Abstract
The concentrations of trace metals (As, Cd, Co, Cu, Fe, Hg, Pb, and Zn) were measured in muscle and hepatopancreas of two Chionoecetes crabs (Chionoecetes japonicus and C. opilio) caught from the East/Japan Sea (EJS) in order to assess the potential health risk by the consumption of deep sea crabs. The highest metal concentrations in muscle and hepatopancreas were As and Fe, respectively, while the lowest metal concentration in two tissues was Pb. The mean concentrations of Cd, Co, Cu, Fe, and Pb in Chionoecetes crabs were one or two orders of magnitude higher in hepatopancreas than in muscles. The mean concentrations of As, Cu, and Hg in muscle and hepatopancreas were relatively higher in C. japonicus than in C. opilio. The estimated daily intakes (EDI) of all metals in muscle were below 0.1% of the provisional tolerable daily intake (PTDI) adopted by the Joint FAO/WHO Expert Committee on Food Additives. Similarly, the target hazard quotient (THQ) of all trace metals in muscle was less than 1.0. These results imply that Chionoecetes crabs caught from EJS do not have an adverse impact on the Korean health. Based on the mean metal concentrations, PTDI, and THQ, the daily maximum permissible intakes of C. japonicus and C. opilio were estimated to be approximately 240 and 410 g/day, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace metals in the ocean play an important role in serving as micronutrients. However, some trace metals are classified as toxic substances for marine organism since nonbiodegradable trace metals such as cadmium and mercury are accumulated in the tissues of marine organisms and the elevated levels of trace metals can cause adverse effects on the growth, breeding, and metabolism of marine organisms (Bryan 1979; Turoczy et al. 2001; Kim and Yoon 2011). Especially, Cd and Hg are accumulated at high levels in crabs compared to other marine organisms (fish and shellfish) due to the binding to metallothionein in tissues (mainly hepatopancreas) of crabs (Chen et al. 2005; Reed et al. 2010; Mok et al. 2014). In addition, these metals are transferred to high tropic levels through the food chain and eventually could be threatened to the human health through the consumption of contaminated seafood (Wang 2002; Tsuchiya et al. 2008; Qiu et al. 2011).
Crabs (Decapoda, Crustacea) are widely distributed in deeper open sea as well as the shallow coastal ocean. The human and industrial activities associated with urbanization and industrialization increased over the last few decades. The potential for metal pollution of benthic crabs in coastal ocean is very high relative to other marine organisms because benthic crabs with restricted mobility mainly reside on the bottom sediment which contains high metal concentrations compared to any other medium in coastal environment and can accumulate high levels of trace metals in their tissues both from water, sediment, and prey (Chen et al. 2005; Reed et al. 2010; Chiarelli and Roccheri 2014). Therefore, trace metal concentrations in benthic crustaceans reflect the status of the pollution of marine environment, and benthic crabs in some coastal oceans have been used as biomonitoring indicators of metal pollution in marine environment (Jewett and Naidu 2000; Burger et al. 2002; Reed et al. 2010; Julshamn et al. 2015). However, the accumulation of trace metals in tissues of deep sea crab species is less well known (Turoczy et al. 2001; Kim and Yoon 2011; Perry et al. 2015).
Chionoecetes crab is a large, long-lived, and deep sea crab, and five species of Chionoecetes crabs live mainly in deep cold waters of the North Pacific Ocean including the East/Japan Sea (EJS), the Okhotsk Sea, the Bering Sea, adjacent to the Aleutian Islands, and the Kamchatka Peninsula and the northwest Atlantic Ocean (from Greenland south to Casco Bay, Maine) (Jadamec et al. 1999). In these Chionoecetes crab species, two species of Chionoecetes crabs, beni-zuwai crab (Chionoecetes japonicus) and snow crab (Chionoecetes opilio), are distributed in the EJS, a marginal sea of the northwestern Pacific Ocean (Chun et al. 2009). C. opilio occurs along the continental shelf at depths ranging from 200 to 450 m, while C. japonicus is observed along the continental slope at depths ranging from 400 to 2000 m in the bottom sediment (Yosho and Hayashi 1994; Kon 1996; Jadamec et al. 1999).
