Abstract
Substantial percentage of world food production depends on pollinating service of honeybees that directly depends on their health status. Among other factors, the success of bee colonies depends on health of developed larvae. The crucial phase of larval development is the first 6 days after hatching when a worker larva grows exponentially and larvae are potentially exposed to xenobiotics via diet. In the present study, we determined the lethal concentration LC50 (72 h) following single dietary exposure of honeybee larvae to formetanate under laboratory conditions, being also the first report available in scientific literature. Activities of antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT) and glutathione-S-transferase (GST) were also measured in the homogenates of in vitro reared honeybee larvae after single formetanate exposure. Decreased specific activity of SOD and increased activities of CAT and GST suggest the induction of oxidative stress. Higher levels of thiobarbituric reactive species in all samples supported this fact. Comparing determined larval toxicity (LC50 of 206.01 mg a.i./kg diet) with adult toxicity data, we can suppose that the larvae may be less sensitive to formetanate than the adult bees.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extensive use of pesticides raises many problems due to their potential harmful effects on non-target organisms, persistence and combined effects with other agrochemicals and environmental factors. Insecticides are thought to be among the major factors contributing to current declines in honeybee populations. Their residues were reported in the wax, honey, beebread and pollen usually taken from in-hive environment (Johnson et al. 2010; Mullin et al. 2010; Pisa et al. 2015, Gómez-Ramos et al. 2016). Among other factors, the success of bee colonies depends on health of developed larvae. Larvae, far from being protected from pesticides in the colony, may be chronically exposed to an accumulation of chemical residues (Human et al. 2014). The first 6 days after hatching are very important because the larvae are potentially exposed to xenobiotics via diet. There are few data concerning the effect of pesticides on honeybee larvae.
Formetanate and formetanate hydrochloride, respectively, belong to the class of a formamidine acaricide and a carbamate insecticide. Both functional groups (formamidine and carbamate) act by permitting constant impulse transmission at synapses leading to convulsions in insects. Good water solubility and poor soil adsorption give to formetanate hydrochloride a high environmental contamination potential. The presence of two functional groups means that selective degradation of only one group may yield decomposition products with an intact pesticide-active site (Divito et al. 2007). The hazard of pesticide poisoning to honeybees results not only from direct contact poisoning but also from the intake of certain contaminated nectar, pollen and water and the transport of contaminated products into the hive (Suchail et al. 2001). The hazard of different chemicals is commonly expressed in terms of acute toxicity (LD50). According to EFSA scientific report (2006), pesticides containing formetanate should not be applied later than 2 weeks before flowering of ornamental shrubs to protect honeybees because of potential harmful effects to adult bees and their developmental stages. Kinetics of formetanate in insects is not fully elucidated. In mammals, formetanate is rapidly and extensively absorbed after oral administration (>96% within 120 h). The major route of excretion is urine (>92% of the dose), predominantly during the first 24 h postadministration. No evidence of bioaccumulation was observed (EFSA 2006). The metabolites formed in plants are very similar to those formed in mammals and insects (Kuhr 1970), but the toxicological impact of residues resulting from the use of formetanate is essentially due to the parent compound (EFSA 2006).
Metabolism or biotransformation does not necessary result in less toxic compounds (Park et al. 2014). It has been reported that many organic cyclic compounds can induce oxidative stress by enhancing reactive oxygen species (ROS) in tissues (Bus and Gibson 1984; Cheung et al. 2001). Organisms have a variety of antioxidant and detoxifying enzymes (e.g. superoxide dismutase, catalase and glutathione-S-transferase). They represent one part of the natural endogenous defence system and are expressed in a response to ROS production to neutralize the oxidative effect of oxygen and its reactive metabolites (Sies 1993). All of these enzymes have been reported to occur in insects (Weirich et al. 2002). Carbamates can generate ROS and can change the antioxidant enzyme activities (Sobeková et al. 2009).
The potential hazard to honeybees from the use of the pesticide is identified in risk assessment. Risk assessment is a simple calculation of likelihood that “bad things” will happen to honeybees based on a specific hazard or dose. The honeybee is generally considered as extremely sensitive to pesticides compared to other insect species, making this species a good environmental indicator of pesticide pollution (Porrini et al. 2003). The high sensitivity of honey bees seems to be confirmed by the lower number of genes encoding xenobiotic detoxifying enzymes in the Apis mellifera genome compared with other insect species (Claudianos et al. 2006; Arena and Sgolastra 2014). Despite that, Hardstone and Scott (2010) who compared the relative sensitivity of A. mellifera to insecticides using adult available data (overall across the six classes of insecticides) observed no evidence that A. mellifera is more sensitive to insecticides relative to other insects. Even though honey bees have a lower number of cytochrome P450 genes, this does not reflect a greater sensitivity to insecticides. No data are available about the effect of formetanate on the enzyme activities in insect species, particularly in the honeybee larvae. Only some reports on insect susceptibility to formetanate are available (Bielza et al. 2008; Contreras et al. 2010; Guillén et al. 2014).
The goal of the present study was to determine the dietary toxicity of formetanate (LC50, 72 h) on honeybee larvae reared in vitro and to assess the effect of formetanate on the antioxidant enzyme activities of honeybee larvae after single dietary exposure.
