Abstract
Concurrent coastal seawater (n = 22), sediment (n = 22), and atmospheric samples (n = 10) were collected in the Aliaga industrial region, Turkey, to explore the spatial variation, sources, and air-seawater exchange of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Seawater Σ16PAH concentrations (particle + dissolved) ranged between 5107 and 294,624 pg L−1, while Σ41PCB concentrations were in the range of 880–50,829 pg L−1. Levels in sediments were highly variable ranging between 35.5–49,682 and 2.7–2450 μg kg−1 in dry weight for Σ16PAHs and Σ41PCBs, respectively. Atmospheric concentrations varied between 1791–274,974 and 104–20,083 pg m−3 for Σ16PAHs and Σ41PCBs, respectively. Sediment organic matter (OM) content and levels of Σ16PAHs and Σ41PCBs correlated weakly (r 2 = 0.19–0.23, p < 0.05) suggesting that the spatial variations of PAHs and PCBs were mainly affected by local sources rather than their sorption to OM. The geographical distribution of PAH and PCB concentrations in air, seawater, and sediment and factor analysis on the sediment levels pointed out that the major sources in the region are steel plants, petroleum refinery, petrochemical complex, ship breaking, loading/unloading activities at the ports, vehicular emissions, and fossil fuel combustion emissions. The direction of the air-seawater exchange was also explored by estimating seawater fugacity fractions of PAHs and PCBs. For PAHs, the number of cases implying deposition (43.0%) and volatilization (39.5%) was similar, while for PCBs, the number of cases implying volatilization (60.4%) was much higher compared to deposition (21.6%). Fugacity fractions were generally <0.36 (deposition) at the sites close to industrial and ship breaking activities where the highest seawater and sediment levels were measured, implying that atmospheric deposition is an important mechanism affecting seawater and sediment PAH and PCB levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent organic pollutants (POPs) enter to the surface waters by dry deposition (particle phase), air-water exchange (gas phase), and wet deposition from the atmosphere, and by wastewater discharges (Odabasi et al. 2008; Cetin et al. 2016). In the water column, hydrophobic compounds are adsorbed to the particulate matter, and they are eventually deposited to bottom sediments (Bigus et al. 2014; Kwan et al. 2014). Along with the physicochemical properties of the pollutants, accumulation in the sediment mainly depends on physical properties and adsorptive capacity of the sediment and the partitioning coefficient between the water and sediment (Bigus et al. 2014). Therefore, sediments are an important reservoir for POPs providing information on their sources, geographical and historical variations.
Recently conducted studies based on soil, ambient air, and plant (pine barks and needles) sampling have shown that there are several important local sources of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in Aliaga industrial region, Izmir, Turkey, and the region is heavily polluted (Aydin et al. 2014; Odabasi et al. 2015). Although atmospheric and soil levels and their exchange between these compartments have been studied well in the region, seawater and sediments that play important roles in environmental dynamics and fate of the pollutants have not been investigated. The objectives of the present study were (1) to determine the spatial variations and sources of PAHs and PCBs in coastal atmosphere, seawater, and sediments and (2) to investigate their air-sea exchange in the Aliaga industrial region, Turkey.
Materials and methods
Study area and sample collection
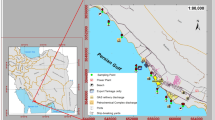
The study region (Aliaga, 38° 42′–38° 55′ N and 26° 50′–27° 04′ E) situated on the Aegean Sea coast (western Turkey) has a number of pollutant sources, i.e., scrap processing iron-steel plants (n = 5), steel rolling mills, a large petroleum refinery, a petrochemical complex, a natural gas-fired power plant, a fertilizer plant, several scrap and slag storage piles, heavy traffic of scrap iron trucks, ship breaking yards, and active ports importing/exporting products and raw materials. Aliaga town (population of ∼60,000), a number of villages, and agricultural areas are also included within the study region.
Concurrent samples were collected from coastal sediment and seawater between July 13 and 20, 2009 from 22 sites along the seashore (∼60 km) in the Aliaga industrial region (Fig. 1). Surface sediment samples were collected manually with a Van Veen grab sampler (2 L) from 22 different sites. Water depth at sediment sampling sites varied between 3 and 19 m. At each point, approximately 350 g of the homogenized sediment sample was transferred into a glass jar capped with Teflon-lined lid and stored in the laboratory at 4 °C as until sample preparation. Seawater samples (∼10 L) were collected from 30 cm under the surface using precleaned polyethylene containers without allowing a headspace. Ambient air samples were also collected during mid-July–mid-August 2009 at ten coastal sites close to the 15 seawater sampling sites (distance <1 km) using polyurethane foam (PUF) disk passive air samplers (PAS). Average sampling time was 30 days, and the average air temperature was 27.1 °C. Before sampling, depuration compounds (13C-PCB 3, 13C-PCB 9, 13C-PCB 15, PCB 30, PCB 107, and PCB 198), 17.5–20 ng per sample were spiked onto PUF disks to determine the compound-specific sampling rates.
