Abstract
Mercury (Hg) reduction by humic substances (HS) in the aquatic medium under the dark condition is a poorly understood but important process in Hg biogeochemical cycling. In this study, an effort was made to provide a better understanding of Hg(II) reduction by well-characterized humic substances under dark condition. Reduction of Hg(II) by dissolved HS in aquatic systems increases with increasing Hg loading. However, Hg(II) reduction gradually decreases with the increasing total S content and oxygen containing functional groups in the dissolved HS under dark condition. Increasing major cation concentration decreases the rate of Hg(II) reduction in aquatic systems. High concentration of Ca2+ ion slows down the intermolecular electron transfer from HS to Hg(II) and inhibits the formation of Hg0 in absence of light. This study indicates that complexation of Hg(II) and HS is essential for Hg reduction under dark condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a high priority global pollutant (Zhu et al. 2016; Chakraborty 2017). The high toxicity and hazardous impacts of Hg on the environment have increased awareness on Hg pollution (Boening 2000; Jiang et al. 2015). Mercury is released into the environment as the results of different natural and anthropogenic processes, and these processes play a significant role in Hg biogeochemical cycling (Lamborg et al. 2012; Rocha et al. 2000; Kim and Fitzgerald 1986). Abiotic and biotic reductions of Hg(II) are the two important routes for Hg emission from the aquatic systems. Mercury undergoes both complexation and reduction reactions with humic substances (HS, a major component of dissolved organic carbon) in the marine environments (Chakraborty et al. 2015a; Chakraborty and Babu 2015). Complexation reaction of Hg with HS may lead to forming thermodynamically stable Hg–humate complexes in the natural environment (Vudamala and Chakraborty 2016). HS in the aquatic system can also reduce Hg(II) to Hg0 and control Hg speciation in the aquatic environments (Zheng et al. 2012; Zheng and Hintelmann 2010; Gu et al. 2011; Chakraborty et al. 2015b; Allard and Arsenie Arsenie 1991). Although HS acts as the electron donor in natural system, the mechanisms of HS mediate Hg(II) reduction with or without light are different and not exactly known (natural system, the mechanisms of HS mediate Hg(II) reduction with or without light are different and not exactly known (Zheng and Hintelmann 2010). Abiotic photo-reduction of Hg(II) by HS in the aquatic environment has been well documented in the literature (O'Driscoll et al. 2006; Lanzillotta et al. 2002; Tseng et al. 2004; Bonzongo and Donkor 2003). Photo-reduction of Hg(II) has been reported to decrease with increasing concentration of dissolved organic carbon (DOC) in aquatic systems (Rolfhus and Fitzgerald 2001; Amyot et al. 1997). However, significant increase in Hg(II) reduction (with increasing reduction rates) has been reported with increasing concentration of DOC in an aquatic system (O’Driscoll et al. 2004; Costa and Liss 1999; Xiao et al. 1995). Aquatic DOC has also been reported to have an inhibitory effect on Hg(II) reduction (Rocha et al. 2000; Mauclair et al. 2008). These apparently opposing results (from the literature) indicate an incomplete understanding of the exact mechanism of Hg–DOC interactions in aquatic environments.
The ratio of –COOH/–OH groups and the sulphur content in HS has been reported to influence Hg(II) reduction process in the aquatic systems (Chakraborty et al. sulphur content in HS has been reported to influence Hg(II) reduction process in the aquatic systems (Chakraborty et al. 2015b). Abiotic reduction of Hg(II) by HS has been reported to depend on pH, salinity and redox condition of aqueous medium (Zheng et al. 2012; Chakraborty et al. aqueous medium (Zheng et al. 2012; Chakraborty et al. 2015b; Chakraborty et al., 2014). Abiotic reduction of Hg(II) in the natural aquatic environment by HS under the dark condition is an important but poorly understood process. The exact mechanism of Hg(II) reduction by HS in aquatic environment under dark condition is not known.dark condition is not known.aquatic environment under dark condition is not known.