Materials and methods
Acquisition and grafting of the first instar larvae and test units
The honeybee larvae were reared in vitro using the methodology described by Aupinel et al. (2007) and OECD 237 (2013). Synchronized first instar larvae of A. mellifera carnica were collected separately from three healthy queen-right colonies (each representing a replicate) reared in experimental apiary of University of Veterinary Medicine and Pharmacy in Košice (Slovakia) during the summer of 2015. Each queen was confined to a defined area on a comb containing empty cells for 30 h to control the age of the brood. At day 1 the combs, containing the synchronized first instar larvae (no more than 24 h), were transferred to experimental laboratory for grafting of the larvae into the testing units. During the test, larvae were maintained in 48-well culture plates with commercial grafting cells with an internal diameter of 9 mm (ref CNE/3, NICOTPLAST/France). Before grafting, the grafting cells were disinfected for a minimum of 2 h in a methylbenzethonium chloride (MBC) (Sigma Aldrich). The disinfected grafting cells were dried and distributed thereafter into the wells of a 48-well culture plate, which were previously half-filled with a piece of a dental roll (cellulose, wetted with a 15% glycerol solution (Sigma Aldrich) in aqueous solution of MBC (4 g L−1)). Single first instar larvae were placed onto the surface of the diet A (20 μL) in each grafting cell using sterile grafting spoon for transfer.
Diet preparation and larval feeding
Honey bee larval diet was prepared using royal jelly (obtained from Institute of Apiculture in Liptovský Hrádok, Slovakia), D-glucose (mikroCHEM), D-fructose (mikroCHEM), yeast extract (Fisher Scientific) and distilled water. The diet compositions (A, B, C) used for feeding the bee larvae and feeding scheme used in our experiment were identical as described by Aupinel et al. (2007) and OECD 237 (2013) as follows:
Diet A (day 1) is 50% weight of fresh royal jelly +50% weight of an aqueous solution containing 2% weight of yeast extract, 12% weight of glucose and 12% weight of fructose.
Diet B (day 3) is 50% weight of fresh royal jelly +50% weight of an aqueous solution containing 3% weight of yeast extract, 15% weight of glucose and 15% weight of fructose.
Diet C (from day 4 to day 6) is 50% weight of fresh royal jelly +50% weight of an aqueous solution containing 4% weight of yeast extract, 18% weight of glucose and 18% weight of fructose.
Multi-residues analysis of royal jelly originated from Institute of Apiculture in Liptovský Hrádok (Slovakia) was not conducted. Royal jelly was freshly collected from healthy colonies kept in Tatra National Park (TANAP) with known treatment history.
Preparation of the spiking solutions and spiked diets
Test item formetanate hydrochloride (analytical standard in 99.7% purity, Fluka) in five test concentrations plus control group (Co) and reference item group (Co+) dimethoate (analytical standard in 99.5% purity, Supelco) were tested in range finding bioassay. All spiking solutions for the preparation of spiked diets were prepared freshly on the day of spiking. First, the stock solution of formetanate (24.24 mg mL−1) in distilled water was prepared. Subsequently, a serial dilution was made from the stock solution using the freshly prepared diet C as a solvent. In such way, five final concentrations of formetanate (0, 151.52, 303.03, 606.06, 1212.12 and 2424.24 mg a.i./kg) in the diet were obtained. The choice of tested concentrations was based on result obtained from independent preliminary test with nominal concentrations of 3030.3, 432.90, 61.80 and 8.80 mg a.i./kg in a diet on day 4 (OECD 237, 2013). The preparation of spiked diets was the same as in range finding test.
The larvae in preliminary and range finding bioassays were fed with untreated diets A and B on day 1 and day 3, respectively. The larval food contaminations were done from one respective solution from five solutions previously prepared on the day 4 of the experiment. For each concentration, three replicates of 12 larvae were used and larval mortality was recorded 24, 48 and 72 h post application. Each day, larval survival was recorded and dead individuals together with commercial grafting cells were removed. Dead larvae were recognized by immobility of their stigmata, and dead prepupae and pupae by occasional black or white subdermal necrotic stains or visible wilting (by using stereomicroscope). On day 7, the uneaten diet was weighed after pipeting from the cells of the alive larvae in both experiments. Uneaten diet is expressed as a proportion (%) of diet offered during the whole bioassay per one tested individual (i.e. according to OECD 237 (2013), single larva should be fed with total volume of 160 μL, i.e. with density of about 1.1 mg μL−1 (OECD 239 2016), it is 176 mg/larva for the whole bioassay). The diet of the control group was prepared in the same way as described for the test item treatment level, but instead of test item containing spiking solution an equivalent quantity of distilled water was added to the untreated diet (= diet C) of the control group.
For the reference item group, in preliminary and range finding tests, one dimethoate-containing spiking solution was prepared (OECD 237 2013). From spiking solution, a defined quantity was used to spike untreated diet (= diet C) in order to obtain a dimethoate-containing diet of nominally 266.7 mg dimethoate a.i./kg diet, corresponding to a feeding dose of nominally 8.8 μg dimethoate a.i./larva.
On days 5 and 6, all the larvae in the different test groups were fed with untreated diet C (OECD 237 2013).