The temperatures of seawater and sediment samples were measured onsite using a thermometer. For seawater and sediment samples, the average observed temperatures were 26.5 ± 0.5 °C and 25.8 ± 0.8 °C, respectively. pH of seawater was measured using a pH meter (WTW pH 720), while salinity was determined with a conductivity meter (WTW Cond 720). The average salinity and pH of the seawater were 0.65 M and ∼8.0, respectively.
Sample preparation and analysis
Before extraction, PCB and PAH surrogate standards were added to all samples. Ambient air PUFs were Soxhlet extracted for 12 h with 1:1 acetone/hexane (ACE/HEX). Approximately 3–5 g of sodium sulfate was added to the 5 g of sediment sample, and the mixture was homogenized with a glass bar. Samples were soaked in 50 mL of a 1:1 ACE/HEX overnight. Then, they were ultrasonically extracted for 1 h. Water samples were filtered through a glass fiber filter (0.7 μm pore size, 47 mm diameter) to retain particle phase compounds followed by a resin column (∼10 g XAD-2) to sorb dissolved phase. Dissolved and particle phase samples were ultrasonically extracted for 1 h with 1:1 ACE/HEX. Water in extracts was removed by addition of 2 g sodium sulfate. Sediment samples were treated with 1.5 g of activated copper powder to remove elemental sulfur. Volumes of all extracts were reduced to 2 mL, and they were switched into HEX utilizing a rotary evaporator and a stream of high purity N2. Samples were cleaned up and separated into two fractions using an alumina-silicic acid column packed with 3 g silicic acid (4.5% water) and 2 g alumina (6% water). PCBs were eluted with 35 mL petroleum ether (fraction 1), while PAHs were eluted with 20 mL dichloromethane (fraction 2). The solvent of the extracts was switched into HEX, and their volume was reduced to 1 mL under a stream of N2.
All samples were analyzed for 16 PAHs and 41 PCBs with an Agilent 6890 N gas chromatograph (GC)-mass spectrometer (Agilent 5973 inert MSD) using electron impact ionization and selected ion monitoring mode (SIM). An HP5-MS capillary column (30 m, 0.25 mm, and 0.25 μm) was used for the analysis, and helium was the carrier gas. Fraction 1 was analyzed for PCBs. Then, for PAH analysis, equal volumes of fractions 1 and 2 were mixed and analyzed as lighter PAHs are eluted in part with fraction 1. The identification of PAH compounds and PCB congeners was based on their retention times, target, and qualifier ions, and their quantification was performed using the internal standard calibration procedure.
Sediment water content was determined gravimetrically using sub-samples after drying at 103 °C in an oven for 24 h. The organic matter (OM) content was also determined gravimetrically by baking in a furnace at 600 °C for 4 h (Wang et al. 2011).
Quality control
Average recoveries for the surrogate standards ranged between 60 and 74% (acenaphthene-d10), 75–78% (phenanthrene-d10), 67–79% (chrysene-d12), 63–76% (perylene-d12), 71–86% (PCB-14), 70–88% (PCB-65), and 71–89% (PCB-166), and sample amounts were corrected for surrogate recoveries. For 1 μL injection, instrumental detection limits (IDL) were 0.15 and 0.10 pg for PAHs and PCBs, respectively. PAH and PCB levels in blank samples (n = 4 for each sample type) were also determined. Definition of the method detection limit (MDL) was MDL = mean blank value + 3SD. For the compounds that could not be detected in blank samples, IDLs were substituted. Mean analyte quantities in blanks were generally <5% of the sample quantities. Sample amounts above the MDL were quantified, and they were blank-corrected by subtracting the average blank levels from those in samples.
Sampling rates (R, m3/day) for atmospheric samples were determined based on the loss of spiked depuration compound amounts, and they averaged as 2.14 ± 0.46 m3/day. The effective air sampling volumes (V air, m3) for individual compounds/congeners during the sampling period were estimated using the methodology developed by Shoeib and Harner (2002). Sampling rate and effective sampling volume calculations were detailed by Kaya et al. (2012). For the calculation of V air, it was assumed that both gas phase and particle phase compounds have similar sampling rates (Harner et al. 2014; Harner et al. 2013).
