorate, chlorfenvinphos, pirimiphos-methyl, chlorpyrifos, ethoprophos, methamidophos and malathion), 8 synthetic pyrethroids (lambda-cyhalothrin, cypermethrin, allethrin, cyfluthrin, deltamethrin, bifenthrin, permethrin and fenvalerate) and 15 organochlorines (beta-endosulfan, gamma chlordane, lindane, aldrin, beta-hexachlorocyclohexane (HCH), methoxychlor, p,p′-dichlorodiphenyldichloroethane (DDD), endosulfan sulphate, endrin, p,p′-dichlorodiphenyltrichloroethane (DDT), p,p′-dichlorodiphenyldichloroethylene (DDE), heptachlor, dieldrin, alpha-endosulfan and delta-HCH). Standard mixture stock solutions were prepared in ethyl acetate at 1000 μg/g for each pesticide. This solution was used for spiking and also to prepare the daily standard working solutions at various concentrations by appropriate dilution of aliquots of the stock solution in ethyl acetate.
Sample collection
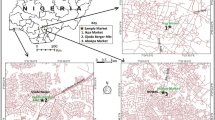
A total of 3483 fruit and vegetable samples comprising tomato (Solanum lycopersicum), watermelon (Citrullus lanatus var. lanatus), green pepper (Capsicum annuum), eggplant (purple, Solanum melongena), squash (Cucurbita sp.), chilli pepper (C. annuum), eggplant (light green, S. melongena), cauliflower (Brassica oleracea), long green beans (Phaseolus vulgaris), short green beans (P. vulgaris), red bell pepper (Capsicum sinense Jacq-red, locally called kpakposhito), green bell pepper (C. sinense Jacq, locally called kpakposhito), cabbage (B. oleracea), okra (Hibiscus esculentus), lettuce (Lactuca sativa), carrot (Daucus carota) and cucumber (Cucumis sativus) were collected from the most popular markets (Agbogbloshie, Mallam Junction, Dome, Mallam Atta and Madina) in Accra Metropolis, Ghana (Fig. 1) between the months of January 2010 to December 2012. All samples were taken according to the recommended methods of sampling for the determination of pesticide residues for compliance with MRLs (CAC/GL 33-1999). Samples were randomly taken at retail points within the various markets. The sample size was at least 1 kg for small- and medium-sized fresh products. The minimum weight for a large product sample (for example, cabbage and watermelon) was 2 kg. Samples were immediately placed in plastic bags and then in an ice chest kept at 4 °C and sent to the laboratory. All samples in their original state were analysed and unwashed, whole and with the peel or skin intact within 24 h after collection. Samples waiting for analysis were stored at 4 °C. Organophosphates and synthetic pyrethroid pesticides in present use together with organochlorines applied in the past were ascertained (the 36 pesticides listed above). These OPs and SPs are registered in Ghana for fruit and vegetable cultivation. The banned organochlorines were also studied to check for their presence or absence.
Method validation
In this study, quick, easy, cheap, effective, rugged and safe (QuEChERS) method was modified for the determination of 36 pesticides fortified at 0.01–1.0 mg/kg in three vegetables and a fruit (lettuce, carrot, tomatoes and pineapples, respectively). Carrot is a root and tuber vegetable with less water content, pineapple has high acid content, tomato is a fruiting vegetable with high water content and lettuce has high water and chlorophyll contents. These were used in the fortification experiments. The method involved extraction, phase separation and the final injection with ethyl acetate as described in Donkor et al. (2015); the entire procedure is outlined below.
Extraction and clean-up
An amount of 10.0 g of the comminuted homogenous samples was weighed into a 50-ml polypropylene (PP) centrifuge tube, extracted with 10 ml acetonitrile and vortexed for 1 min. After that, a mixture of 4.0 g anhydrous MgSO4, 1.0 g NaCl, 1.0 g trisodium citrate dihydrate and 0.5 g disodium hydrogen citrate sesquihydrate was added, immediately vortexed for 1 min and centrifuged for 5 min at 3000 rpm.