Experimental conditions
From day 1 to day 7, the larvae were incubated in a hermetic container (Sicco) at +34.5 °C, containing a Petri dish filled with a saturated solution of potassium sulphate (mikroCHEM) in order to keep a relative humidity of 90 ± 5%. In order to avoid any potential contamination of the control larvae or cross-contamination of the reference item larvae, the control group and reference item group were stored in separate containers with the same test conditions as described above. The desired test conditions were recorded with suitable and calibrated instruments. The preliminary and range finding bioassays were done under the same testing conditions.
Mortalities were expressed in number of dead larvae and in a corrected percentage according to OECD 237 (2013). Determination of growth delay degree was not part of these bioassays.
Enzyme assays
Alive larvae of three concentration groups in range finding bioassay were frozen (−50 °C) and then homogenized in 5 mmol L−1 TRIS-HCl buffer pH 7.8 containing 0.15 mol L−1 KCl, 1 mmol L−1 Na2EDTA and 2 mmol L−1 reduced glutathione using Ultra-Turrax T25 homogenizer. Homogenates (25% w/v) were ultracentrifuged at 105,000×g for 1 h (4 °C). Supernatants were stored at −50 °C until using for enzyme assays. The total proteins were quantified according to Bradford (1976) using bovine serum albumin as a standard.
Enzyme assays were performed in a UV-Vis spectrophotometer Helios-beta. Superoxide dismutase activity (SOD, EC 1.15.1.1) was determined by measuring the inhibition of cytochrome c reduction using xanthine/xanthine oxidase O2 •– generating system at 550 nm (25 °C) (Flohé and Ӧtting 1984). Aliquots of the supernatants were added to a cuvette containing a 50 mmol L−1 phosphate buffer supplemented 0.1 mmol L−1 Na2EDTA (pH 7.8), 20 μmol L−1 cytochrome c and 50 μmol L−1 xanthine. Final volume of reaction mixture was 1 mL. The reaction was initiated by addition of xanthine oxidase. One unit of SOD activity was defined as the amount of enzyme that causes 50% inhibition of cytochrome c reduction under the assay conditions. Catalase activity (CAT, EC 1.11.1.6) was assayed by monitoring the decrease in absorbance at 240 nm (30 °C) due to the consumption of H2O2 (Sizer and Beers 1952) in a medium containing 10 mmol L−1 H2O2 and 20 mmol L−1 phosphate buffer at pH 7.0. Final volume of reaction mixture was 3 mL and quartz cuvette was used for determination. Glutathione-S-transferase activity (GST, EC 2.5.1.18) was measured at 340 nm (30 °C) according to Habig and Jacoby (1981). Reaction medium with total volume 1 mL was containing 1 mmol L−1 of 1-chloro-2,4-dinitrobenzene as the substrate and 1 mmol L−1 of reduced glutathione in 0.2 mol L−1 phosphate buffer supplemented 1 mmol L−1 Na2EDTA (pH 6.5). One unit of enzyme activity was defined as the amount of enzyme that catalyses the formation of 1 μmol of product per minute under assay conditions. Specific enzyme activities were expressed in units per milligram of protein. Lipid peroxidation products were measured as thiobarbituric acid reactive substances (TBARS) that absorb at 535 nm (Gutteridge 1984). The content of TBARS was expressed as A535/mg of protein. All used reagents were of the highest purity from Sigma, Merck and Boehringer.
Statistical evaluation
The statistical processing of the obtained data was performed by using ToxRat Professional, Version 3.2.1, (® ToxRat Solutions GmbH) and EPA Probit Analysis, version 1.5. The data of the test item treatment group (formetanate) were statistically evaluated and compared to the control data by using a Fisher’s exact binomial test with Bonferroni correction, one-sided greater, α = 0.05.
The results of the enzyme analysis were expressed as mean ± SD (n = 6) of three independent determinations. Statistical analysis was done by Student’s t test (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
The effects of formetanate exposure on honeybee larvae reared with contaminated food were assessed according to the OECD 237 (2013). The results of the independent preliminary test showed 100% mortality on day 5 in tested nominal concentration of 3030.3 mg a.i./kg diet and no mortality at lowest tested concentration of 8.80 mg a.i./kg diet. Based on mortality observed in preliminary test, the LC50 was expected to be between concentrations of 303.03 and 606.06 mg a.i./kg diet in a range finding test. Because range finding test showed cumulative mortality on day 7 over 50% in all tested concentrations (Table 1), we decided to pool mortality data obtained from our preliminary test together with those obtained in range finding test in a final LC50 calculation. We tried to use as originally data as possible and thereby, the controls were also pooled, i.e. there were total 72 larvae introduced. The established LC50 (72 h) for formetanate for A. mellifera larvae was 206.01 mg a.i./kg diet with a 95% confidence interval of 114.51–316.36 mg a.i./kg diet (χ2 = 18.3; df = 7; P < 0.001) (Fig. 1).
Observed range of uneaten diet on day 7 (Table 2) was from 51.9 to 81.0 mg/larva in test concentration of 432.9 and 1212.12 mg a.i./kg diet, respectively. During the both bioassays, larval mortality in the control group from day 4 to day 7 was <15% (no larvae died until day 7) and the larval mortality in the reference group was >50% from day 4 until day 7, thus validating the test (Table 1). On day 7, no statistical significance between the control and the lowest tested concentration was shown (Table 1). Determined no observed effect concentration (NOEC) of 8.8 mg a.i./kg diet was based on cumulative mortality observed on day 7.