Estimation of gas phase concentrations
PUF disk passive air samplers mainly target gas phase compounds. However, recent studies have implied that PUF disk passive air samplers also collect particles, and the sampling rates are typically in the range 4 ± 2 m3 day−1 for both gas and particle phase compounds (Markovic et al. 2015; Harner et al. 2014; Harner et al. 2013). Since gas phase concentrations are needed to assess air-seawater gas exchange of pollutants, atmospheric total (gas + particle phase) PAH and PCB levels measured with passive samplers were used to estimate the gas phase concentrations in the present study.
Distribution of atmospheric semivolatile organic compounds between the gas and particle phases is explained using the gas-particle partition coefficient, K p (m3 μg−1) (Harner and Bidleman 1998):
where C p and C g are the concentrations in the particle and gas phases, respectively (ng m−3), and C TSP is the atmospheric total suspended particulate concentration (μg m−3).
The octanol-air partition coefficient (K OA) and organic matter fraction of particles (f OM) were used to estimate K p assuming that the major distribution process is absorption (Harner and Bidleman 1998):
Gas phase concentrations were estimated as:
where C t is the total (gas + particle phase) concentration measured with the PUF disk passive air sampler.
Temperature (T) dependent K OA values of the PAHs and PCBs (log K OA = A + B/T) were calculated using the constants (A and B) reported by Odabasi et al. (2006a), Odabasi et al. (2006b), Harner and Bidleman (1996), Chen et al. (2002), Zhang et al. (1999). Recently measured average TSP concentration in Aliaga region (95 μg m−3) and its OM content (50%) were used for the calculation of K p and gas phase fractions (Bozlaker et al. 2008a; Cetin and Odabasi 2008).
Results and discussions
PAH and PCB concentrations in seawater, sediment, and air
Total (particle + dissolved) Σ16PAH concentrations in the seawater samples ranged between 5107 and 294,624 pg L−1, while Σ41PCB concentrations were in the range of 880–50,829 pg L−1 (Fig. 2). Seawater PAH and PCB levels observed in this study were within the ranges reported previously (Totten et al. 2001; Nizzetto et al. 2008; Valavanidis et al. 2008). It was reported that the average aqueous Σ14PAH concentration in Izmir Bay, Turkey, was 12,700 pg L−1, while Σ41PCB concentrations were 250–390 pg L−1 (Demircioglu et al. 2008; Odabasi et al. 2008). Izmir Bay is not influenced by any industrial PAH/PCB source. Most of the PAH and PCB levels observed in the present study were substantially higher than those reported for Izmir Bay, further indicating that surface waters in Aliaga region are polluted by the industrial sources.
Concentrations in sediments varied highly and ranged between 35.5–49,682 and 2.7–2450 μg kg−1 in dry weight for Σ16PAHs and Σ41PCBs, respectively (Fig. 3). Observed sediment PAH concentrations were generally within the ranges reported previously. ΣPAH concentrations reported for Hong Kong sediment ranged between 22.4 and 4420 μg kg−1 dry weight (Zheng and Richardson 1999), and for coastal sediments in the Pearl River Delta in China, they were 94–4300 μg kg−1 dry weight (Fung et al. 2005). Feo et al. (2011) have reported a wider interval for Naples harbor sediments (12–21,740 μg kg−1 dry weight). Observed sediment PCB concentrations were comparable to Σ28PCB concentrations reported for the Pearl River Delta sediments (6.0–290 μg kg−1 dry weight) (Fung et al. 2005) and for the Venice Lagoon, Italy (89–717 μg kg−1 dry weight) (Frignani et al. 2005), and they were substantially higher than those measured for Singapore’s coastal sediments where Σ28PCBs ranged between 0.339 and 1.356 μg kg−1 dry weight (Wurl and Obbard 2006).
Seawater PAH and PCB compound/congener profiles were dominated by low to medium molecular weight (MW) compounds for PAHs (phenanthrene, fluorene, acenaphthene, fluoranthene, and pyrene) and PCBs (PCB-118, 18, 138, 105, 110, 28, 101, and 70). Pyrene, phenanthrene, fluoranthene, benz(a)anthracene, and chrysene were dominant PAHs for sediment samples, and PCB-18, 138, 44, 70, 209, 18, 153, and 52 were the congeners having the highest levels. In general, seawater and sediment profiles were similar. However, the contributions of high molecular weight compounds were higher to total PAHs and PCBs in sediment relative to seawater (Figs. 4 and 5). The difference in seawater and sediment PAH and PCB profiles may be ascribed to differential partitioning of the individual compounds (i.e., high MW compounds have relatively higher octanol-water partition coefficients (K OW); thus, their sorption to organic matter is relatively higher).
Distribution of PAHs and PCBs between particle and dissolved phases in the seawater is mainly decided by their physicochemical properties (i.e., solubility, octanol-water partition coefficient-K OW, and organic carbon partition coefficient (K OC) (Bigus et al. 2014). PAHs and PCBs were distributed between particle and dissolved phases in the present study. Dissolved phase fraction ranged between 24–65% and 0–59% for PAHs and PCBs, respectively, and decreased with K OW. Dissolved phase fraction and K OW correlated well (p < 0.01) and K OW explained the 77 and 89% of variability in dissolved phase fractions for PAHs and PCBs, respectively (Fig. 6).