Major cations in the aquatic environment have been reported to screen binding sites of HS and prevent other trace/heavy metals to undergo complexation reactions (Chakraborty and Chakrabarti 2006). Thus, the presence of major cations in an aqueous medium is expected to influence Hg–HS complexation in the aquatic medium. There is no information available on the effect of varying concentrations of Ca2+ (a major cation) on Hg(II) complexation and reduction by HS under dark condition. The impact of varying concentrations of Ca2+ ion is expected to provide a better understanding of Hg(II) reduction processes in an aqueous medium under dark condition. The aims of this study were to (1) estimate Hg(II) reduction rate by different well-characterized HS at varying [Hg]/[HS] ratio, and (2) understand the impact of varying major cation concentrations on Hg(II) reduction. An effort was made to understand the mechanism of Hg(II) reduction by HS under the dark condition in the aquatic environments.
Materials and methods
Cleaning procedures for containers
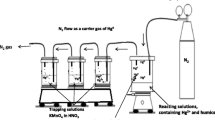
Teflon containers used in this study were to minimize Hg adsorption on the wall of the containers (Chakraborty et al. 2015b). All the containers, cleaned with soap and then with ultrapure water, were filled with 10% (v/v) nitric acid (HNO3) and kept at the room temperature for 7 days. The acid filled containers were then rinsed with ultrapure water, and filled with ultrapure water. The filling water was replaced daily. Quartz boats, used for Hg measurement, were pre-cleaned by heating at 800 °C.
Reagents
The stock solutions (1000 mg dm−3) of Hg and Ca (ICP standards) were obtained from Merck, Germany. Ten percent (v/v) of HNO3 solution was prepared from the ultrapure HNO3 obtained from Merck, Darmstadt, Germany. Potassium permanganate (KMnO) crystal, analytical grade, was obtained from Merck, Mumbai, India.
Preparation of well-characterized humic and fulvic acid solution
The well-characterized humic substances, obtained from the International Humic Substance Society (IHSS; MN, USA), were used in this study. Suwannee River humic acid (catalogue no. 2S101H) (SRHA), Suwannee River fulvic acid (catalogue no. 1S101F) (SRFA), and Eliot soil humic acid (catalogue no. 1S102H) (ESHA) were used as representatives of DOC in this investigation. Table 1 presents elemental compositions, concentrations of carbohydrate, carboxylic (−COOH), and phenolic–OH groups in SRHA, SRFA, ESHA. The stock solutions of SRHA, SRFA, and ESHA were prepared by dissolving approximately 0.1 g of the HS (individually and separately) in 100 cm3 of ultrapure water. The pH of the solutions adjusted to 10 by adding NaOH. The HS completely dissolved at pH 10. The solutions further filtered through 0.45-μm cellulose acetate filters, and pH was adjusted to 8.0 by using ultrapure HNO3 (as described in Chakraborty et al. 2015b). The solutions stored in the dark at 4 °C.
Reduction experiments of Hg(II)
A series of model solutions were prepared to know about Hg(II)–HS interaction and Hg(II) reduction in the aquatic environment under dark condition. The model solutions contained 5.0 mg dm−3 of HS (SRHA, SRFA, ESHA) (separately) in ultrapure water with varying concentrations of Hg(II). The concentrations of all these HS were kept constant. The concentration of Hg(II) in model solution varied from 5 × 10−9 to 125 × 10−9 M. The concentration range of Hg used in this study was high but comparable to some of the polluted coastal waters of India (Table 2) (Balchand and Nambisan 1986; Krishnakumar and Pillai 1990; Selvaraj et al. 1999; Satpathy et al. 2008b). The solution was prepared and kept under dark condition. The concentration of major cation (Ca2+) was varied from 0 to 0.1 × 10−3 M in solutions containing constant [Hg]/[HS] ratio. The concentrations of Hg (∼25 × 10−9 M) and HS (5 mg kg−1) in solutions were kept constant to maintain a constant [Hg]/ [HS] ratio. The pH of the solutions, measured by using a pH meter (Metrohm, 827 pH Lab, Switzerland), was adjusted by using ultrapure NaOH and HNO3 (as described in Chakraborty et al. 2015). The reductions of Hg(II) by different HS were monitored continuously for 144 h. The pH of each solution was checked yet again immediately at the end of the experiments. Each experiment also included a control. The control solution contained all the reagents except HS. Elemental mercury was trapped in oxidizing solution (containing KMnO4 and HNO3). The detailed description of Hg-trap system has been described in Chakraborty et al. (2015b). The trapping system was kept in dark condition. Total concentrations of reduced Hg in oxidizing solutions were determined by direct mercury analyser (DMA-80, Milestone, Italy).