Crabs are one of most preferred seafood products in the whole world. Chionoecetes crab (especially, C. japonicus) is a representative commercial species caught in the EJS and is consumed in the neighboring country, such as Korea, Russia, and Japan, either as boiled or steamed cooked product. Generally, mainly the muscle tissue of legs and claws of the Chionoecetes crabs is consumed; however, sometimes the hepatopancreas of Chionoecetes crabs is also consumed in Korea. According to Statistics Korea, the production of Chionoecetes crabs has gradually increased from ~25,000 t in the 1990s to ~40,000 t in 2010’s in Korea (KMOF 2015). However, Korea during the last 23 years has been discharging various kinds of liquid and solid wastes at two dumping sites of EJS located within the habitat of Chionoecetes crabs (KMOF 2004; Song et al. 2015). Recently, as the consumption of Chionoecetes crabs increases, Koreans have become increasingly aware of the safety of Chionoecetes crabs.
In this study, we investigated the concentrations of eight trace metals (As, Cd, Co, Cu, Fe, Hg, Pb, and Zn) in tissues of Chionoecetes crabs collected in the EJS. The primary purpose of our study is to evaluate to what degree these Chionoecetes crabs are contaminated with these selected trace metals. Potential human health risk assessment is also conducted to evaluate whether Chionoecetes crab consumption presented any potential hazard to humans.
Materials and methods
Sample collection and analytical methods
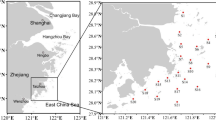
Five male beni-zuwai crabs and snow crabs were collected from each of the four and three fishing ports, respectively, along the eastern coast of Korea in March 2011 (Fig. 1). These ports were the representative distributing centers of Chionoecetes crabs. After collection, Chionoecetes crabs were kept alive in a cool box filled with ice and were transferred to the laboratory within 2 days. In the laboratory, these crabs were stored in a freezer at −20 °C and were euthanized by chilling. Before the crabs were dissected, crab sizes were measured. Crab sizes (carapace length, carapace width, body thickness, and weight) of these Chionoecetes crabs collected at each sampling site are shown in Table 1.
The trace metals in the tissues of Chionoecetes crabs were measured by an analytical procedure modified from Kim et al. (2011). Firstly, Chionoecetes crabs were washed with deionized water (DIW) passed through a Milli-Q water purification system (Elix 5, Millipore, USA) and were dissected using a precleaned ceramic knife over a clean ceramic chopping board. Muscle samples in body, claw and leg, and hepatopancreas samples were taken from each individual and then were freeze-dried at −80 °C with a vacuum freeze dryer. The dried tissues were pulverized and homogenized with a mixer mill in a jar.
Approximately 1.0 g of the powdered tissue samples was placed in an acid-cleaned 60-mL digestion vessel (Teflon, Savillex Corp., USA) with 10 mL of nitric acid (HNO3, Suprapur grade, Merck, Germany) and then was settled at room temperature for 3 h. After prereaction, the tissue samples were digested with lids at 80 °C for 7 h and were dried by heating the vessel without lids at 100 °C for 3 h. This procedure was repeated until only a negligible amount of white residue remained, and the residues were dissolved in 80 mL of 2% HNO3. The solutions were filtered using a filter paper (Advantec, 5C, 110 mm), and the filtered solutions were made up to 100 mL with 2% HNO3 into a 100-mL volumetric flask. The As, Cd, Cu, Co, Fe, Pb, and Zn concentrations except Hg in the solution were measured by using an inductively coupled plasma–mass spectrometer (ICP-MS, ELAN DRC-e, PerkinElmer, USA). Approximately 0.1 g of homogenized dry tissues was used for the Hg analysis. Total Hg concentrations in the dried tissue samples were measured directly without pretreatment using an automated mercury analyzer (DMA 80, Milestone, Italy).