Adsorption of POPs onto sediments strongly depends on the properties of both the pollutants (adsorbate) and sediments (adsorbent), the contaminant concentration, and sediment OM content (Bigus et al. 2014). OM contents of the sediment samples collected in the present study varied highly and ranged from 2.6 to 17.6% (dry weight, 9.4 ± 4.8%, average ± SD). Sediment OM content and concentrations of Σ16PAHs and Σ41PCBs correlated significantly (p < 0.05). However, OM content explained only the 19 and 23% of variability in PAH and PCB concentrations, respectively. This suggested that the spatial variations of Σ16PAHs and Σ41PCBs in sediments were mainly affected by local sources rather than their sorption to organic matter.
Atmospheric concentrations were also highly variable and ranged between 1791–274,974 and 104–20,083 pg m−3 for Σ16PAHs and Σ41PCBs, respectively (Fig. 7). Low molecular weight compounds, i.e., phenanthrene, fluorene, fluoranthene, and pyrene, dominated the observed Σ16PAH levels. Low molecular weight congeners (PCB-18, 28, 31, 33, 52, and 49) also dominated the atmospheric Σ41PCB levels.
Sources of PAHs and PCBs
For all sample types (air, seawater, and sediment), the lowest PAH and PCB concentrations were measured at sites far from industrial sources. However, the highest levels were detected at sampling points located at Nemrut Bay where there are active ports for product and raw material import/export for steel plants, refinery/petrochemical plant, and near the ship breaking yards. In a recent study in the study area, using Positive Matrix Factorization, five sources for atmospheric PAHs were identified as biomass and coal combustion, iron-steel production, unburned crude oil and petroleum products, and diesel and gasoline exhaust emissions with contributions of 40, 27, 27, 3, and 3%, respectively (Aydin et al. 2014). Identified sources of atmospheric PCBs were iron-steel production and ship breaking activities, coal and wood combustion, and evaporative emissions from technical PCB mixtures with contributions of 57, 31, and 12%, respectively (Aydin et al. 2014). The spatial distribution of PAH and PCB levels observed in the present study are in agreement with the previous studies reporting that steel plants, petroleum refinery, petrochemical complex, and ship breaking activities are the primary sources of atmospheric PAHs and PCBs in the region (Aydin et al. 2014; Odabasi et al. 2015). These findings further suggest that these sources may substantially affect the PAH and PCB levels in coastal seawater and sediments in the region.
Possible sources of PAHs and PCBs measured in sediments were further investigated by factor analysis. Principal component analysis was used for extraction, and the raw calculated factor loading coefficients were rotated by Varimax with Kaiser Normalization (SPSS software version 20.0 for Windows by SPSS Inc.). Only major principal components (PCs) having eigenvalues >1.0 were extracted and significant clusters having factor loadings >0.5.
The number of factors extracted for PAHs were two. These factors accounted for the 99.5% of the total variance (Table 1). The first factor (56.3% of the variance) had high loadings on acenaphthylene, acenaphthene fluorene, phenanthrene, anthracene, fluoranthene, pyrene, banz[a]anthracene, and chrysene. Low molecular weight PAHs, especially acenaphthylene, were reported as markers for scrap processing iron-steel plants by Odabasi et al. (2009). Therefore, factor 1 could be attributed to iron-steel production. The second factor (43.2% of the variance) was dominated by non-volatile PAHs like benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene, and benzo[g,h,i]perylene. These compounds were indicated as source fingerprints for vehicular exhaust emissions (Motelay-Massei et al. 2003; Callen et al. 2012; Tian et al. 2013). Carbazole had also a high loading in factor 2. Carbazole is found in fossil fuels (i.e., crude oil, coal), and it is primarily emitted from coal combustion (Odabasi et al. 2006b). These results indicate that factor 2 is not a fully resolved factor, and therefore, it could be attributed to vehicular exhaust and other fossil fuel combustion emissions (i.e., crude oil and coal).
