Abstract
Chlordecone is an organochlorine pesticide, used in the Lesser Antilles from 1972 to 1993 to fight against a banana weevil. That molecule is very persistent in the natural environment and ends up in the sea with runoff waters. The objective of the present study is to evaluate the level of contamination in several trophic groups of marine animals according to their distance from the source of pollution. Samples of suspended matter, macroalgae, herbivorous fishes, detrivorous crustaceans, zooplanktivorous fishes, first- and second-order of carnivorous fishes, and piscivorous fishes have been collected in two sites, located downstream the contaminated sites (Goyave and Petit-Bourg), in three marine habitats (coastal mangroves, seagrass beds located 1.5 km from the shoreline, and coral reefs at 3 km offshore). Animals collected in mangroves were the most contaminated (mean concentrations 193 μg kg−1 in Goyave and 213 μg kg−1 in Petit-Bourg). Samples from seagrass beds presented intermediate concentrations of chlordecone (85 μg kg−1 in Goyave and 107 μg kg−1 in Petit-Bourg). Finally, samples from coral reefs were the less contaminated (71 μg kg−1 in Goyave and 74 μg kg−1 in Petit-Bourg). Reef samples, collected 3 km offshore, were two to three times less contaminated than those collected in mangroves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlordecone is an organochlorine pesticide, used from 1972 to 1993 in the Lesser Antilles, to fight again the banana weevil. The manufacturing of this chemical (commercialized as Kepone® and then as Curlone®) was first done in Virginia and stopped in 1975, when workers from the site of production began to show severe and diverse pathologies associated to their exposure. Due to the sewage system of the factory, the local environment and wildlife was also impacted (Epstein 1978; Huff and Gerstner 1978). In the French West Indies, the use of this chemical, however, continued until 1993. As a consequence, approximately 6200 ha is moderately to heavily polluted by chlordecone in Guadeloupe (Cabidoche and Lesueur Jannoyer 2011), which represents about 25% of the land surface used for agriculture. Chlordecone is a very persistent molecule in the environment with a half-life estimated to 600 years (Cabidoche et al. 2009).
In Guadeloupe, banana plants grow in the southern part of Basse-Terre (one of the two islands of Guadeloupe), which is mountainous and, as a consequence of tropical humid weather, characterized by intense rainfall events. Organochlorine molecules are hydrophobic and adsorbed onto organic matter of the soil. With the erosion of soil particles, desorption phenomena, slow solubilization, and infiltration processes, these compounds reach runoff and ground waters that end up directly into the sea (Cattan et al. 2006; Coat et al. 2006; Cabidoche et al. 2009).
Since 2003, several sampling surveys have been conducted in Guadeloupe to evaluate the level of contamination by chlordecone of some species of fishes, crustaceans, and mollusks (Bouchon and Lemoine 2003, 2007; Bertrand et al. 2013; Dromard et al. 2016a, b). In 2008, the French food and safety authorities lowered the maximal residue limit (MRL) for chlordecone, authorized for human consumption and commercialization of sea products, from 200 to 20 μg kg−1 of wet weight and regulated the fishing activities around the island. The most contaminated marine areas, located downstream of the banana plantations, are now totally closed to fishing activities. The boundary areas are classified as areas of fishing restrictions in which it is not possible to fish a list of targeted species. These rules have been established to protect the health of the local population, especially because seafood represents a large part of the Caribbean food trade.
Studying the evolution of pollution within an inshore-offshore gradient, with different habitats and different species in the trophic food-web, is necessary to understand their dispersion mechanism. Few studies have been conducted to evaluate the dispersion of pesticide in marine environment with an inshore-offshore gradient (Rato et al. 2006; Briand et al. 2014; Dromard et al. 2016b). Organochlorine pollution in marine food-webs has been studied in mangroves (Paez-Osuna et al. 2002; Bayen et al. 2005), seagrass beds (Haynes et al. 2000; Bouchon et al. 2016), and coral reefs (Glynn et al. 1995; Haynes and Johnson 2000), but few works analyzed the dynamics of transfer of an organochlorine contamination in the continuum “mangrove-seagrass beds-coral reefs” (Schaffelke et al. 2005). The degradation of these three interlinked habitats has dramatic ecological and economical consequences (Wilkinson and Salvat 2012).