The accuracy and precision of the analytical procedures were checked using certified reference material (CRM). DOLT-4 (dogfish liver, National Research Council Canada) for trace metals except Hg and ERM-CE278 (mussel, European Commission—Directorate General Joint Research Centre) for Hg was used as CRM. These reference materials were treated and measured with the same procedures as the sample, and the mean recovery of each metal was 118% for As, 81% for Cd, 94% for Co, 95% for Cu, 86% for Fe, 102% for Hg, 87% for Pb, and 91% for Zn. In this study, the concentrations of trace metal are expressed as micrograms per gram wet weight (hereafter μg/g).
Potential risk assessment
In order to evaluate the harmful effect of Korean health by Chionoecetes crab consumption, the potential risk assessments were performed for the muscle of Chionoecetes crabs. In this study, we used the provisional maximum tolerable daily intake (PMTDI) of Cd, Cu, Fe, Hg, and Zn suggested by the Joint Food and Agriculture Organization (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) (WHO 2016) and reference dose (RfD) recommended by the US Environmental Protection Agency (Onsanit et al. 2010; USEPA 2016a). Here, PMTDI for Cd and Hg was calculated from the provisional tolerable monthly intake (PTMI) and provisional tolerable weekly intake (PTWI) established by the JECFA, respectively.
Firstly, the estimated daily intake (EDI) was calculated and then was compared with PMTDI. The EDI (μg/kg·bw/day) depends on the metal concentrations in Chionoecetes crabs and the amount of Chionoecetes crab consumption, which was calculated using Eq. (1):
where Ccrab is the mean concentration of trace metals in the muscle of Chionoecetes crabs (μg/g ww), DCcrab is the daily consumption of Chionoecetes crabs by Korean adults (g/day), and BW is the mean body weight (kg) of Korean adults.
We also assessed the health risk for the consumption of Chionoecetes crabs using the target hazard quotient (THQ). THQ indicates the ratio between exposure and the reference dose (RfD) and is based on the USEPA Human Health Risk Assessment approach (Copat et al. 2013; USEPA 2016b). This assessment approach provides useful and valuable information about the safety of consuming marine products including fish and shellfish (Mok et al. 2014). THQ can be expressed by Eq. (2):
where EF is the exposure frequency (from 52 days/year for people who eat crab once a week to 365 days/year for people who eat crab seven times a week), ED is the exposure duration (about 80 years as the average lifetime of adults in Korea), and ET is the average exposure time for noncarcinogens and is equal to EF × ED. Therefore, THQ was simply calculated by dividing the RfD suggested by USEPA (2016a) by the EDI as below (Eq. (3)):
On the other hand, the value of THQ less than 1 can be interpreted to be no obvious risk.
Statistical analysis
The Shapiro–Wilk test was used to assess normality of metal concentrations in tissues of Chionoecetes crabs. The Student’s t test and the Mann–Whitney U test were also performed to identify any significant differences in metal concentrations between species or tissues of Chionoecetes crabs. Statistical significance was set at p < 0.05. All statistical analysis of data was carried out using SPSS version 11.0 software package for Windows (SPSS, Chicago, IL, USA).
Results and discussion
Accumulation pattern of trace metals in tissues of Chionoecetes crabs
The concentrations of trace metals in the muscle and hepatopancreas of Chionoecetes crabs caught from the EJS waters are shown in Table 2. The trace metal concentrations in muscle of C. japonicus decreased in the order As > Zn > Cu > Fe > Hg ≥ Cd ≥ Co > Pb, and the decreasing order has statistically significant difference except Cd (p < 0.05). The trace metal concentrations in muscle of C. opilio decreased in the order As, Zn > Cu > Fe > Hg, Cd, Co > Pb, and the decreasing order has statistically significant difference (p < 0.05) although no significant differences were found in the concentrations between As and Zn or among Cd, Co, and Hg.