Four factors were extracted for PCBs accounting for the 98.8% of the total variance (Table 2). The first factor (47.2% of the variance) was highly loaded on PCB-17, PCB-18, PCB-28, PCB-31, and PCB-33. It was reported that these low molecular weight congeners dominate the iron-steel plant emissions (Odabasi et al. 2009). This factor had also high loadings on PCB-52, PCB-49, PCB-44, PCB-74, PCB-70, PCB-95, PCB-101, PCB-99, PCB-87, PCB-110, PCB-118, and PCB-105. Lee et al. (2005) have reported that coal and hardwood combustion emissions are dominated by PCB-49, PCB-52, PCB-28, PCB-44, PCB-101, PCB-110, and PCB-118. These indicate that factor 1 is not a fully resolved factor, and therefore, it could be attributed to iron-steel plant and combustion (i.e., coal and wood) emissions. Highly chlorinated congeners (PCB-158, PCB-183, PCB-177, PCB-171, PCB-156, PCB-199, PCB-194, and PCB-206) showed high correlations for factor 2 (26.2% of the variance). Highly chlorinated congeners (PCB-149, PCB-153, PCB-138, PCB-187, PCB-180, and PCB-170) were also associated with factor 3 (21.4% of the variance). Most of these congeners are major components of technical PCB mixtures like Aroclor 1254, Arochlor 1260, and Kanechlor 600 (Takasuga et al. 2005; Takasuga et al. 2006; Jin et al. 2012). Therefore, factors 2 and 3 could be attributed to technical PCB mixtures. The last factor (4.0% of the variance) was highly loaded on PCB-209. PCB209 is not a technical mixture component, but it is present in phthalocyanine green and titanium dioxide white pigments that are used for various applications (Hu et al. 2011). These pigments are likely to be contained in ferrous scrap that is used as the raw material in iron-steel production in the region. Scrap is produced by ship breaking, and it is also imported through the ports in the area. The highest sediment PCB-209 levels were measured at sites close to ports (sites 4, 5, 6, 7, and 21) and ship breaking yards (sites 12, 16, and 23). Thus, factor 4 could be attributed to ship breaking and scrap loading/unloading activities at the ports.
Air-seawater exchange of PAHs and PCBs
Measured air and seawater concentrations were used to determine the direction of PAH and PCB transfer between the air and seawater interface. The seawater fugacity fraction [f W/(f W + f A) = (H'C w)/(H'C w + C g)] > 0.5 implies net contaminant volatilization from seawater; values <0.5 indicate net gas phase deposition from air, while in the case of equilibrium, f W/(f W + f A) value is ∼0.5. Here, C w and C g are the dissolved phase water and gas phase air concentrations (pg m−3), and H′ is the dimensionless Henry’s law constant for PAHs (Ten Hulscher et al. 1992; Bamford et al. 1999) and PCBs (Schenker et al. 2005). Salting-out constants (K s, L mol−1) were estimated using the method by Ni and Yalkowsky (2003) and the K OW values for PAHs (Finizio et al. 1997; Jonker and Koelmans 2002; VCCL 2016; NLM 2016) and PCBs (Han et al. 2006). H * is the Henry’s law constant in saline water. It was determined through [log (H */H) = K s C s] where C s is the molar salt concentration of seawater (measured as 0.65 M). The uncertainty of the calculated fugacity fractions was estimated using a propagated error analysis. Uncertainty in H values was estimated as <25% (Ten Hulscher et al. 1992; Bamford et al. 1999; Schenker et al. 2005). The uncertainties of C g and C w were taken as 10% (Cetin and Odabasi 2007; Odabasi et al. 2008). It should be noted that the estimation of gas phase concentrations introduces additional errors that could not be assessed easily. Previously collected atmospheric concentration data in Izmir region based on active sampling (Odabasi et al. 2008; Bozlaker et al. 2008a; Bozlaker et al. 2008b; Demircioglu et al. 2011) was evaluated in terms of the partitioning model used in the present study. It was found that the modeling errors are variable for different compounds having different volatilities and for sample sets collected at different locations (i.e., suburban, urban). Model performs well (errors 1.0–20%) for compounds with log K OA values <9.0, while errors increase up to ∼50% for those with log K OA values >12.0. Variability based on site characteristics does not allow assigning certain values for modeling errors associated with C g. If a conservative modeling error of 50% is taken into account, error propagation related to estimation of f W/(f W + f A) implies that the equilibrium is denoted by a fraction of 0.5 ± 0.14 (a range of 0.36–0.64). It should be noted that the seawater samples were grab samples, while air samples were 1-month integrated samples. Therefore, possible variations in seawater concentrations during the air sampling period introduce additional uncertainty to fugacity calculations. The fugacity fractions for most of the PAHs (82.5%) and PCBs (82.0%) indicated non-equilibrium conditions (Fig. 8). For PAHs, the number of the cases implying deposition (43.0%) and volatilization (39.5%) were similar. However, for PCBs, the number of cases implying volatilization (60.4%) was higher compared to deposition (21.6%). Low molecular weight PAHs (i.e., acenaphthylene, acenaphthene, fluorene, phenanthrene, and anthracene) volatilized at all sites, while the remaining compounds volatilized or deposited at different sites. However, a similar pattern was not observed for PCB congeners that were volatilized at the majority of the sampling sites.