In the present study, we examined the level of contamination in several trophic groups of marine animals in relation to their distance from the source of pollution. Concentrations of chlordecone have been measured in three marine habitats: mangroves, seagrass beds, and coral reefs.
Materials and methods
Study sites
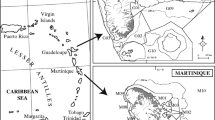
Two study sites (Goyave and Petit-Bourg) were chosen in the eastern coast of Basse-Terre in Guadeloupe (Fig. 1). These two sites are located in an area of fishing restriction due to their position downstream the contaminated rivers and agricultural plots. These two sites include three types of marine habitats: coastal mangroves, seagrass beds (located approximately at 1.5 km from the coast), and coral reefs (around 3 km offshore). Depths were comprised between 1 m in mangroves and 5 m in coral reefs ecosystems.
Sample collection and preparation
The sampling survey was carried out from January 2014 to February 2015. For this study, 205 samples were collected, 113 at Goyave, and 92 at Petit-Bourg (Tables 1 and 2). Macroalgae, fishes, and crustaceans were collected by hand, spearfishing or using nets in seagrass beds and mangroves. Fishes and crustaceans were clustered in trophic groups: detritivorous crustaceans (Crust Det), herbivorous fishes (Fish HB), zooplanktivorous fishes (Fish PK), first-order carnivorous fishes (Fish CA1: invertebrate feeders), second-order carnivorous fishes (Fish CA2: invertebrates and fish feeders), and piscivorous fishes (Fish PV: fish feeders). Each sample was rinsed, weighted to insure the minimal quantity required for chlordecone analysis (10 g wet weight), and frozen (−18 °C) until analyses.
For sampling the suspended matter, seawater was collected in the three habitats of each site in plastic drums. Water was then filtered at the laboratory on Whatman GF/F 47-mm filters.
Chlordecone extraction and analysis
The laboratory Labocea conducted the quantitative analyses of chlordecone. Molecules of chlordecone were extracted from homogenized samples tissues with a solution of organic solvents (hexane-acetone) and turned into chlordecone hydrate (hydrosoluble) in the presence of soda. The aqueous phase was rinsed with hexane to eliminate fats. Chlordecone was then reassembled in acid conditions, extracted with a solution of hexane and acetone. Concentrations of chlordecone were quantified with liquid chromatography coupled to mass spectrometry in tandem (UPLC-MS/MS). Chlordecone was extracted following the method recommended by ANSES (“Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail,” French organization in charge of the sanitary security). The lower limit of quantification with this method was 1 μg kg−1, and the concentrations of chlordecone were expressed in μg kg−1 (wet weight).
Statistical analysis
Shapiro-Wilk’s tests attested of the non-normality of data distribution. Then, concentrations of chlordecone were compared between types of habitat (mangrove, seagrass beds, and coral reefs) with Kruskal-Wallis tests. All statistical analyses were performed using the software package R.
Results
Concentrations of chlordecone according to the habitats
Concentrations of chlordecone measured in this study varied from 1 μg kg−1 (the limit of quantification) to 1034 μg kg−1. Concentrations of chlordecone were significantly different between the three types of habitats at Goyave (X 2 = 18.9, p < 0.001) and at Petit-Bourg (X 2 = 5.5, p < 0.05), and an inshore-offshore gradient of contamination was found (Fig. 2). Samples collected in mangroves were the most contaminated with a mean concentration of chlordecone equal to 193 μg kg−1 at Goyave and 213 μg kg−1 at Petit-Bourg. Marine organisms sampled in seagrass beds presented intermediate concentrations of chlordecone (85 μg kg−1 at Goyave and 107 μg kg−1 at Petit-Bourg). Finally, vegetal and animal samples from coral reefs were the less contaminated (71 μg kg−1 at Goyave and 74 μg kg−1 at Petit-Bourg).