An amount of a 6.0-ml aliquot of the extract was transferred into a 15-ml PP centrifuge tube containing 150 mg primary-secondary amine (PSA) and 900 mg MgSO4. The tube was closed and shaken vigorously, vortexed for 30 s and centrifuged for 5 min at 3000 rpm; 4.0 ml of the cleaned extract was transferred into a round bottom flask and adjusted quickly to a pH of 5 by adding 40 μl of 5% formic acid solution in acetonitrile (v/v). The filtrate was concentrated to just dryness on a pressure-reduced rotary evaporator with a water bath temperature below 40 °C. The extract was re-dissolved by adding 1.0 ml of ethyl acetate and was then transferred into a 1.8-ml standard opening vial for quantitation with gas chromatography-electron capture detector (GC-ECD) and gas chromatography-pulsed flame photometric detector (GC-PFPD).
GC analysis of pesticide residues
Organochlorines and synthetic pyrethroids
The organochlorine (OC) and SP residues were analysed with Varian Gas Chromatograph CP-3800 equipped with 63Ni ECD. The GC conditions used for the analysis were as follows: capillary column coated with VF-5ms (30 m + 10 m EZ-Guard, 0.25 mm, 0.25 μm film thickness), and carrier gas and make-up gas were nitrogen at a flow rate of 1.0 and 29 ml/min, respectively. The temperature of injector operating in splitless mode was held at 270 °C, and ECD was set at 300 °C. The column oven temperature was programmed as follows: 70 °C for 2 min and increased steadily at a rate of 25 °C/min to 180 °C and increased at 5 °C/min up to 300 °C. The injection volume of the GC was 1.0 μl. The residues detected by the GC analysis were confirmed on Varian GC CP-3800/Saturn 2200 MS/MS.
Organophosphorous
The OP residues were analysed by Varian Gas Chromatograph CP-3800 equipped with PFPD. The GC conditions were capillary column coated with VF-1701 (30 m, 0.25 mm, 0.25 μm film thickness), and the carrier gas was nitrogen at a flow rate of 2.0 ml/min with air 1, air 2 and H2 flow rate of 17, 10 and 14 ml/min, respectively. The temperature of injector operating in splitless mode was held at 270 °C, and the PFPD was set at 280 °C. The column oven temperature was programmed as follows: 70 °C for 2 min and increased steadily at a rate of 25 °C/min to 200 °C and increased at 20 °C/min up to 250 °C. The injection volume of the GC was 2.0 μl. Similar to the OC and SP, the residues detected by the GC analysis were confirmed on the mass spectrometer.
Quality control and quality assurance
The quality control/assurance carried out was analysis of blank samples, fortified samples and replicates. Each kind of fruit and vegetable samples was fortified at 0.05 mg/kg by adding intermediate pesticide solutions, and then the average recovery rate and relative standard deviations (RSDs, %) of pesticides were calculated. The recovery checks were carried out in replicates. The limit of detection (LOD) was calculated by using signal to noise ratio of 3, and the limit of quantification (LOQ) was also calculated by using a signal-to-noise ratio of 10. If the residue result was above the EU MRL, the sample was considered as an exceedance.
Estimation of short-term intake (acute intake)
In exposure assessment of pesticides, the regulatory threshold risk is the highest concentration of the exposure distribution; hence, these values from the monitoring results for this study were used to assess the acute dietary exposure of the pesticides and the commodities in adults and children. The estimated short-term intake (ESTI) was used to estimate acute dietary exposure. The calculation of the short-term intake was based on IESTI calculation (version 15) from the FAO/WHO acute dietary intake assessment. The average body weight of 60 kg for adults and 15 kg for children was used in the calculation.