Enzymes that protect against xenobiotics are recognized as sensitive biomarkers, especially those involved in oxidative stress and detoxification processes. The effect of formetanate (151.52–2424.24 mg a.i./kg diet) and dimethoate (266.7 mg a.i./kg diet) on antioxidative enzyme system of in vitro reared honeybee larvae was tested 72 h after single exposure to contaminated diet. Because of high cumulative mortality observed on day 7 (Table 1), activities of antioxidant enzymes were tested only for three different concentrations. In this study, homogenates of whole insects were used to obtain the enzyme preparations.
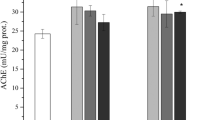
Significantly lower activity of SOD (3.3–4.5 times) was determined in homogenates of larvae fed on treated diet (dimethoate—organophosphate as positive control Co+—and formetanate—carbamate as tested active ingredient) when compare with the control Co (untreated diet) (Fig. 2). Specific activity of catalase statistically decreased (237 U mg−1) at the lowest tested concentration of formetanate when compared with the control (288 U mg−1). The activity of CAT was rising up with the tested concentrations of formetanate (305 and 334 U mg−1 , respectively). All tested concentrations of formetanate caused increasing activity of glutathione-S-transferase (Fig. 2). TBARS is a marker of lipid peroxidation. Higher TBARS content in tested homogenates of honeybee larvae were in good correlation with changed enzymes activities (Fig. 2). The highest changes of all determined parameters were observed at formetanate concentration of 303.03 mg a.i./kg diet.
Specific activities of antioxidant and detoxifying enzymes and TBARS content in honeybee larvae homogenates after single exposure to formetanate. Co control group, Co+ positive control (dimethoate). Values are means ± SD (n = 6). Student t test was used for evaluation; *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
All the developmental stages of honeybee are exposed to a wide range of agrochemicals and veterinary medicinal products used in agriculture and apiculture through contaminated food, wax, etc. Multiple chemical residues present in wax may interact to cause a delay in the development of larvae reared in old combs (Wu et al. 2011).
In the present study, we determined the lethal concentration seventy-two hours (72 h, LC50) following single exposure of honeybee larvae to formetanate in the laboratory conditions, being also the first report available in the scientific literature. Determined LC50 was calculated by using a pooled mortality data obtained from two bioassays (preliminary and range finding tests), both done in summer 2015 (June and July) using fresh hatched larvae from the same colonies (the same biological material). During both bioassays, observed larval mortality in the control group was 0% and in the reference group was >50% from day 4 until day 7. In our opinion, observed cumulative mortality of 17 larvae in concentration of 432.9 mg a.i./kg diet on day 7 in a preliminary test is maybe just “accidental” and that is why we decided to pool data in LC50 determination. As for mortality, 100% larval mortality was observed on day 5 in tested nominal concentration of 3030.3 mg a.i./kg diet, in lower tested concentration of 2424.24 mg a.i./kg diet on day 6 and no mortality at lowest tested concentration of 8.80 mg a.i./kg diet on day 7. The highest larval mortality and visible growth delay in both bioassays were observed between days 5 and 6 (Table 1). The same finding was observed in positive control (pooled data) on day 6.
Comparing determined LC50 (72 h) for larvae (206.01 mg a.i./kg diet) with those one for adult of A. mellifera 72 h LC50 of 11.17 mg a.i./kg diet (data not published), we can suppose that the larvae seem to be less sensitive to formetanate than the adults. This finding is in line with results presented by Tavares et al. (2015), who observed that the larvae were more tolerant to thiamethoxam when compared with the adults of Africanized A. mellifera. Similarly, Yang et al. (2012) observed that larvae were more tolerant to the imidacloprid when compared with adults of A. mellifera. The second-instar larva of western flower thrips, Frankliniella occidentalis (Pergande), was also less susceptible to the formetanate than the adults (Contreras et al. 2010). Identical principle of different sensitivity of larva vs. adult bee can be observed for toxic standard dimethoate preferred in both OECD 213 (1998) (acute oral adult bee) and OECD 237 (2013) (honeybee larvae) tests. Reported oral LD50 (24 h) for adult worker honeybee is in the range of 0.10–0.35 μg a.i./bee and for honeybee larvae is 8.8 ± 0.5 μg a.i./larva. In contrary to that findings, approximately 50 times higher LC50 for selenium were observed for adult foragers compared with larvae (Hladun et al. 2013).
Different susceptibility observed between larvae and adults can be explained by the presence of the insect fat body, a unique tissue to insects, which plays major role in intermediary metabolism and it is the central storage depot of nutrients and energy reserves. The storage function of the fat body is essential for the life of holometabolous insects (butterflies, flies, fleas, bees, ants, beetles etc.), which must accumulate at least a minimal amount of nutrients in the larval stages to survive during periods of starvation and metamorphosis. Lipids always represent the major component of the fat body and the main source of metabolic fuel (Arrese and Soulages 2010). The absolute amounts of lipids increase in both worker and drone larva as they increase in weight in the course of larval development, and the amounts decrease during metamorphosis (Hrassnigg and Crailsheim 2005). For a number of other insect species, the lipid content corrected for dry weight is higher in larvae compared with adults (Fast 1964; Hardstone et al. 2010). According to Hardstone and Scott (2010), to gain a greater understanding of the relative sensitivity of honey bees, it would be desirable to evaluate other life stages, e.g. larval and/or bioassays endpoints other than mortality.