Concentrations of chlordecone according to the trophic group
The level of contamination according to the habitat was studied for the different categories of samples independently. At Goyave, a decreasing gradient of contamination was found for four trophic groups: suspended matter (X 2 = 6.0, p < 0.05), macroalgae (X 2 = 8.9, p < 0.001), detritivorous crustaceans (X 2 = 6.7, p < 0.05), second-order carnivorous fishes (X 2 = 5.7, p < 0.05), and planktivorous fishes (X 2 = 4.7, p < 0.05, Fig. 3). The concentrations of chlordecone were not significantly different according to the habitat for the herbivorous fishes, first-order carnivorous fishes, and piscivorous fishes. Herbivorous fishes presented low and similar concentrations in the three habitats (42, 19, and 10 μg kg−1 in mangrove, seagrass bed, and reef, respectively). First-order carnivorous and piscivorous fishes were highly contaminated in mangrove (295 and 338 μg kg−1, respectively) but showed similar levels of contamination in seagrass bed and coral reef (114 and 112 μg kg−1 for CA1; 154 and 134 μg kg−1 for PV).
Mean concentrations of chlordecone by trophic groups (in μg kg−1 ± SE) measured at Goyave in mangroves, seagrass beds, and coral reefs. SM suspended matter, Crust Det detritivorous crustaceans, Fish HB herbivorous fishes, Fish PK planktivorous fishes, Fish CA1 carnivorous fishes 1, Fish CA2 carnivorous fishes 2, Fish PV piscivorous fishes
At Petit-Bourg, a decreasing gradient of contamination was found for the suspended matter (X 2 = 5.5, p < 0.05), macroalgae (X 2 = 10.4, p < 0.001), first-order carnivorous fishes (X 2 = 9.7, p < 0.001), and piscivorous fishes (X 2 = 12.1, p < 0.001, Fig. 4). No significant difference was found for second-order carnivorous fishes. For the latter, concentrations of chlordecone were close between mangrove, seagrass bed, and reef: 160, 203, and 160 μg kg−1, respectively.
Discussion
The concentrations of chlordecone measured in the present study indicate a high contamination of marine organisms located in the coastal marine habitats in Guadeloupe. A decreasing inshore-offshore gradient of contamination by chlordecone was found at both sites. Samples collected in mangroves, located along the shore, were the most contaminated and presented the highest concentrations of chlordecone measured in this study. Samples from seagrass beds showed intermediate concentrations, while samples from coral reefs were the less contaminated. Concentrations of chlordecone in organisms were two to three times higher in mangroves than in coral reefs.
In previous studies on chlordecone pollution in the James River (Virginia), mean concentrations of chlordecone reached 4800 μg kg−1 in zooplankton and 1700 μg kg−1 for the zooplanktivorous white perch, Morone americana (Nichols 1990; Luellen et al. 2006). In Guadeloupe, the highest concentration measured was 1034 μg kg−1 for Pomadasys corvinaeformis, which is a first-order carnivorous fish (CA1), and the concentration of chlordecone in zooplankton averaged 20 μg kg−1 at Petit Bourg and 6 μg kg−1 at Goyave. In comparison, the level of contamination of marine organisms in Guadeloupe appears low.
However, in coral reefs, located approximately at 3 km offshore, concentrations of chlordecone measured were still three times higher than the maximal residue limit authorized for human consumption and commercialization of sea products (20 μg kg−1). These results justify the interdiction of fishing on the continental shelf located on the eastern coast of Basse-Terre due to high levels of contamination by chlordecone.
In Florida, Glynn et al. (1995) studied the dispersion of marine fauna contamination by pesticides at three sites distributed on a 5 km distance from the coast and found no spatial variation of the level of pollution between the sites. In other studies, the dispersion of organochlorine pollutants was generally demonstrated over larger distances. Rato et al. (2006) studied the dispersion of a pesticide over 25 km of the continental shelf in Portugal. In the south of New Caledonia, a decreasing gradient of pollution was also found from the coast to the reef barrier on a 45 km distance (Briand et al. 2014). In Guadeloupe, the width of the continental shelf in front of Petit Bourg and Goyave is narrow (around 5 km) and exposed to eastern winds and swell, which prevents the dispersion of the pollutants seaward. Indeed, pollution is concentrated on this small area.