Results and discussion
Table 1 gives a summary of the commodities studied and their regions of origin. The referenced markets in Accra Metropolis in the study are the points of consumer purchase, hub and also recipients for the distribution and sale of fruits and vegetables produced in localities particularly the Greater Accra (GA), Ashanti (AS) and Eastern Region (ER) of the country (Fig. 2). Limited quantities also arrive from Central Region (CR), Brong-Ahafo (BA), Western Region (WR), Upper East (UE), Upper West (UW), Volta Region (VR) and Northern Region (NR). Moreover, about 11% of the total bulk originates from neighbouring countries, Togo, Niger and Burkina Faso (OT). Retailers, hawkers and consumers/buyers within and outside the Metropolis all gather at these markets to obtain their stock from the farmers or middle men. The origins of samples purchased were authenticated from the vendors/farmers. Further, these fruits and vegetables were selected because of their widespread acceptance in the Ghanaian diet; there is not much or virtually no processing before consumption.
Overall, 3483 samples were analysed; 2334 samples were obtained from the Greater Accra, Eastern and Ashanti regions (Table 1). Over 67% of the entire samples analysed were provided by the Greater Accra, Eastern and Ashanti regions with 11% coming from neighbouring countries. Contributions from the other regions were minimal with no samples from Western and Upper West regions; these regions are not popular for the growth of these commodities.
Table 2 summarizes the validity of the method. In all, 36 pesticides were detected, employing the modified QuEChERS procedure. The method was validated by evaluating linearity, recovery, correlation coefficient, analytical limit of quantification (LOQ) and RSD. The pesticide residues at or above the LOQ were reported. The LOQ varied between 0.005 and 0.01 mg/kg for most of the compounds. The MRLs were accessed from the EU pesticide database (December 2014). The GC with PFPD and ECD chromatograms of the blank tomato sample, standards and spiked tomato are shown in Figs. 3 and 4, respectively.
Concentrations of pesticide residues in fruits and vegetables
Table 3 outlines the data obtained after the analysis of 3483 samples of fruits and vegetables for 36 different pesticide residues (OPs, OCs and SPs). Overall, pesticide residues were detected or not detected among the various commodities examined. Others were also below the detection limit of <0.01 mg/kg. The concentration ranges and the corresponding EU MRLs for each pesticide residue and the commodity are described in Table 3. Aldrin was not detected in all the commodities except egg plant (0 < 0.01 mg/kg). On the other hand, gamma-HCH and delta-HCH were not found in green pepper and tomato, respectively. The highest range for these two OCs was found in the range not detectable (ND)–0.40 mg/kg for carrots and ND–0.62 mg/kg for cauliflower, respectively. Likewise, the metabolite p,p′-DDD was present in all the commodities studied with the highest range occurring in carrots. In the case of OPs, chlorpyrifos and fenitrothion residues were noted in all the commodities, whereas dimethoate and pirimiphos-methyl were the only pesticides not detected in cauliflower and squash, respectively. The highest concentration of dimethoate was noticed in lettuce (7.88 mg/kg). For the eight SPs examined, all of them were present in not detectable to detectable amounts in most commodities except kpakposhito (green) which had no allethrin. However, some of the commodities had higher maximum concentrations for all the SPs considered, for example lettuce and cabbage.
Pesticide residues above the EU MRLs
Out of the 3483 samples, 28 different pesticides exceeded their MRLs in the fruits and vegetables (Table 4). Each commodity contained at least two pesticides. Fenvalerate exceeded in 11.3% samples, fenitrothion and lambda-cyhalothrin exceeded the limit in 5.6 and 3.6% samples, respectively, whereas parathion, aldrin, endrin, endosulfan sulphate, methamidophos, heptachlor, phorate and ethoprophos had no exceedance. Besides, fenvalerate and fenitrothion were the most frequently found pesticides and were detected in most of the samples. The highest concentration of fenvalerate was found in short green beans (I) and garden egg (J) whereas, with fenitrothion, it was chilli pepper (A). This might be a result of their readily availability on the market as these pesticide-active ingredients are registered in Ghana for fruit and vegetable cultivation (Ghana Gazette 2012).
Parathion, aldrin, endrin, endosulfan sulphate, methamidophos, heptachlor, phorate and ethoprophos were not detected in any of the samples. DDT and its metabolites (DDD and DDE) were found in four commodities. This finding suggested that most OCs had been phased out in the Ghanaian environment.
In all, the highest exceedance was found in long green bean (containing four OPs, four SPs and four OCs) and lettuce (five OPs, four SPs and one OC) samples. Green pepper (P) and water melon (Q) were the least contaminated.