In our opinion, larval phase is crucial from toxicological point of view. A worker larva grows about 900–1100 times the weight of an egg or newly hatched larva coupled with increasing fat body. During pupal phase, fat body energy reserves are mobilized in response to the energy demands of other tissues. At the same time, the fat body responds to the metabolic requirements of the organ itself. Therefore, the mobilization of energy stores must be tightly coupled to a number of metabolic pathways (Arrese and Soulages 2010).
Repeated exposure scenario according to OECD 239 (2016) seems to be more realistic, if in reality, potential residues present in larval diet are consumed daily over the first 6 days after hatching where except for the larval mortality recorded from day 4 to day 8, a mortality of non-emerged bees (pupal mortality) are counted on day 22 of bioassay. Appropriateness of chronic exposure scenario was confirmed in a study using larval rearing method adapted by Zhu et al. (2014) to assess the chronic oral toxicity to honeybee larvae of the four most common pesticides detected in pollen and wax (fluvalinate, coumaphos, chlorothalonil and chloropyrifos). Authors observed a significant increase in larval mortality at/or beyond day 4 of feeding. According to these authors, chronic toxicity is likely to be undetected in a conventional acute toxicity study, resulting in potential underestimation of pesticide effects to larvae.
Even with the discrepancy in the sensitivity of the honeybee during its development, Yang et al. (2012) highlight that the toxic effect of imidacloprid at low doses may be harmful and may affect the larvae. Results obtained from the study done by Tavares et al. (2015) support this finding, since the sublethal doses of thiamethoxam caused atypical progression of developmental stages and the development of the optic lobes larvae.
Beside the larval mortality, also the presence of uneaten diet of alive larvae on day 7 was observed (Table 2). The range of uneaten diet ranged from 29.5 to 46.0% of total weight of diet one larva should be fed with during the bioassay (i.e. total weight of 176 mg diet for one larva during the whole bioassay). The uneaten diet was present with alive larvae with inhibited growth. The lower cumulative mortality observed at concentration of 432.9 mg a.i./kg diet (preliminary test) in comparison to mortality observed at two lower concentrations can be explained by higher number of larvae with uneaten diet on the bottom of their cells (Table 2). At this concentration, 19 out of 36 larvae survived on day 7 but as much as 12 of them were inhibited in their growth, probably due to the insufficient intake of contaminated diet. There are some not answered questions. How to handle with these data when assessing acute/dietary toxicity to in vitro reared larvae? Would in vitro reared larvae inhibited in growth develop to mature stage? More important question is how to apply this observation to reality, from organism level (larva) to colony level? And to which extent will be relevant? This should be reflected in some next studies.
Exposure to sublethal concentrations of pesticides and their metabolites within brood tissues may result in continuous pesticide stress (Wu et al. 2011) or reduction of bee’s lifespan (Oliveira et al. 2014). Some data on formetanate hydrochloride residues in pomes, citrus, strawberries and stone fruits were reported (Niemann 1993; Hu et al. 1996). Undeterminably long dissipation value of the residues on citrus foliage (given as formetanate equivalents with dissipation curve followed first-order kinetics) was observed (Hadjidemetriou et al. 1985). The metabolites formed in plants (foliar application) are very similar to those formed in mammals and insects. The major constituent of the residue was formetanate in all the investigated crops (tomatoes, lemons, peaches and alfalfa) and the main found metabolites resulting from the hydrolysis of formetanate were N′-(3-hydroxyphenyl)-N′,N-dimethylformamidine, 3-formamidophenylmethyl-carbamate and 3′-hydroxyformanilide (Kuhr 1970; EFSA 2006). No data on presence of formetanate in wax, pollen, nectar, royal jelly or propolis are published in scientific studies to that time, so it is difficult to estimate potential exposure to larvae. Our data on mortality in honeybee’s larvae peaked about 2 days following single exposure. There is no evidence that the observed effects are induced by parent compound or its metabolite(s).
A large number of organic cyclic compounds can stimulate the generation of ROS by redox cycling of biotransformed organic molecules (Park et al. 2014). In insects, increased ROS production has also been associated with detoxification processes (Du Rand et al. 2015). Antioxidant enzymes play key roles in regulation of the intracellular ROS balance to prevent ROS-mediated damage and are produced by cells in response to oxidative stress (Corona and Robinson 2006). Superoxide dismutase and catalase are considered as the primary defence against oxidative damage, and glutathione-S-transferase is an early marker of induction of detoxifying system and also appears to contribute to cellular protection of oxidative damage (Badiou-Bénéteau et al. 2012).
Superoxide dismutase (SOD) has particular value as an antioxidant that can help to protect against cell destruction. The substrate of SOD is the superoxide radical anion. It is responsible both for direct damage of biological macromolecules and for generating other reactive oxygen species. SOD keeps the concentration of superoxide radicals in low level. The inhibition of the SOD activity may result from the increased production of superoxide anion radical. The extent of the decrease of SOD activity depends on the degree of oxidative stress (Pracasam et al. 2001). Accumulation of superoxide anion radical leads to damage of the mitochondrial membrane and apoptosis (Huang et al. 2000). It has been shown that SOD is inhibited by H2O2 because of the reduction of copper ions in the active centre of the enzyme (Hodgson and Fridovich 1975).