Fugacity fractions were generally <0.36 at the sites near the industrial activities and near ship breaking yards where the highest seawater and sediment concentrations were observed, indicating that gas phase deposition from the atmosphere might be an important mechanism affecting seawater and sediment PAH and PCB levels. Dry particle deposition was not determined in the present study. However, recent studies in the Aliaga region based on measurement of dry deposition fluxes and estimation of those for air-soil exchange have reported that both mechanisms are equally important for the transfer of PAHs and PCBs to the terrestrial surfaces (Bozlaker et al. 2008a; Bozlaker et al. 2008b).
Conclusions
The spatial variation, sources, and air-seawater exchange of PAHs and PCBs were explored in the Aliaga industrial region, Turkey. Σ16PAHs (particle + dissolved) in the seawater ranged between 5107 and 294,624 pg L−1, while Σ41PCB levels were in the range of 880–50,829 pg L−1. Levels in sediments were highly variable ranging between 35.5–49,682 and 2.7–2450 μg kg−1 in dry weight for Σ16PAHs and Σ41PCBs, respectively. Atmospheric concentrations varied between 1791–274,974 and 104–20,083 pg m−3 for Σ16PAHs and Σ41PCBs, respectively.
Sediment OM content and levels of Σ16PAHs and Σ41PCBs correlated weakly suggesting that the spatial variations of PAHs and PCBs were mainly affected by local sources rather than their sorption to OM. Previous atmospheric studies in the region, the observed geographical distribution of PAH and PCB levels in air, seawater, and sediment, and factor analysis conducted on the sediment levels in the present study pointed out that the major sources in the region are steel plants, petroleum refinery, petrochemical complex, ship breaking, loading/unloading activities at the ports, vehicular emissions, and fossil fuel combustion emissions.
The direction of the air-seawater exchange was also explored by estimating seawater fugacity fractions of PAHs and PCBs. For PAHs, the cases showing deposition and volatilization were similar, while for PCBs, the number of cases implying volatilization was higher compared to deposition. Fugacity fractions for most of the PAHs and PCBs indicated deposition at the sites close to industrial and ship breaking activities where the highest seawater and sediment levels were observed, indicating that gas phase transfer is an important mechanism affecting seawater and sediment PAH and PCB levels.
References
Aydin YM, Kara M, Dumanoglu Y, Odabasi M, Elbir T (2014) Source apportionment of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in ambient air of an industrial region in Turkey. Atmos Environ 97:271–285
Bamford HA, Poster DL, Baker JE (1999) Temperature dependence of Henry’s law constants of thirteen polycyclic aromatic hydocarbons between 4 degrees C and 31 degrees C. Environ Toxicol Chem 18:1905–1912
Bigus P, Tobiszewski M, Namiesnik J (2014) Historical records of organic pollutants in sediment cores. Mar Pollut Bull 78:26–42
Bozlaker A, Odabasi M, Muezzinoglu A (2008a) Dry deposition and soil-air gas exchange of polychlorinated biphenyls (PCBs) in an industrial area. Environ Pollut 156:784–793
Bozlaker A, Muezzinoglu A, Odabasi M (2008b) Atmospheric concentrations dry deposition and air-soil exchange of polycyclic aromatic hydrocarbons (PAHs) in an industrial region in Turkey. J Hazard Mater 153:1093–1102
Callen MS, Lopez JM, Mastral AM (2012) Apportionment of the airborne PM10 in Spain episodes of potential negative impact for human health. J Environ Monit 14:1211–1220
Cetin B, Odabasi M (2007) Air-water exchange and dry deposition of polybrominated diphenyl ethers at a coastal site in Izmir Bay Turkey. Environ Sci Technol 41:785–791
Cetin B, Odabasi M (2008) Atmospheric concentrations and phase partitioning of polybrominated diphenyl ethers (PBDEs) in Izmir Turkey. Chemosphere 71:1067–1078
Cetin B, Odabasi M, Bayram A (2016) Wet deposition of persistent organic pollutants (POPs) in Izmir Turkey. Environ Sci Pollut Res 23:9227–9236
Chen J, Xue X, Schramm KW, Quan X, Yang F, Kettrup A (2002) Quantitative structure-property relationship for octanol-air partition coefficients of polychlorinated biphenyls. Chemosphere 48:535–544