QA/QC
One reagent blank and two certified reference materials (MESS3 and PACS 2, obtained from National Research Council, Canada) were periodically analysed to check the sensitivity of the instrument. Continuous monitoring of spiked Hg recovery in the liquid sample was checked to maintain the quality of the data.
Results and discussion
Reduction of Hg(II) by different well-characterized humic and fulvic acids in aquatic medium under dark condition
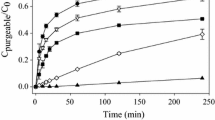
Reduction of Hg(II) in aqueous medium by different well-characterized HS under dark condition (as a function of time) is presented in Fig. 1. The production of Hg0 was monitored for 144 h. Figure 1a–c shows the changes in production of Hg0 by SRHA, SRFA, and ESHA, respectively, at varying [Hg]/[HS] ratio at constant pH (pH = 8). It has been reported that Hg(II) reduction by HS is based on a uniform reaction mechanism and controlled by slow transport of Hg(II) towards reactive groups within the humic hydrocolloids under study (Rocha et al. 2003). Hg ions initially prefer to bind with functional groups of HS that are easily accessible.
The rate of production of Hg0 by the HS was quick at the beginning of the each experiment but gradually declined with the progress of time as reported in our previous study (Chakraborty et al. 2015b). Figure 1a–c shows that different HS had different Hg(II) reduction capacity and reduction rate in the aqueous medium (at a fixed [Hg]/[HS] ratio) under dark condition. The differences of Hg(II) reduction by different HS in the aquatic environment depend on the concentration and availability of reactive functional groups in the HS. Increasing [Hg]/[HS] ratio in the test solutions increased Hg(II) reduction at pH 8.0. At low Hg loading, minor metal complexing sites of HS (mainly composed of S, N, and halogen containing functional groups/soft acid) plausibly underwent strong complexation reactions with Hg(II) (soft base) to form thermodynamically stable complexes. Thus, less amount of Hg(II) got reduced (Vudamala and Chakraborty 2016) at low [Hg]/ [HS] ratio. Zheng et al. (2012) describe the importance of Hg/DOC ratio in regulating Hg(II) photo-reduction in the aquatic environment. Hg(II) reduction has been reported to occur only at high Hg/DOC ratio which is very similar to the observation made in this study. At higher Hg loading, Hg(II) interacts with the reactive groups much faster. The major binding sites include –COOH and phenolic –OH groups (hard acid) that are considered as the reactive groups in HS. These functional groups interact weakly with Hg(II) (a soft base) and is responsible for Hg(II) reduction, and thus, more production of Hg0 was observed at higher [Hg]/[HS] ratio system.
Table 3 shows that ESHA had the highest production of Hg0 in the aquatic environment followed by SRHA and SRFA. The rates of production of Hg0 by the SRFA and SRHA (both aquatic humic substances) were comparable. This difference of Hg0 production probably indicates that sources of HS may play important role in controlling Hg(II) reduction in aquatic environment. The rates of production of Hg0 by HS under dark condition are in agreement with the Hg0 production rate reported in the literature (Baohua et al. 2011). The production of Hg0 in an aquatic system depends on [Hg]/[HS] ratio and the nature and composition of HS within the system. Hg(II) photo-reduction is a well-studied phenomenon, where sunlight causes dissolved gaseous Hg0 production in aquatic systems (Nriagu 1994; Amyot et al. 2000; Garcia et al. 2005; Dill et al. 2006; O’Driscoll et al. 2008; Amyot et al. 1997) but information on abiotic reduction process of Hg(II) in aquatic systems under the dark condition is scarce. An effort was made to recognize the impact of varying concentration of major cation on Hg0 production by different well-characterized HS in absence of light. The intention of this study was to provide a better understanding of Hg(II) reduction processes by HS under dark condition.