Based on the overall metal concentrations in muscle tissue, the accumulation patterns of trace metals in muscles of C. japonicus and C. opilio are very similar to each other although the habitat depth of two Chionoecetes crabs from the EJS waters is different. The highest and lowest metal concentrations in the muscle were As and Pb, respectively, which imply that As relative to other metals is selectively absorbed very well in the muscle of two Chionoecetes crabs. This trend is similar to the accumulation pattern of metals in the muscle of stone crab (Menippe mercenaria) from South Carolina coastal waters (Reed et al. 2010).
The trace metal concentrations in hepatopancreas of C. japonicus decreased in the order Cu, Fe > As > Zn, Cd > Co > Hg > Pb, and the decreasing order has statistically significant difference (p < 0.05) although no significant differences were found in the concentrations between As and Zn or between Hg and Pb. The trace metal concentrations in the hepatopancreas of C. opilio decreased in the order Fe > Cu > As, Zn > Cd > Co > Pb, Hg, and the decreasing order has statistically significant difference (p < 0.05) although no significant differences were found in the concentrations between As and Zn or between Hg and Pb.
On the basis of the metal concentrations in hepatopancreas tissue, the accumulation patterns of trace metals in the hepatopancreas of C. japonicus and C. opilio are very similar with statistically significant difference. The highest metal concentrations were Fe or Cu, while the lowest metal concentrations were Hg or Pb. This indicates that Fe or Cu relative to other metals is selectively absorbed very well in the hepatopancreas of two Chionoecetes crabs. These results of Chionoecetes crabs in the EJS were similar to those of stone crabs in the South Carolina coastal area (Reed et al. 2010).
Comparison of metal concentrations in tissues of Chionoecetes crabs
Generally, trace metals are heterogeneously distributed among tissues in the crab (Nissen et al. 2005; Reed et al. 2010). The concentrations of trace metals in the tissues of C. japonicus and C. opilio showed statistically significant difference (p < 0.05) between muscle and hepatopancreas (Table 2). The average concentrations of As, Hg, and Zn in C. japonicus were relatively higher in muscle than in hepatopancreas. This result is consistent with that in tissues of intertidal burrowing crab (Scylla serrata) found from estuaries on the eastern coast of Australia (Mortimer 2000). In C. opilio, there was no significant difference between muscle and hepatopancreas in the levels of As, Hg, and Zn. However, the average concentrations of Cd, Co, Cu, Fe, and Pb were much higher in hepatopancreas than in muscle in both species. Especially, the concentrations of Cd and Fe in hepatopancreas were one or two orders of magnitude higher than those in muscle. The higher concentrations of trace metals in the hepatopancreas relative to other tissues are due to detoxification function of the hepatopancreas (Rainbow 2007; Reed et al. 2010).
Inter-species difference for the average concentrations of trace metals in muscle and hepatopancreas of two Chionoecetes crabs showed a statistically significant difference (p < 0.05) (Fig. 2). The average concentrations of As, Cu, Hg, and Zn in muscle were higher in C. japonicus than in C. opilio, while the average Pb concentrations were about two times of magnitude higher in C. opilio than in C. japonicas (Fig. 2a). Similarly, the average concentrations of As, Cu, and Hg in hepatopancreas were relatively higher in C. japonicus than in C. opilio, while the average Fe concentrations were higher in C. opilio than in C. japonicas (Fig. 2b). These results appear to be due to the difference of prey organisms and uptake (or excretion and detoxification) rate for each metal between two Chionoecetes crab species because it has become appreciated that uptake of trace metals through diet could be the major source of metals for many aquatic invertebrates (Wang 2002; Rainbow 2007).