In the present study, the gradient of contamination was analyzed for different trophic groups of marine organisms. The majority of the studied trophic groups showed a decreasing gradient of the contamination from the coast seaward. However, some trophic groups presented a different pattern. In Goyave, the gradient of contamination was not observed for the first-order carnivorous fishes (CA1: invertebrates feeders) and piscivorous fishes (Table 1). These species were highly contaminated in mangrove but showed similar levels of contamination in seagrass bed and coral reef habitats. This could result from the mobility of these species between seagrass beds and coral reefs (for example: Sphyraena barracuda). The absence of gradient for some trophic groups can also be explained by the fact that the same trophic group can be constituted by different species in the three habitats. Moreover, the lack of samples in some habitat could lead to a bias in the comparison (for example, there is a single fish species of CA1 represented in the reef habitat). Still in Goyave, herbivorous fishes presented a low and similar level of contamination in the three habitats (Table 1). That result could be partially explained by their feeding patterns, as they consume macroalgae that were faintly contaminated. At Petit Bourg, the gradient of contamination was not significant for the second-order carnivorous fishes (CA2: invertebrates and fish feeders, Table 2). The movements of some of these species across the different habitats (for example: Lutjanus apodus and L. griseus) could explain the similar level of contamination between the three habitats. These movements can be carried out for dietary purposes or during post-settlement migrations (Chapman and Kramer 2000; Cocheret de la Morinière et al. 2002).
To conclude, considering all the data combined, this study evidences a decreasing gradient of the contamination by chlordecone from the coast to the coral reefs 3 km away from the source of pollution. This spatial variation in chlordecone concentration suggests that uptake from the water column is a significant source of contamination. Uptake through the trophic food web via bioamplification is another potential source of contamination, but this hypothesis requires further investigation. Future research will evaluate the relative contribution of uptake from the water column vs. the food web.
References
Bayen S, Wurl O, Karuppiah S, Sivasothi N, Kee Lee H, Obbard JF (2005) Persistent organic pollutants in mangrove food webs in Singapore. Chemosphere 61:303–313
Bertrand JA, Guyader O, Reynal L (2013) Caractérisation de la contamination de la faune halieutique par la chlordécone autour de la Guadeloupe. Projet CarGual. http://archimer.ifremer.fr/doc/00136/24762/
Bouchon C, Lemoine S (2003) Niveau de contamination par les pesticides des chaînes trophiques des milieu marins côtiers de la Guadeloupe et recherche de biomarqueurs de génotoxicité. Rapport UAG-DIREN, 33 pp
Bouchon C, Lemoine S (2007) Contamination par les pesticides des organisms marins de la baie du Grand Culd-de-Sac Marin (île de la Guadeloupe). Rapport UAG-DIREN, 39 pp
Bouchon C, Lemoine S, Dromard C, Bouchon-Navaro Y (2016) Level of contamination by metallic trace elements and organic molecules in the seagrass beds of Guadeloupe Island. Environ Sci Pollut Res 23:61–72
Briand MJ, Letourneur Y, Bonnet X, Wafo E, Fauvel T, Brischoux F, Guillou G, Bustamante P (2014) Spatial variability of metallic and organic contamination of anguilliform fish in New Caledonia. Environ Sci Pollut Res 21:4576–4591
Cabidoche YM, Achard R, Cattan P, Clermont-Dauphin C, Massat F, Sansoulet J (2009) Long-term pollution by chlordecone of tropical volcanic soils in the French West Indies: a simple leaching model accounts for current residue. Environ Pollut 157:1697–1705