Impact varying major cation (Ca2+) concentration on Hg(II) reduction by humic and fulvic acids (HS) under dark condition
Figure 2a–c presents the impacts of varying Ca2+ concentrations on Hg(II) reduction by HS in aquatic systems under the dark condition. The production of Hg0 gradually increased, reached maxima and remained constant with the progress of time. Highest Hg(II) reduction by HS was observed when there was no Ca2+ in the solution. The release of Hg0 decreased with increasing Ca2+ concentration in the solution. Figure 3a–c shows the impact of varying concentrations of Ca2+ on Hg(II) reduction rate. The reduction rate of Hg(II) by HS in aquatic system gradually decreased with increasing Ca2+ ion concentration. The decreasing Hg(II) reduction by HS with increasing Ca2+ concentration can be attributed to the screening/shielding effect of Ca2+ ion (Chakraborty and Chakrabarti 2006; Chakraborty et al. 2006). Under dark condition, Ca2+ prevented Hg(II) to undergo complexation reaction with the major binding sites in the HS by shielding the negative charges of HS and prevented electron transfer from HS to Hg(II) and decreased Hg(II) reduction. This observation suggests that complexation of Hg(II) with HS is necessary for Hg(II) reduction under dark condition. High concentration of Ca2+ prevented intermolecular electron transfer from HS to Hg(II) and formation of Hg0, the major pathways for gaseous Hg formation, under dark condition. Lamborg (2003) has suggested that Hg complexation with dissolved organic matter might enhance Hg(II) reduction under dark condition. The intra-molecular electron transfer from dissolved organic matter to Hg(II) has been recognized as an important process for abiotic Hg reduction. The impact in variation of Ca2+ concentrations on Hg(II) reduction by humic and fulvic acids (HS) under dark condition (in this study) also indicates that complexation of Hg(II) with HS is necessary for Hg(II) reduction under dark condition.
Impact of varying S content of HS on Hg(II) reduction in the presence of major cation (Ca2+) in aquatic environment
Mercury prefers to form strong complexes with those ligand containing less electronegative halides and nitrogen over other ligands containing oxygen (Chakraborty et al. 2015b). The strong interaction of Hg with reduced sulphur has been reported in the literature (Xia et al. 1999). It has been well documented that inorganic sulphide controls the speciation of Hg in anoxic environments. Strong interactions between reduced HS and Hg0 have been reported to occur through thiolate ligand-induced oxidative complexation (Gu et al. 2011). Hg(II) can be effectively reduced to Hg0 in the presence of small concentration of reduced HS in the solutions. The production of Hg is expected to inhibit as the complexation with HA concentration increases. However, under oxic environment, the concentrations of reduced sulphur group are low or negligible. Thus, binding of Hg to humic and fulvic acids (HS) is expected to influence by their sulphur containing functional groups. Hg–organic sulphur complexes have been reported to be thermodynamically stable (Chakraborty et al. 2016). Gerbig et al. (2011) have shown that Hg(II) prefers to bind with an average of 2.4 sulphur atoms in absence of reduced sulphur. In this study, an effort was made to check the impact of the varying sulphur content of HS (reported by IHSS) on Hg(II) reduction in the aquatic environment.
Figure 4a shows the impact of varying total sulphur content in well-characterized humic and fulvic acids on Hg(II) reduction at different Hg loadings under dark condition. Reduction of Hg(II) decreased with the increasing total S content in the humic and fulvic acids. The effect of Hg loading on Hg reduction was more noticeable in the presence of SRHA, which contained the highest concentration of total S.
The increasing Hg loading increases complexation with S-binding ligands in SRHA. Further increase in Hg concentration saturates all the strong sites and Hg further move towards other binding sites (such as –COOH, –OH groups) to form thermodynamically weak complexes. These complexing sites are known to reduce Hg(II) in aquatic medium. Thus, reduction of Hg(II) by SRHA gradually increased with increasing Hg loading under dark condition. However, with low total S-content in ESHA and SRFA, the effect of Hg loading on Hg(II) reduction was not prominent. Zheng et al. (2012) have suggested that the reduction of Hg(II) depends on natural organic matter (NOM) source, oxidation state, and NOM/Hg ratio. Rapid reduction of Hg(II) by HS is counterbalanced by oxidation of Hg0 by HS. The dual role of HS controls the availability of reactive Hg in a system.