Catalase activity has been reported to be present in honey. Honey contains H2O2 produced by glucose oxidase, an enzyme present in honey (Weirich et al. 2002). Catalase perhaps acts to keep H2O2 levels in honey below toxic level. The enzyme is very active in the honeybee and can be modulated by environmental pollutants (Carvalho et al. 2013). Catalase prevents hydroxyl radical formation by breaking down hydrogen peroxide into oxygen and water (Corona and Robinson 2006). Increased activity of catalase (Fig. 2) could provide protection against H2O2 generated internally or entering the cell by diffusion. Higher activity of CAT suggested a high dietary exposure to H2O2 or H2O2-generating chemicals. Increased CAT activity could be partially explained by the ability of formetanate to induce oxidative stress.
The GST family is a multifunctional cytosolic enzyme superfamily that plays important roles in the detoxification of insecticides and secondary metabolites, as well as in protection against oxidative stress (Badiou-Bénéteau et al. 2012). GSTs act by conjugating the thiol group from glutathione to ROS and wide variety of xenobiotics, resulting in more polar compounds being excreted or further metabolized (Du Rand et al. 2015; Mittapalli et al. 2007). Some isoenzymes of GST also have glutathione peroxidase activity (Corona and Robinson 2006). This activity is particularly important in invertebrates because they are deficient in the other (vertebrate-type, selenium-dependent) glutathione peroxidases (Pardini 1995; Weirich et al. 2002). Insect GSTs are classified into at least six classes: delta, epsilon, omega, sigma, theta and zeta, of which the delta and epsilon classes are unique to insects (Du Rande et al. 2015). Delta GSTs have been implicated in insecticide resistance in several insects. At the metabolic level, resistance mechanisms of insects to both plant toxins and synthetic insecticides involve the induction of detoxification enzymes which transform xenobiotics into less toxic and more soluble compounds that can be excreted (Human et al. 2014). In our experiment, formetanate increased total GST activity from 0.21 U mg−1 in control group to 0.33; 0.38 and 0.33 U mg−1, respectively, in experimental groups. This could be explained by the fact that some part of formetanate metabolism involves conjugation to glutathione. Increased specific activity of GST was also observed in honeybees poisoned with permethrin, deltamethrin and flumethrin (Yu et al. 1984; Nielsen et al. 2000; Papadopoulos et al. 2004). GSTs from the sigma class have a high affinity for lipid peroxidation products and because of their localization in metabolically active tissues in flies it can be supposed that these enzymes may play a role in protection against oxidative stress (Du Rand et al. 2015). The enzyme activities were determined in vitro reared larvae on day 7. Maximum absolute amounts of lipids are characterized for this larval stage (Hrassnigg and Crailsheim 2005). Lipids are considered to be the most sensitive macromolecules to oxidative damage. Increased levels of TBARS (marker of lipid peroxidation) in experimental groups pointed to harmful effect of formetanate to larval tissue.
Enzymatic detoxification in the eusocial A. mellifera may be complemented by behaviours comprising a “social detoxification system”, including forager discrimination, dilution by pollen mixing and colony food processing via microbial fermentation that reduces the number or quantity of ingested chemicals requiring detoxification (Berenbaum and Johnson 2015).
Conclusion
To study the life cycle of honeybee is still a challenge for scientists. Xenobiotics with long half-life of degradation are potentially hazardous for the larvae despite that they are not directly exposed in contrary to adult foraging honeybees. The crucial phase of larval development is the first 6 days after hatching when a worker larva grows exponentially and larvae are potentially exposed to xenobiotics via diet. Pesticide residues in larval diet/wax can potentially have adverse effects on larvae (e.g. growth delay), which may secondary inhibit colony development, health and overwintering success. While adult foraging bees are targeted directly during the collection of nectar or pollen, the larvae are protected from high xenobiotics exposure using the “social detoxification system”. Only a few data on acute or chronic toxicity of pesticides orally administered to honeybee larvae are known. In the present study, we determined dietary LC50 (72 h) following single dietary exposure of honeybee larvae to formetanate under laboratory conditions. The decrease in SOD activity and increase in CAT and GST activity suggest the induction of oxidative stress in honeybee larvae after single exposure to formetanate. Higher level of TBARS in samples strongly supports this hypothesis.
Because the success of colonies depends on the health of developed larvae, potential adverse effects of pesticides must be considered for a complete analysis. Our findings can potentially contribute to policies regarding pesticide registration purposes by the regulatory agencies.