Cabidoche YM, Lesueur Jannoyer M (2011) Pollution durable des sols par la chlordécone aux Antilles: comment la gérer ? Innovations Agronomiques 16:117–133
Cattan P, Cabidoche YM, Lacas JG, Voltz M (2006) Occurrence of runoff on high infiltrability under two banana cropping systems. Soil Till Res 86:38–51
Chapman MR, Kramer DL (2000) Movements of fishes within and among fringing coral reefs in Barbados. Environ Biol Fish 57:11–24
Coat S, Bocquené G, Godard E (2006) Contamination of some aquatic species with the organochlorine pesticide chlordecone in Martinique. Aquat Living Resour 19:181–187
Cocheret de la Morinière E, Pollux BJA, Nagelkerken I, van der Velde G (2002) Post-settlement life cycle migration patterns and habitat preference of coral reef fish that use seagrass and mangrove habitats as nurseries. Estuar Coast Shelf Sci 55:309–321
Dromard CR, Bodiguel X, Lemoine S, Bouchon-Navaro Y, Reynal L, Thouard E, Bouchon C (2016a) Assessment of the contamination of marine fauna by chlordecone in Guadeloupe and Martinique (Lesser Antilles). Environ Sci Pollut Res 23:73–80
Dromard CR, Bouchon-Navaro Y, Cordonnier S, Bouchon C (2016b) The invasive lionfish, Pterois volitans, used as a sentinel species to assess the organochlorine pollution by chlordecone in Guadeloupe (Lesser Antilles). Mar Pollut Bull 107:102–106
Epstein SS (1978) Kepone—hazard evaluation. Sci Total Environ 9:1–62
Glynn PW, Rumbold DG, Snedaker SC (1995) Organochlorine pesticide residues in marine sediment and biota from the Northern Florida Reef Tract. Mar Pollut Bull 30:397–402
Haynes D, Johnson JE (2000) Organochlorine, heavy metal and polyaromatic hydrocarbon pollutant concentrations in the Great Barrier Reef (Australia) environment: a review. Mar Pollut Bull 41:7–12
Haynes D, Muller J, Carter S (2000) Pesticide and herbicide residues in sediments and seagrasses from the Great Barrier Reef World Heritage Area and Queenland Coast. Mar Pollut Bull 41:279–287
Huff JE, Gerstner HB (1978) Kepone: a literature summary. J Environ Pathol Toxicol 1:377–395
Luellen DR, Vadas GG, Unger MA (2006) Kepone in James River fish: 1976-2002. Sci Total Envion 358:286–297
Nichols MM (1990) Sedimentologic fate and cycling of Kepone in an estuarine system: example from the James River estuary. Sci Total Environ 97(98):407–440
Paez-Osuna F, Ruiz-Fernández AC, Botello AV, Ponce-Vélez G, Osuna-López JI, Frías-Espericueta MG, López-López G, Zazueta-Padilla HM (2002) Concentrations of selected trace metals (Cu, Pb, Zn), organochlorines (PCBs, HCB) and total PAHs in mangrove oysters from the Pacific Coast of Mexico: an overview. Mar Pollut Bull 44:1296–1313
Rato M, Sousa A, Quinta R, Langston W, Barroso C (2006) Assessment of inshore/offshore tributylin pollution gradients in the Northwest Portugal continental shelf using Nassarius reticulatus as a bioindicator. Environ Toxicol Chem 25:99–106
Schaffelke B, Mellors J, Duke NC (2005) Water quality in the Great barrier reef region: responses of mangrove, seagrass and macroalgal communities. Mar Pollut Bull 51:279–296
Wilkinson C, Salvat B (2012) Coastal resource degradation in the tropics: does the tragedy of the commons apply for the coral reefs, mangrove forests and seagrass beds. Mar Pollut Bull 6:1096–1105
Acknowledgements
We are grateful to the anonymous reviewer for his valuable suggestions which have enabled us to improve this article. This research was funded by the “Contrat de Recherche Développement CHLOHAL 2” coordinated by the Préfecture of Martinique Island.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Dromard, C.R., Guéné, M., Bouchon-Navaro, Y. et al. Contamination of marine fauna by chlordecone in Guadeloupe: evidence of a seaward decreasing gradient. Environ Sci Pollut Res 25, 14294–14301 (2018). https://doi.org/10.1007/s11356-017-8924-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8924-6