The impact of varying concentration of major cation (Ca2+) on Hg(II) reduction is presented in Fig. 4b. Increasing Ca2+ ion concentration in solution gradually decreased Hg(II) reduction (as discussed above). It is suggested that Ca2+ (Hard acid) screened Hg(II) to undergo complexation reaction with major binding sites (COOH and phenolic –OH groups) in HS and decreased Hg(II) reduction. Hg(II) reduction gradually decreased with increasing total S content of the HS. This observation suggests that total S-binding sites help Hg(II) to form strong complexes with HS and decreased Hg(II) reduction in aquatic medium under dark condition.
Impact of varying O-containing groups in HS on Hg(II) reduction in the presence of major cation (Ca2+) in aquatic environment
It is well known that oxygen-containing major functional groups in HS (–OH and –COOH) helps photo-reduction of Hg(II) (Chakraborty et al. 2015b; Fantozzi et al. 2007; Zheng and Hintelmann 2010). Reduction process of Hg(II) to Hg0 by dissolved organic acids in the aquatic environment has been identified as a significant pathway and important to consider for a better understanding of Hg cycling between the atmosphere and aqueous systems (Si and Ariya 2008). Zheng and Hintelmann (2010) have shown the influences of different functional groups of various low-molecular weight organic compounds on Hg(II) reduction in the aquatic medium. Hg(II) reduction process is dependent on several factors such as (i) nature of bonds between Hg(II) and dissolved organic matter, (ii) O/N ratio in dissolved organic matter and (iii) concentration of reduced S donor groups. Reduced dissolved organic matter has been reported to be more reactive than oxidized dissolved organic matter. The more abundant electron-donating functional groups such as hydroquinones or semiquinones in reduced dissolved organic matter were probably responsible for more reduction. HSAB theory also predicts that oxygen containing function groups are responsible for Hg(II) reduction.
Impacts of varying –OH/–COOH ratio of the different HS on Hg(II) reduction in an aquatic environment under the dark condition is shown in Fig. 5a. Increasing interaction of Hg (a soft base) with oxygen-containing ligands (hard acid) is expected to increase Hg0 production in a system. The heterogeneity of humic substances makes it extremely difficult to understand the exact mechanism of Hg(II) reduction process by –OH/−COOH functional groups.
Increasing [Hg]/[HS] ratio increased the production of Hg0 in the aquatic system. Increase in Hg loading is expected to increase the chance of Hg–oxygen containing ligands (–COOH, –OH) interactions in the aquatic environment and, thus, increased Hg0 production. Figure 5b shows the changes in Hg(II) reduction with the varying –OH/–COOH ratio (Table 1) in the studied HS at different concentration of Ca2+ under dark condition. The rate of Hg(II) reduction gradually decreased with the increasing Ca2+ concentration. This observation can be justified by considering the screening effect of Ca2+ in the aquatic system. Ca2+ probably prevented Hg(II) to undergo complexation reactions with –COOH, –OH functional groups of HS and decreased its reduction under dark condition. Hg(II) reduction has been reported to influence by the ratio of –OH/–COOH in aquatic medium (Rocha et al. 2000).
Mechanisms of abiotic Hg(II) reduction under dark condition
There are three general pathways by which Hg0 may form in natural waters. (i) Hg(II) species (not associated directly with an organic complex, either free or inorganically complexed) may get reduced to Hg0 by reactive intermediate generated in solution. This intermediate could be an organic or inorganic radical or a reduced metal generated by photo-oxidation of natural organic matter. (ii) Direct photo-reduction of Hg(II) may occur in an aquatic system. In this case, Hg(II) is reduced by electrons supplied by DOC (due to photo-oxidation). (iii) An autocatalytic reaction may also reduce Hg(II) to form Hg0 in an aquatic system.
Here, we propose that chances of formation of reactive intermediate by photo-oxidation of natural organic matter and the possibility of electrons transfer to Hg(II) is less under dark condition.
This study suggests that Hg(II) reduction by HS in aquatic systems decreases with increasing Ca2+ concentrations under dark condition.