References
Arena M, Sgolastra F (2014) A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology [Internet] [cited 2016 March 2] 23(3):324–334. doi:10.1007/s10646-014-1190-1
Arrese EL, Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55:207–225. doi:10.1146/annurec-ento-112408-085356
Aupinel P, Fortini D, Michaud B, Marolleau F, Tasei JN, Odoux JF (2007) Toxicity of dimethoate and fenoxycarb to honey bee brood (Apis mellifera), using a new in vitro standardized feeding method. Pest Manag Sci 63:1090–1094
Badiou-Bénéteau A, Carvalho SM, Brunet JL, Carvalho GA, Buleté A, Giroud B, Belzunces LP (2012) Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: application to the systemic insecticide thiamethoxam. Ecotox Environ Safe 82:22–31
Berenbaum MR, Johnson RM (2015) Xenobiotic detoxication pathways in honey bees. Curr Opin Insect Sci 10:51–58
Bielza P, Quinto V, Gravalos C, Fernandez E, Abelian JJ (2008) Impact of production system on development of insecticide resistance in Frankliniella occidentalis (Thysanoptera: Thripidae). J Econ Entomol 101:1685–1690
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bus JS, Gibson JE (1984) Paraquat: model for oxidant-initiated toxicity. Environ Health Persp 55:37–46
Carvalho SM, Belzunces LP, Carvalho GA, Jean-Luc Brunet JL, Badiou-Beneteau A (2013) Enzymatic biomarkers as tools to assess environmental quality: a case study of exposure of the honeybee Apis mellifera to insecticides. Environ Toxicol Chem 32(9):2117–2124
Cheung CCC, Zheng GJ, Li AMY, Richardson BJ, Lam PKS (2001) Relationship between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquatic toxicol 52:189–203
Claudianos C, Ranson H, Johnson RM, Biswas S, Schuler MA, Berenbaum MR, Feyereisen R, Oakeshott JG (2006) A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol 15(5):615–636
Contreras J, Espinosa PJ, Quinto V, Abella J, Gravalos NC, Fernandez E, Dielza P (2010) Life-stage variation in insecticide resistance of the western flower thrips (Thysanoptera: Thripidae). J Econ Entomol 103:2164–2168
Corona M, Robinson GE (2006) Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol Biol 15(5):687–701
Divito CB, Davies S, Masoudi S, Muhoro CN (2007) Relative stability of formamidine and carbamate groups in the bifunctional pesticide formetanate hydrochloride. J Agric Food Chem 55:5377–5382
Du Rand EE, Smit S, Beukes M, Apostolides Z, Pirk CWW, Nicolson SW (2015) Detoxication mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci Rep 5:11779. doi:10.1038/srep11779
European Food Safety Authority . 2006. Conclusion regarding the peer review of the pesticide risk assessment of the active substance formetanate. The EFSA Journal, [Internet]. [cited2016 Oct 10] 4(6):RN-69, 1–78 Available from: doi:10.2903/j.efsa.2006.69r
Fast PG (1964) Insect lipids: a review. Mem Entomol Soc Can 37:1–50
Flohé L, Ötting F (1984) SOD assays. Method Enzymol 105:93–104
Gómez-Ramos MM, García-Valcárcel AI, Tadeo JL, Fernández-Alba AR, Hernando MD (2016) Screening of environmental contaminants in honey bee wax comb using gas chromatography–high-resolution time-of-flight mass spectrometry. Environ Sci Pollut Res 23:4609. doi:10.1007/s11356-015-5667-0
Guillén J, Navarro M, Bielza P (2014) Cross-resistance and baseline susceptibility of spirotetramat in Frankliniella occidentalis (Thysanoptera: Thripidae). J Econ Entomol 107(3):1239–1244
Gutteridge JMC (1984) Ferrous ion-EDTA-stimulated phospholipid peroxidation. Biochem J 224:697–701
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione-S-transferases. Method Enzymol 77:398–405
Hadjidemetriou DG, Iwata Y, Gunther FA (1985) Analysis and dissipation of dislodgable residues of acephate, dimethoate and formetanate hydrochloride on citrus foliage. Pestic Sci 16:302–310
Hardstone MC, Scott JG (2010) Is Apis mellifera more sensitive to insecticides than other insects? Pest Manag Sci 66:1171–1180
Hardstone MC, Huang X, Harrington LC, Scott JG (2010) Differences in development, glycogen, and lipid content associated with cytochrome P450-mediated permethrin resistance in Culex pipiens Quinquefasciatus (Diptera: Culicidae). J Med Entomol 47:188–198
Hladun KR, Kaftanoglu O, Parker DR, Tran KD, Trumble JT (2013) Effects of selenium on development, survival, and accumulation in the honeybee (Apis mellifera L.) Environ Toxicol Chem 32(11):2584–2592
Hodgson EK, Fridovich I (1975) The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of enzyme. Biochemistry 14(24):5294–5949
Hrassnigg N, Crailsheim K (2005) Differences in drone and worker physiology in honeybees (Apis mellifera). Apidologie 36:255–277
Hu RW, Petay V, Fournier J (1996) Determination of formetanate hydrochloride in strawberries. J Agric Food Chem 44:181–184
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W (2000) Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407(6802):390–395
Human H, Archer CR, du Rand EE, Pirk CWW, Nicolson SW (2014) Resistance of developing honeybee larvae during chronic exposure to dietary nicotine. J Insect Physiol 69:74–79
Johnson RM, Ellis MD, Mullin CA, Frazier M (2010) Pesticides and honey bee toxicity—USA. Apidologie 41(3):312–331