Demircioglu E, Sofuoglu A, Odabasi M (2008) Air-water exchange of polycyclic aromatic hydrocarbons at a coastal site in Izmir Bay (in Turkish) National Air Pollution and Control Symposium 220-235 October 22-25, 2008, Hatay, Turkey
Demircioglu E, Sofuoglu A, Odabasi M (2011) Atmospheric concentrations and phase partitioning of polycyclic aromatic hydrocarbons in Izmir Turkey. Clean-Soil Air Water 39:319–413
Feo ML, Sprovieri M, Gherardi S, Sammartino S, Marsella E (2011) Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in the harbour of Naples (Southern Italy): time and spatial distribution patterns. Environ Monit Assess 174:445–459
Finizio A, Mackay D, Bidleman TF, Harner T (1997) Octanol–air partition coefficient as a predictor of partitioning of semivolatile organic chemicals. Atmos Environ 31:289–2296
Frignani M, Bellucci LG, Favotto M, Albertazzi S (2005) Pollution historical trends as recorded by sediments at selected sites of the Venice Lagoon. Environ Int 31:1011–1022
Fung CN, Zheng GJ, Connell DW, Zhang X, Wong HL, Giesy JP, Fang Z, Lam PKS (2005) Risks posed by trace organic contaminants in coastal sediment on the Pearl River Delta, China. Mar Pollut Bull 50:1036–1049
Han XY, Wang ZY, Zhai ZC, Wang LS (2006) Estimation of n-octanol/water partition coefficients (Kow) of all PCB congeners by ab initio and a Cl substitution position method. QSAR Comb Sci 25:333–341
Harner T, Bidleman TF (1996) Measurements of octanol-air partition coefficients for polychlorinated biphenyls. J Chem Eng Data 41:895–899
Harner T, Bidleman TF (1998) Octanol–air partition coefficient for describing particle/gas partitioning of aromatic compounds in urban air. Environ Sci Technol 32:1494–1502
Harner T, Su K, Genualdi S, Karpowicz J, Ahrens L, Mihele C, Schuster J, Charland J-P, Narayan J (2013) Calibration and application of PUF disk passive air samplers for tracking polycyclic aromatic compounds (PACs). Atmos Environ 75:123–128
Harner T, Mitrovic M, Ahrens L, Schuster J (2014) Characterization of PUF disk passive air samplers for new priority chemicals: a review. Organohalogen Compd 76:11–29
Hu D, Martinez A, Hornbuckle KC (2011) Sedimentary records of non-Aroclor and Aroclor PCB mixtures in the Great Lakes. J Great Lakes Res 37:359–364
Jin RH, Park SU, Park JE, Kim JG (2012) Polychlorinated biphenyl congeners in river sediments: distribution and source identification using multivariate factor analysis. Arch Environ Contam Toxicol 62:411–423
Jonker MTO, Koelmans AA (2002) Sorption of polycyclic aromatic hydrocarbons and polychlorinated biphenyls to soot and soot-like materials in the aqueous environment: mechanistic considerations. Environ Sci Technol 36:3725–3734
Kaya E, Dumanoglu Y, Kara M, Altiok H, Bayram A, Elbir T, Odabasi M (2012) Spatial and temporal variation and air-soil exchange of atmospheric PAHs and PCBs in an industrial region. Atmos Pollut Res 3:435–449
Kwan CS, Takada H, Boonyatumanond R, Kato Y, Mizukawa K, Ito M, Dung LQ, Zakaria MP, Santiago EC (2014) Historical occurrences of polybrominated diphenyl ethers and polychlorinated biphenyls in Manila Bay Philippines and in the upper Gulf of Thailand. Sci Total Environ 470-471:427–437
Lee RGM, Coleman P, Jones JL, Jones KC, Lohmann R (2005) Emission factors and importance of PCDD/Fs PCBs PCNs PAHs and PM10 from the domestic burning of coal and wood in the UK. Environ Sci Technol 39:1436–1447
Markovic MZ, Prokop S, Staebler RM, Liggio J, Harner T (2015) Evaluation of the particle infiltration efficiency of three passive samplers and the PS-1 active air sampler. Atmos Environ 112:289–293
Motelay-Massei A, Ollivon D, Garban B, Chevreuil M (2003) Polycyclic aromatic hydrocarbons in bulk deposition at a suburban site: assessment by principal component analysis of the influence of meteorological parameters. Atmos Environ 37:3135–3146
National Library of Medicine (NLM) 2016 Specialized Information Services (SIS) Toxnet Available from: http://toxnet.nlm.nih.gov/
Ni N, Yalkowsky H (2003) Prediction of Setschenow constants. Int J Pharm 254:167–172
Nizzetto L, Lohmann R, Gioia R, Jahnke A, Temme C, Dachs J, Herckes P, Di Guardo A, Jones KC (2008) PAHs in air and seawater along a North-South Atlantic transect: trends processes and possible sources. Environ Sci Technol 42:1580–1585
Odabasi M, Cetin E, Sofuoglu A (2006a) Determination of octanol-air partition coefficients and supercooled liquid vapor pressures of PAHs as a function of temperature: application to gas-particle partitioning in an urban atmosphere. Atmos Environ 40:6615–6625