Percent of samples containing pesticide residues
Table 5 gives the overall statistical distribution of pesticide residue during the monitoring programme. The results revealed that more samples exceeded the MRLs in 2010 (46.8%) than those in 2011 and 2012. In all, 61.5% of the samples had residues below the MRL with 2011 registering the highest. On the other hand, 1128 samples contained levels above the MRL with only 213 samples having no detectable residues. Surprisingly, the number of samples in 2012 was lower than that in 2010 and 2011; this was as a consequence of poor rains affecting growth and harvesting of many of the fruits and vegetables.
Table 6 also shows the number of samples analysed from each region with quantity of samples having residues above or below the MRL. The residues at or below the MRL were detected in 2142 (62%) of the samples, 213 (6%) contained no pesticide residues whereas 1128 (32%) exceeded the MRL levels. Three hundred eighty-four samples from other locations beyond Ghana (Togo, Niger and Burkina Faso) and Upper East contained the highest number of residues (159 (41%) and 48 (47%)) exceeding their MRLs. The CR and BA though growth-specific vegetables had a lower number of samples, yet 28 and 25% of the samples were recorded with residues above the MRL, respectively.
These exceedance levels in most cases could be due to the pesticide residue remaining intact in the commodity. Generally, the number of samples without detectable residues was higher among GA, AS and ER. The same order was observed with samples with residues above or below the MRL for these regions. Likewise, the larger number of samples with residues below the MRL suggested a good agricultural practice could be in place in several of the locations whereas those above the MRL implied the cases where pesticides were applied directly to the edible commodity just before pre-harvest or after harvest to guarantee sound produce.
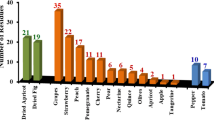
The largest percent violations occurred in long green beans (58%), lettuce (57%), chilli pepper (48%), white cabbage (46%), cucumber (42%) and garden eggs (41%) (Table 7). All the commodities except cauliflower and carrot had over 80% positive detections. Garden eggs, short green beans, green pepper, kpakposhito (green), kpakposhito (red) and white cabbage attained 100%. The recorded violations were perhaps due to non-compliance with the pre-harvest interval for different pesticides. Besides, many of these vegetable or fruit growers have a low level of education, receive limited technical support, do not read the pesticide labels or understand their content and are economically vulnerable as observed in Brazil. Others could also use the pesticide meant for one vegetable for another as reported in a Brazilian case study (Jardim and Caldas 2012). In all, green pepper (9%) was registered to have the lowest pesticide violation.
Co-occurrence of residue findings
In all, a total of 18,303 pesticide residues were found in 3483 samples (Table 8). A maximum of 18 pesticide residues were detected in a particular sample. On this count, only six samples showed this phenomenon. Four hundred eighty samples registering only five different residues were the most predominant. As much as 219 samples had no pesticide residues present. Most of the vegetables analysed contained two or more multiple residues; however, long green beans were recorded to have the highest multiple residue finding.
Comparison with data from other nations with similar studies
Monitoring results can vary significantly between various studies depending on the goals and objectives of the study, the food commodities analysed, the methods used, the number of samples selected for each commodity, the use of pesticides on the commodity, the analytical capabilities, the choice of pesticides examined in the study, the detection or reporting limit for the pesticide residues and the MRL allowed for a particular pesticide. Thus, comparing this study to other nations (Table 9), the results varied significantly between the different countries. The incidence of violative residues (61.5%) and their detection rates (32.4%) were significantly higher with Ghana’s samples than those reported for other countries in spite of the number of samples involved. The values in the various countries suggest good agricultural practices coupled with effective monitoring programmes could account for the lower residual level of pesticides in fruits and vegetables. A case in point is that of Korea, Egypt and China which have such programmes in place. On the contrary, Belgium and Denmark reported relatively higher pesticide detections. Besides the programmes for monitoring, these nations also focus on compliance with MRLs or, like the USDA, studies are designed to determine residues that may be present at any concentration, even orders of magnitude below the MRL (Ripley et al. 2000). Overall, the most frequently detected pesticides in the Ghanaian samples were fenvalerate and fenitrothion whereas, in the other nations, iprodione and boscalid in Belgium, malathion and fenthion in Spain, chlorpyrifos and imazalil in Croatia and dimethoate and dicofol in Egypt were the most frequently detected pesticides. The difference in the pesticides detected may be due to the crop/pesticide application in the various countries.