It is suggested that Ca2+ prevented Hg(II) to undergo complexation reactions with different major functional groups in HS under dark condition. Ca2+, thus, prevented intermolecular electron transfer from HS to Hg(II) and inhibited Hg0 formation. The impact of increasing Ca2+ concentrations on Hg(II) reduction by HS in the aqueous medium is presented in Fig. 6. Figure 6a describes more complexation of Hg with the major functional groups of HS in the presence of low concentration of Ca2+. More complexation leads to more Hg(II) reduction under dark condition. Figure 6b shows the impact of high concentration of Ca2+ ion on Hg(II) reduction. The large screening effect prevents Hg(II) to undergo complexation with major functional groups which inhibit Hg(II) reduction. It is suggested that complexation of Hg(II) and HS in an aqueous medium is one of the requirements for Hg(II) reduction under dark condition.
Conclusions
The reduction of Hg(II) by HS in the aquatic medium is an important process under dark condition. The major outcomes of this research are summarized below:
-
(i)
Abiotic Hg(II) reduction in an aquatic system may increase with increasing Hg(II) loading under dark condition.
-
(ii)
Abiotic Hg(II) reduction decreases with increasing Ca2+ concentration in an aquatic system. Intermolecular electron transfer from HS to Hg(II) is screened by Ca2+ which prevents Hg0 formation in an aquatic medium under dark condition.
-
(iii)
Reduction of Hg(II) gradually decreases with the increasing total S content in the HS. However, Hg(II) reduction is influenced by changing –OH/–COOH ratio in dissolved HS under dark condition.
-
(iv)
This study also suggests that complexation of Hg(II) and HS is a requirement for Hg(II) reduction under dark condition.
References
Allard B, Arsenie I (1991) Abiotic reduction of mercury by humic substances in aquatic system—an important process for the mercury cycle. Water Air Soil Pollut 56:457–464. doi:10.1007/BF00342291
Amyot M, Mierle G, Lean D, Mc Queen DJ (1997) Effect of solar radiation on the formation of dissolved gaseous mercury in temperate lakes. Geochim Cosmochim Acta 61(5):975–987
Amyot M, Lean DR, Poissant L, Doyon MR (2000) Distribution and transformation of elemental mercury in the St. Lawrence River and Lake Ontario. Can J Fish Aquat Sci 57(S1):155–163
Balchand AN, Nambisan PNK (1986) Effect of pulp-paper effluents on the water quality of Muvattupuzha river emptying into Cochin backwaters. Indian J Mar Sci 15:253–259
Baohua G, Bian Y, Miller CL et al (2011) Mercury reduction and complexation by natural organic matter in anoxic environments. Proc Natl Acad Sci U S A 108:1479–1483. doi:10.1073/pnas.1008747108
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351. doi:10.1016/S0045-6535(99)00283-0
Bonzongo JCJ, Donkor AK (2003) Increasing UV-B radiation at the earth’s surface and potential effects on aqueous mercury cycling and toxicity. Chemosphere 52(8):1263–1273
Chakraborty P (2017) Mercury exposure and Alzheimer's disease in India—an imminent threat? Sci Total Environ. 589, 232-235. doi:10.1016/j.scitotenv.2017.02.168
Chakraborty P, Babu PR (2015) Environmental controls on the speciation and distribution of mercury in surface sediments of a tropical estuary, India. Mar Pollut Bull 95(1):350–357
Chakraborty P, Chakrabarti CL (2006) Chemical speciation of Co, Ni, Cu, and Zn in mine effluents and effects of dilution of the effluent on release of the above metals from their metal-dissolved organic carbon (DOC) complexes. Anal Chim Acta 571:260–269. doi:10.1016/j.aca.2006.04.069