Odabasi M, Cetin B, Sofuoglu A (2006b) Henry’s law constant octanol-air partition coefficient and supercooled liquid vapor pressure of carbazole as a function of temperature: application to gas/particle partitioning in the atmosphere. Chemosphere 62:1087–1096
Odabasi M, Cetin B, Demircioglu E, Sofuoglu A (2008) Air–water exchange of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) at a coastal site in Izmir Bay Turkey. Mar Chem 109:115–129
Odabasi M, Bayram A, Elbir T, Seyfioglu R, Dumanoglu Y, Bozlaker A, Demircioglu H, Altiok H, Yatkin S, Cetin B (2009) Electric arc furnaces for steel-making: hot spots for persistent organic pollutants. Environ Sci Technol 43:5205–5211
Odabasi M, Ozgunerge Falay E, Tuna G, Altiok H, Kara M, Dumanoglu Y, Bayram A, Tolunay D, Elbir T (2015) Biomonitoring the spatial and historical variations of persistent organic pollutants (POPs) in an industrial region. Environ Sci Technol 49:2105–2114
Schenker U, MacLeod M, Scheringer M, Hungerbuhler K (2005) Improving data quality for environmental fate models: a least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds. Environ Sci Technol 39:8434–8441
Shoeib M, Harner T (2002) Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ Sci Technol 36:4142–4151
Takasuga T, Kumar KS, Noma Y, Sakai S (2005) Chemical characterization of polychlorinated biphenyls,-dibenzo-p-dioxins, and-dibenzofurans in technical Kanechlor PCB formulations in Japan. Arc Environ Con Tox 49:385–395
Takasuga T, Senthilkumar K, Matsumura T, Shiozaki K, Sakai SI (2006) Isotope dilution analysis of polychlorinated biphenyls (PCBs) in transformer oil and global commercial PCB formulations by high resolution gas chromatography-high resolution mass spectrometry. Chemosphere 62:469–484
Ten Hulscher TEM, Van Der Velde LE, Bruggeman WA (1992) Temperature dependence of Henry’s law constant for selected chlorobenzenes, polychlorinated biphenyls and polycyclic aromatic hydrocarbons. Environ Toxicol Chem 22:1179–1188
Tian YZ, Li WH, Shi GL, Feng YC, Wang YQ (2013) Relationships between PAHs and PCBs and quantitative source apportionment of PAHs toxicity in sediments from Fenhe reservoir and watershed. J Hazard Mater 248:89–96
Totten LA, Brunciak PA, Gigliotti CL, Dachs J, Glenn TR IV, Nelson ED, Eisenreich SJ (2001) Dynamic air-water exchange of polychlorinated biphenyls in the New York-New Jersey Harbor Estuary. Environ Sci Technol 35:3834–3840
Valavanidis A, Vlachogianni T, Triantafillaki S, Dassenakis M, Androutsos F, Scoullos M (2008) Polycyclic aromatic hydrocarbons in surface seawater and in indigenous mussels (Mytilus galloprovincialis) from coastal areas of the Saronikos Gulf (Greece). Estuar Coast Shelf Sci 79:733–739
Virtual Computational Chemistry Laboratory (VCCL) 2016 Available from: http://146.107.217.178/lab/alogps/starthtml
Wang DG, Alaee M, Byer J, Liu YJ, Tian CG (2011) Fugacity approach to evaluate the sediment–water diffusion of polycyclic aromatic hydrocarbons. J Environ Monit 13:1589–1596
Wurl O, Obbard JP (2006) Distribution of organochlorine compounds in the sea-surface microlayer water column and sediment of Singapore’s coastal environment. Chemosphere 62:1105–1115
Zhang X, Schramm KW, Henkelmann B, Klimn C, Kaune A, Kettrup A, Lu P (1999) A method to estimate the octanol-air partition coefficient of semivolatile organic compounds. Anal Chem 71:3834–3838
Zheng GJ, Richardson BJ (1999) Petroleum hydrocarbons and polycyclic aromatic hydrocarbons (PAHs) in Hong Kong marine sediment. Chemosphere 38:2625–2632
Acknowledgments
The present study was supported in part by the “Assessment of current status of Aliaga industrial region for air pollution” project conducted by Dokuz Eylul University for the Turkish Ministry of Environment and Urbanism and for the industries located in Aliaga region.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Rights and permissions
About this article
Cite this article
Odabasi, M., Dumanoglu, Y., Kara, M. et al. Spatial variation of PAHs and PCBs in coastal air, seawater, and sediments in a heavily industrialized region. Environ Sci Pollut Res 24, 13749–13759 (2017). https://doi.org/10.1007/s11356-017-8991-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8991-8