Estimation of short-term intake (acute intake)
Short-term exposure expressed as an acute hazard index for adults and children based on the highest concentration of pesticide residues detected in fruits and vegetables is presented in Tables 10 and 11.
Estimated short-term intake and risk assessment based on commodities
The contributions of each commodity to the total ESTI as well as the percentage acute reference dose (% ARfD) calculated are presented in Table 10. The % ARfD of the commodities ranged from 47,189.9 (in lettuce) to 3.2 (in green and chilli peppers). Lettuce was recorded to have the highest % ARfD and ESTI value of 47,189.9% and 1.22 × 101 mg/kg bw/day and 47,344.9% and 1.23 × 101 mg/kg bw/day for adults and children, respectively. Additionally, lettuce, carrot, squash, eggplant (light green), eggplant (purple), watermelon, tomato, cabbage and long green beans showed % ARfD values greater than 100% contributing much more than any other commodity to both intake and the risk. Chilli pepper was recorded to have the least % ARfD and ESTI values of 3.2% and 1.69 × 10–3 mg/kg bw/day and 0.8% and 4.19 × 10–4 mg/kg bw/day for both adults and children, respectively. Likewise, the remaining commodities from cucumber to green pepper are listed in Table 10. Therefore, these commodities with % ARfD values <100 would not pose a risk to consumers for short-term exposure.
Estimated short-term intake and risk assessment based on pesticides
The contribution of each pesticide to the total ESTI as well as the % ARfD were calculated and are presented in Table 11. The estimated short-term exposures ranged from 1.12 × 101 mg/kg bw/day in dimethoate to 1.75 × 10–4 mg/kg bw/day in ethoprophos for adults and from 7.53 × 100 mg/kg bw/day in dimethoate to 5.53 × 10–4 mg/kg bw/day in methamidophos for children. Higher short-term exposure values were obtained for dimethoate, lambda-cyhalothrin, chlorpyrifos, phorate, beta-endosulfan, fenvalerate, bifenthrin, cyfluthrin, fenitrothion, cypermethrin and parathion for both adults and children. The high exposure value for dimethoate was mainly due to the regular consumption of cabbage, watermelon and lettuce. On the contrary, low short-term exposure values were also obtained for malathion, ethoprophos, profenofos, methamidophos and organophosphate insecticides which are not registered in Ghana for fruit and vegetable cultivation.
Conclusion
This present study covered a 3-year period from 2010 to 2012 for fruits and vegetables sold in most popular markets in Accra Metropolis. Pesticide residues were detected in fruit and vegetable (3483) samples purchased from five major markets in Accra. The relative occurrence of pesticides was fenvalerate 11.3%, fenitrothion 5.6%, lambda-cyhalothrin 3.6%, dimethoate 3.2%, permethrin 2.7%, and deltamethrin 2.2%. These pesticides have been registered in Ghana for vegetable and fruit cultivation as per the EPA register of 2013. The commodities with the greatest concentrations exceeding the EU MRLs were long green beans (61%) and lettuce (57%) while watermelon (10%) and green pepper (9%) contained the least. It was also apparent from the study that the leafy and fleshy vegetables were very much at risk of pesticide contamination than the others. However, for short-term exposure, some of the pesticides (e.g. dimethoate) and commodities (e.g. lettuce) had higher % ARfD values. Accordingly, there is the need for continuous regular monitoring programmes to ensure minimal pesticide residues in fruits and vegetables to safeguard the health of the consuming populace. These results also call for improved residue control at production levels, stiffer control of pesticide spraying as well as regulation of pesticide sales. Additionally, well-developed and coordinated training programmes should be initiated to enhance farmers’ knowledge of pesticide application rate and risk and other non-chemical means of pest eradication on farms.