Comparison of average concentration between beni-zuwai crab and snow crab for trace metals (As, Cd, Co, Cu, Fe, Hg, Pb, and Zn) in a muscle and b hepatopancreas. The error bar and asterisk mark represent the standard deviation of metal concentrations and significant difference (p < 0.05), respectively
Comparing the concentrations of trace metals in the muscle and hepatopancreas of other crabs from various oceanic environments in the whole world (Table 3), the average concentration of As in muscle of C. japonicus was higher than that of horseshoe crab from USA, red king crab from Barents Sea, and stone crab from USA, and that of C. opilio was higher than that in other studies of snow crab, but lower than that of horseshoe crab and stone crab from the USA (references in Table 3). The average concentrations of Co in muscle of two Chionoecetes crabs were higher than those of rock crab from Taiwan and stone crab from USA, while those of Fe in muscle of two Chionoecetes crabs were lower than those of rock crab from Taiwan and stone crab from USA (references in Table 3). The average concentrations of Cd, Cu, and Zn in muscle of two Chionoecetes crabs are much lower than those of king crab from Australia and blue crab of Yellow Sea, the continental shelf located in the western sea of Korea (references in Table 3). However, the average concentrations of Cd, Hg, and Pb in muscle of two Chionoecetes crabs are much higher than those of red king crab from Norway (references in Table 3).
The average concentrations of As, Co, Cu, Fe, and Zn in hepatopancreas of two Chionoecetes crabs were much lower than those of stone crab from USA (references in Table 3). The average concentration of Cd in hepatopancreas of C. japonicus was higher than that of king crab from Australia and stone crab from USA, while that of C. opilio was lower than that of king crab from Australia and stone crab from USA (references in Table 3). The average concentration of Hg in hepatopancreas of C. japonicas was similar to that of king crab from Australia, and that of C. opilio was similar to that of stone crab from USA (references in Table 3). The average concentration of Pb in hepatopancreas of C. japonicas was lower than that of stone crab from USA, while that of C. opilio was higher than that of stone crab from USA (references in Table 3). These results appear to be associated with the difference in ecological needs, feeding, and metabolic activities among different crab species and various pollution sources such as urban and agricultural activities, electrical power station, and international shipping activities.
Potential risk assessment by the consumption of Chionoecetes crabs
Some metals, such as Cu, Fe, and Zn, are present in high concentration as the essential element in the human body because these metals play an important role in the metabolism and immunity system of the human body (Prashanth et al. 2015). However, excessively high metal concentrations can adversely affect the human body. In addition, the toxic metals, such as Cd, Hg, and Pb, can act as a hazard factor to human health although a small amount of these metals remain in the human body (Bilandžić et al. 2011; Mok et al. 2014). Therefore, we assessed the potential health risk of trace metals by the consumption of two Chionoecetes crabs caught from the EJS. Here, we only used the concentrations of metal in muscle tissue of two Chionoecetes crabs to evaluate the health risk assessment since most Korean people only consume the muscle tissue of Chionoecetes crabs.
Firstly, the metal concentrations (except Co and Fe) in the muscle of two Chionoecetes crabs were compared with the values of the regulatory limit set by each country for ensuring the human health and the safety of marine products (references are shown in Kim and Han (1999) and Mok et al. (2014)). The As and Cu concentrations in muscles of two Chionoecetes crabs were in the range of 2.7–78.2 μg/g (mean 22.5 ± 14.4 μg/g) and 0.9–18.3 μg/g (mean 6.2 ± 3.7 μg/g), respectively. Although the standards of As and Cu for crustaceans are not yet established in the whole world, the As and Cu concentrations in all samples were much lower than the regulatory limits (86 μg/g and 20 μg/g) of As and Cu for mollusk and shellfish applied in USA and UK, respectively. In addition, for arsenic, nontoxic arsenobetaine is the most common As species in marine organisms and inorganic As, the most toxic form, has generally been found in very low levels in marine organisms, such as in red king crab from the Barents Sea (Julshamn et al. 2015).