Chakraborty P, Gopalapillai Y, Murimboh J, Fasfous II, Chakrabarti CL (2006) Kinetic speciation of nickel in mining and municipal effluents. Anal Bioanal Chem 386(6):1803–1813
Chakraborty P, Babu PR, Vudamala K, Ramteke D, Chennuri K (2014) Mercury speciation in coastal sediments from the central east coast of India by modified BCR method. Mar pollut bull, 81(1):282--288
Chakraborty P, Sarkar A, Vudamala K, Naik R, Nath BN (2015a) Organic matter—a key factor in controlling mercury distribution in estuarine sediment. Mar Chem 173:302--309. doi:10.1016/j.marchem.2014.10.005
Chakraborty P, Vudamala K, Coulibaly M et al (2015b) Reduction of mercury (II) by humic substances—influence of pH, salinity of aquatic system. Environ Sci Pollut Res 22:10529–10538. doi:10.1007/s11356-015-4258-4
Chakraborty P, Vudamala K, Chennuri K et al (2016) Mercury profiles in sediment from the marginal high of Arabian Sea: an indicator of increasing anthropogenic Hg input. Environ Sci Pollut Res 23:8529–8538. doi:10.1007/s11356-015-5925-1
Costa M, Liss PS (1999) Photoreduction of mercury in sea water and its possible implications for Hg 0 air–sea fluxes. Mar Chem 68:87–95. doi:10.1016/S0304-4203(99)00067-5
Dill C, Kuiken T, Zhang H, Ensor M (2006) Diurnal variation of dissolved gaseous mercury (DGM) levels in a southern reservoir lake (Tennessee, USA) in relation to solar radiation. Sci Total Environ 357(1):176–193
Fantozzi L, Ferrara R, Frontini FP, Dini F (2007) Factors influencing the daily behaviour of dissolved gaseous mercury concentration in the Mediterranean Sea. Mar Chem 107:4–12. doi:10.1016/j.marchem.2007.02.008
Garcia E, Amyot M, Ariya PA (2005) Relationship between DOC photochemistry and mercury redox transformations in temperate lakes and wetlands. Geochim Cosmochim Acta 69(8):1917–1924
Gerbig CA, Kim CS, Stegemeier JP, Ryan JN, Aiken GR (2011) Formation of nanocolloidal metacinnabar in mercury-DOM-sulfide systems. Environmental science & technology 45(21):9180–9187
Gu B, Bian Y, Miller CL, Dong W, Jiang X, Liang L (2011) Mercury reduction and complexation by natural organic matter in anoxic environments. Proc Natl Acad Sci 108(4):1479–1483
Jiang T, Skyllberg U, Wei S, Wang D, Lu S, Jiang Z, Flanagan DC (2015) Modeling of the structure-specific kinetics of abiotic, dark reduction of Hg(II) complexed by O/N and S functional groups in humic acids while accounting for time-dependent structural rearrangement. Geochim Cosmochim Acta 154:151–167
Kim JP, Fitzgerald WF (1986) Sea-air partitioning of mercury in the equatorial pacific ocean. Science 231:1131–1133. doi:10.1126/science.231.4742.1131
Krishnakumar PK, Pillai VK (1990) Mercury near a caustic soda plant at Karwar,India. Mar Pollut Bull 21:304–307
Krishnakumar PK, Pillai VK, Valsala KK (1990) Bioaccumulation of trace metals by marine flora and fauna near a caustic soda plant (Karwar, India). Indian J Fish 37:129–137
Lamborg CH, Hammerschmidt CR, Gill GA et al (2012) An intercomparison of procedures for the determination of total mercury in seawater and recommendations regarding mercury speciation during GEOTRACES cruises. Limnol Oceanogr Methods 10:90–100. doi:10.4319/lom.2012.10.90
Lamborg CH, mercury speciation and reactivity in the coastal and estuarine waters of long island sound, 2003, PhD Thesis, University of Connecticut.