References
Abhilash PC, Singh N (2009) Pesticides use and application: an Indian scenario. J Hazard Mater 165:1–12
Afari-Safa V, Asare-Bediako E, Kenyon L, Micah JA (2015) Pesticide use practices and perceptions of vegetable farmers in the cocoa belts of the Ashanti and Western regions of Ghana. Adv. Crop Sci Tech. 3:3. 1000174. doi: 10.4172/2329-8863
Amoah PP, Drechsel RC, Abaidoo RC, Ntow WJ (2006) Pesticide and pathogen contamination of vegetables in Ghana’s urban markets. Arch Environ Contam Toxicol 50:1–6
Andersen JH, Poulsen ME (2001) Results from the monitoring of pesticide residues in fruit and vegetables in the Danish market 1998–99. Food Addit Contam 18:906–931
Bempah CK, Donkor AK (2010) Pesticide residues in fruits at the market level in Accra Metropolis, Ghana, a preliminary study. Environ Monit Assess 175:551–561
Bempah CK, Donkor AK, Yeboah PO, Dubey B, Osei-Fosu P (2011) A preliminary assessment of consumer’s exposure to organochlorine pesticides in fruits and vegetables and the potential health risk in Accra Metropolis, Ghana. Food Chem 128:1058–1065
Bempah CK, Asomaning J, Ansong DA, Boateng J, Boahen Asabere S (2012) Contamination levels of selected organochlorine and organophosphorous pesticides in Ghanaian fruits and vegetables. Emir J Food Agric 24(4):293–301
Berrada H, Fernández M, Ruiz MJ, Moltó JC, Mañes J (2006) Exposure assessment of fruits contaminated with pesticide residues from Valencia, 2001-03. Food Addit Contam 23:674–682
Bošković S, Pucarević M (2012) Monitoring pesticide residues in food of plant origin, Tematski zbornik International Conference on Bioscience: Biotechnology and Biodiversity—step in the future. The Fourth Joint UNS–PSU Conference, Novi Sad, Serbia, 18–20 June 2012. Book of Proceedings. pp. 229–243
Ĉesnik AB, GregorćIć A, Bolta ŜV, Kmecl V (2006) Monitoring of pesticide residues in apples, lettuce and potato of the Slovene origin, 2001–04. Food Add Contam 23(2):164–173
Chun OK, Kang HG (2003) Estimation of risks of pesticide exposure, by food intake, to Koreans. Food Chem Toxicol 41:1063–1076
Damalas CA (2009) Understanding benefits and risks of pesticide use. Sci Res Essay 4(10):945–949
Dogheim SM, Gadalla SA, El-Marsafy AM (1999) Monitoring of pesticide residues in Egyptian fruits and vegetables in 1995. J AOAC Int 82:984–955
Dogheim SM, El-Marsafy AM, Salama EY, Gadalla SA, Nabil YM (2002) Monitoring of pesticide residues in Egyptian fruits and vegetables during 1997. Food Addit Contam 19:1015–1027
Donkor A, Osei-Fosu P, Nyarko S, Kingsford-Adaboh R, Dubey B, Asante I (2015) Validation of QuEChERS method for the determination of 36 pesticide residues in fruits and vegetables from Ghana,using gas chromatography with electron capture and pulsed flame photometric detectors. J Environ Sci Heal B 50:560–570
Donkor A, Osei-Fosu P, Dubey B, Kingsford-Adaboh R, Ziwu C, Asante I (2016) Pesticide residues in fruits and vegetables in Ghana: a review. Environ. Sci. Pollut. Res. Int. Oct. 23 (19). pp. 18966–18987
Donkor A, Addae FL, Tawiah R, Asomaning W, Dubey B, Osei-Fosu P, Ziwu C, Mohammed M (2017) Evaluation of trace metals in vegetables sampled from farm and market sites of Accra Metropolis, Ghana. Int J Environ Stud 74(2):315–324
EU Pesticide Database (2013) http://ec.europa.eu/sanco_pesticides/public/index.cfm. (Accessed on December 2014)
FAO/WHO (2004) Food and Agriculture Organization/World Health Organization Food Standards programme. Codex Alimentarius Commission. Twenty-Seventh Session, Geneva. Switzerland, 28 June–03 July 2004