Kuhr RJ (1970) Metabolism of carbamate insecticide chemicals in plants and insects. J Agric Food Chem 18(6):1023–1103
Mittapalli O, Neal JJ, Shukle RH. 2007. Tissue and life stage specificity of glutathione S-transferase expression in the Hessian fly, Mayetiola destructor: implications for resistance to host allelochemicals. J Insect Sci [Internet]. [cited 2016 March 2]; 7: 1–13. Available from: http://jinsectscience.oxfordjournals.org/content/jis/7/1/20.full.pdf 10.1673/031.007.2001
Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, van Engelsdorp D, Pettis JS (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One 5:e9754
Nielsen SA, Brødsgaard CJ, Hansen H. 2000. Effects on detoxification enzymes in different life stages of honeybees (Apis mellifera L., Hymenoptera: Apidae) treated with a synthetic pyrethroid (flumethrin). Altern Lab Anim 28(3):437–443 [Abstract]
Niemann RA (1993) Determination of formetanate hydrochloride in selected fruits by coupled-column cation exchange liquid chromatography. J AOAC Int 76:1362–1368
OECD. 1998. Guideline for the Testing of Chemicals No. 213: Honey bee, Acute Oral Toxicity Test, Section 2; Effects on Biotic Systems, OECD, Paris 10.1787/9789264070165-en
OECD. 2013. Guideline for the Testing of Chemicals No. 237: Honey Bees (Apis mellifera) Larval Toxicity Test, Single Exposure, Section 2: Effects on Biotic Systems 10.1787/9789264203723-en
OECD. 2016. Guidance Document on Honey Bee Larval Toxicity Test following Repeated Exposure No. 239, [Internet]. [cited 2016 Oct 10] Available at: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2016)34&docLanguage=En.
Oliveira RA, Roat TC, Carvalho SM, Malaspina O (2014) Side-effects of thiamethoxam on the brain and midgut of the Africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environ Toxicol 29:1122–1133
Papadopoulos AI, Polemitou I, Laifi P, Yiangou A, Tananaki C (2004) Glutathione S-transferase in the developmental stages of the insect Apis mellifera macedonica. Comp Biochem Physiol C 139:87–92
Pardini RS (1995) Toxicity of oxygen from naturally occurring redox-active pro-oxidant. Arch Insect Biochem 29:101–118
Park YC, Lee S, Cho MH. 2014. The Simplest Flowchart Stating the Mechanisms for Organic Xenobiotics-induced Toxicity: Can it possibly be accepted as a “Central Dogma” for Toxic Mechanisms? Toxicol Res [Internet]. [cited 2016 May 12]; 30(3): 179–184. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4206744/pdf/toxicr-30-179.pdf doi: 10.5487/TR.2014.30.3.179
Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs A, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, Morrissey CA, Noome DA, Settele J, Simon-Delso N, Stark JD, Van der Sluijs JP, Van Dyck P, Wiemers M (2015) Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res 22:68. doi:10.1007/s11356-014-3471-x
Porrini C, Sabatini AG, Girotti S, Fini F, Monaco L, Celli G, Bortolotti L, Ghini S (2003) The death of honey bees and environmental pollution by pesticides: the honey bees as biological indicators. B Insectol 56(1):147–152
Pracasam A, Sethupathy S, Lalitha S (2001) Plasma and RBCs antioxidant status in occupational male pesticide sprayers. Clin Chim Acta 310:107–112
Sies H (1993) Strategies of antioxidant defence. Eur J Biochem 215:213–219
Sizer IW, Beers RF Jr (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–139
Sobeková A, Holovská K, Lenártová V, Flešárová S, Javorský P (2009) The another toxic effect of carbamate insecticides. Acta Biol Hung 60(1):45–54
Suchail S, Guez D, Belzunces LP (2001) Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ Toxicol Chem 20(11):2482–2486
Tavares DA, Roat TC, Carvalho SM, Silva-Zacarin ECM, Malaspina O (2015) In vitro effects of thiamethoxam on larvae of Africanized honey bee Apis mellifera (Hymenoptera: Apidae). Chemosphere 135:370–378
Weirich GF, Collins AM, Williams VP. 2002. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie [Internet]. [cited 2016 April 7]; 33: 3–14. Available from: https://hal.archives-ouvertes.fr/hal-00891910 doi: DOI: 10.1051/apido:2001001
Yang EC, Chang HC, Wu WY, Chen YW (2012) Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS One 7:e49472
Yu SJ, Robinson FA, Nation JL (1984) Detoxication capacity in the honeybee, Apis mellifera L. Pestic Biochem Physiol 22:360–368
Wu JY, Anelli CM, Sheppard WS (2011) Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS One 6:e14720
Zhu W, Schmehl DR, Mullin CA, Frazier JL (2014) Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS One 9(1):e77547
Acknowledgements
Funding was provided by the Slovak Grant Agency VEGA (grant No. 1/0858/16 and No. 1/0176/16) and by the National Reference Laboratory for Pesticides of University of Veterinary Medicine and Pharmacy in Košice, Slovakia. The authors would like to thank Mgr. Tomáš Plichta for his help during the larvae rearing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Staroň, M., Sabo, R., Sobeková, A. et al. Formetanate toxicity and changes in antioxidant enzyme system of Apis mellifera larvae. Environ Sci Pollut Res 24, 14060–14070 (2017). https://doi.org/10.1007/s11356-017-8966-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8966-9