Lanzillotta E, Ceccarini CA, Ferrara R (2002) Photo-induced formation of dissolved gaseous mercury in coastal and offshore seawater of the Mediterranean basin. Sci Total Environ 300(1):179–187
Mauclair C, Layshock J, Carpi A (2008) Quantifying the effect of humic matter on the emission of mercury from artificial soil surfaces. Appl Geochem 23:594–601. doi:10.1016/j.apgeochem.2007.12.017
Nriagu JO (1994) Mechanistic steps in the photoreduction of mercury in natural waters. Sci Total Environ 154(1):1–8
O’Driscoll NJ, Lean DRS, Loseto LL et al (2004) Effect of dissolved organic carbon on the photoproduction of dissolved gaseous mercury in lakes: potential impacts of forestry. Environ Sci Technol 38:2664–2672. doi:10.1021/es034702a
O’Driscoll NJ, Poissant L, Canario J, Lean DRS (2008) Dissolved gaseous mercury concentrations and mercury volatilization in a frozen freshwater fluvial lake. Environmental science & technology 42(14):5125–5130
O'Driscoll NJ, Siciliano SD, Peak D, Carignan R, Lean DRS (2006) The influence of forestry activity on the structure of dissolved organic matter in lakes: implications for mercury photoreactions. Sci Total Environ 366(2):880–893
Rocha JC, Junior ES, Zara LF et al (2000) Reduction of mercury(II) by tropical river humic substances (Rio Negro)—a possible process of the mercury cycle in Brazil. Talanta 53:551–559. doi:10.1016/S0039-9140(00)00532-4
Rocha JC, Sargentini E, Zara LF, Rosa AH, dos Santos A, Burba P (2003) Reduction of mercury (II) by tropical river humic substances (Rio Negro)—Part II. Influence of structural features (molecular size, aromaticity, phenolic groups, organically bound sulfur). Talanta, 61(5),699--707
Rolfhus KR, Fitzgerald WF (2001) The evasion and spatial/temporal distribution of mercury species in Long Island Sound, CT-NY. Geochim Cosmochim Acta 65:407–418. doi:10.1016/S0016-7037(00)00519-6
Satpathy KK, Natesan U, Sarguru S (2008a) Seasonal variations in mercury concentrations in the coastal waters of Kalpakkam, southeast coast of India. Curr Sci 95:374–381
Satpathy R, Hee T, Esterbrooks D, Mohiuddin S (2008b) Delayed defibrillator lead perforation: an increasing phenomenon. Pacing Clin Electrophysiol 31:10–12. doi:10.1111/j.1540-8159.2007.00919.x
Selvaraj K (1999) Total dissolvable copper and mercury concentrations in innershelf waters, off Kalpakkam. Curr Sci 77:494–497
Selvaraj P, Narayanan PR, Reetha AM (1999) Association of functional mutant homozygotes of the mannose binding protein gene with susceptibility to pulmonary tuberculosis in India. Tuber Lung Dis 79:221–227. doi:10.1054/tuld.1999.0204
Si L, Ariya PA (2008) Reduction of oxidized mercury species by dicarboxylic acids (C2–C4): kinetic and product studies. Environ Sci Technol 42:5150–5155
Tseng CM, Lamborg C, Fitzgerald WF, Engstrom DR (2004) Cycling of dissolved elemental mercury in Arctic Alaskan lakes. Geochim Cosmochim Acta 68(6):1173–1184
Vudamala K, Chakraborty P (2016) Kinetic speciation of mercury–humate complexes in aqueous solutions by using competing ligand exchange method. Microchem J 126:551–557
Xia K, Skyllberg UL, Bleam WF, Bloom PR, Nater EA, Helmke PA (1999) X-ray absorption spectroscopic evidence for the complexation of Hg(II) by reduced sulfur in soil humic substances. Environmental science & technology 33(2):257–261
Xiao ZF, Strömberg D, Lindqvist O (1995) Influence of humic substances on photolysis of divalent mercury in aqueous solution. In: Mercury as a global pollutant. Springer Netherlands, Dordrecht, pp 789–798
Zheng W, Hintelmann H (2010) Nuclear field shift effect in isotope fractionation of mercury during abiotic reduction in the absence of light. J Phys Chem A 114(12):4238–4245
Zheng W, Liang L, Gu B (2012) Mercury reduction and oxidation by reduced natural organic matter in anoxic environments. Environ Sci Technol 46(1):292–299
Zhu W, Lin CJ, Wang X, Sommar J, Fu X, Feng X (2016) Global observations and modeling of atmosphere–surface exchange of elemental mercury: a critical review. Atmos Chem Phys 16(7):4451–4480
Acknowledgements
Authors are thankful to the Director, NIO, Goa, for his encouragement and support. This work is a part of the Council of Scientific and Industrial Research (CSIR) supported GEOSINKS (PSC0106) project. KV is thankful to UGC for providing the Senior Research Fellowship. This article bears NIO contribution number 6032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Severine Le Faucheur
Rights and permissions
About this article
Cite this article
Vudamala, K., Chakraborty, P. & Sailaja, B.B. An insight into mercury reduction process by humic substances in aqueous medium under dark condition. Environ Sci Pollut Res 24, 14499–14507 (2017). https://doi.org/10.1007/s11356-017-8979-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8979-4