FDA Pesticide Monitoring Program Fiscal Year (2012) Pesticide Report. https://www.fda.gov/food/foodborneIllnessContaminants/Pesticides/default.htm
Federal Agency for the Safety of the Food Chain (FASFC) (2006) Report of monitoring results concerning Directives 90/642/EEC, 76/895/EEC and Commission Recommendation 2005/178/EC. Brussels (Belgium). http://www.favv-afsca.be/publications-en/pesticide-residue-monitoring-food-plant-origin.asp. (Accessed on December 2014)
Federal Agency for the Safety of the Food Chain (FASFC) (2008) http://www.favv-afsca.be/publications-en/pesticide-residue-monitoring-food-plant-origin.asp. (Accessed on December 2014)
Fontcuberta M, Arques JF, Villalbi JR, Martinez M, Centrich F, Serrahima E, Pineda L, Duran J, Casas C (2008) Chlorinated organic pesticides in marketed food: Barcelona, 2001-06. Sci Total Environ 389:52–57
Ghana Gazette (2012) Environmental Protection Agency–(Environmental Permitted Project and Revised Register of Pesticides as at December 31st 2012). pp. 830–849
Jardim ANO, Caldas ED (2012) Brazilian monitoring programmes for pesticide residues in food—results from 2001 to 2010. Food Control 25(2):607–616
Knežević Z, Serdar M, Ahel M (2012) Risk assessment of the intake of pesticides in Croatian diet. Food Control 23:59–65
Li W, Tai L, Liu J, Gai Z (2014) Monitoring of pesticide residues levels in fresh vegetables from Heibei Province, North China. Environ Monit Assess 186:6341–6349
Ntow WJ (2005) Pesticide residues in Volta Lake, Ghana. Lakes and reservoirs: Res. Manage.: 243–248
Ntow WJ, Gijzen HJ, Drechsel P (2006) Farmer perceptions and pesticide use practices in vegetable production in Ghana. Pest Manag Sci 62(4):356–365
Osei-Fosu P, Donkor AK, Nyarko S, Nazzah NK, Asante IK, Kingsford-Adaboh R, Arkorful NA (2014) Monitoring of pesticide residues of five notable vegetables at Agblogbloshie market in Accra. Ghana Environ Monit Assess 186(11):7157–7163
Osman MEH, El-Sheekh MM, El-Naggar AH, Gheda SF (2010) Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol Fertil Soils 46:861–875
Poulsen ME, Andersen JH (2003) Results from the monitoring of pesticide residues in fruit and vegetables on the Danish market, 2000-01. Food Addit Contam 20(8):742–757
Ripley DB, Lissemore IL, Leishman DP, Denommé AM (2000) Pesticide residues on fruits and vegetables from Ontario, Canada, 1991–1995. J AOAC Int 83(1):196–213
Ruel MT, Minot N, Smith L (2004) Patterns and determinants of fruit and vegetable consumption in Sub-Saharan Africa: a multi-country comparison. Joint FAO/WHO Workshop on Fruits and Vegetables for Health, 1–3 September, 2004, Kobe, Japan
Swarnam TP, Velmurugan A (2013) Pesticide residues in vegetable samples from the Andaman Islands. India Environ Monit Assess 185(7):6119–6127
Acknowledgements
We are very grateful to our anonymous friends in overseas for the financial support for this study. The authors also thank the Chemistry Department, University of Ghana, and the Pesticide Residues Laboratory, Ghana Standards Authority, Accra, for the technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Fosu, P.O., Donkor, A., Ziwu, C. et al. Surveillance of pesticide residues in fruits and vegetables from Accra Metropolis markets, Ghana, 2010–2012: a case study in Sub-Saharan Africa. Environ Sci Pollut Res 24, 17187–17205 (2017). https://doi.org/10.1007/s11356-017-9287-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9287-8