The Cd concentrations in muscles of two Chionoecetes crabs were in the range of 0.009–0.463 μg/g (mean 0.109 ± 0.110 μg/g). The Cd concentrations in all samples were below the regulatory limits (0.5 to 1.0 μg/g) of Cd established in the European Commission and Korea. Although it is well known that Cd is accumulated in very high levels in hepatopancreas of crab than in muscle, high Cd concentrations above the regulatory limit (0.5 μg/g) set by the European Commission in muscle (claw) of some crab (e.g., edible crab, Cancer pagurus) have been found in Northern Europe such as Norway, Scotland, and the English channel (Maulvault et al. 2012; Wiech et al. 2017), which have raised concern about the safety of crab consumption. However, the Cd concentrations in the muscle of Chionoecetes crabs are much lower than those in other crabs from Europe and thus the muscle of Chionoecetes crabs is much less of a problem about food safety.
The Pb concentrations in the muscles of two Chionoecetes crabs were in the range of ND–0.073 μg/g (mean 0.018 ± 0.018 μg/g). The Pb concentrations in all samples were below the regulatory limits (0.5 to 1.0 μg/g) of Pb established in the European Commission and Korea. The Hg concentrations in muscles of two Chionoecetes crabs ranged from 0.025 to 0.421 μg/g (mean 0.125 ± 0.084 μg/g), which were relatively lower than the regulatory limits (0.5 to 1.0 μg/g) set by many countries including Australia and New Zealand, USA, and the European Commission. The Zn concentrations in muscles of two Chionoecetes crabs ranged from 2.9 to 28.6 μg/g (mean 14.4 ± 5.5 μg/g), which were much lower than the regulatory limits (50 μg/g) established in UK. Therefore, the concentrations of all metals in muscles of two Chionoecetes crabs in the EJS were lower than the standards set by many countries around the world.
As mentioned in the “Potential risk assessment” section, the EDI and THQ are calculated by using Eqs. (1) and (3), respectively, and the values of the calculated EDI and THQ are shown in Table 4. Here, we assessed the potential health risk for only five metals (Cd, Cu, Fe, Hg, and Zn) measured in this study since the previously established PTWIs of Pb and As for the human health protection are withdrawn in the 79th JECFA meeting held in 2014 and the PTWI for Co is absent.
On the basis of the average concentrations of trace metals in the muscle of C. japonicus and C. opilio in this study (average concentrations are in Table 4), the daily consumption of C. japonicus (0.01 g/day) and C. opilio (0.39 g/day) for Korean adults reported by the Korea National Health and Nutrition Examination Survey (KMOHW 2013), and the mean body weight (~64 kg) in Korean adults based on the Korean Health Statistics 2014 (KMOHW 2015), the EDI values of Cd, Cu, Fe, Hg, and Zn for C. japonicus and C. opilio are calculated to be approximately 0.02–2.47 × 10−3 and 0.55–76.2 × 10−3 μg/kg·bw/day, respectively (Table 4). The calculated EDI values for all metals in muscles of C. japonicus and C. opilio were below 0.1% of the PMTDI established by the JECFA (WHO 2016), and the hazardous level decreased in the order Hg > Cd > Zn > Cu > Fe. On the other hand, the EDI values for the essential elements (Cu, Fe, and Zn) in muscle of two Chionoecetes crabs were one or two orders of magnitude higher than those for the toxic metals (Cd and Hg), implying that the muscle of Chionoecetes crabs contributes to the dietary intake of Cu, Fe, and Zn and is a useful source for these elements.
Generally, the THQ proposed by USEPA has been used widely in the whole world for the risk assessment of trace metals in seafood consumption including fish and shellfish (Storelli 2008). In this study, we also used the THQ to assess the health risk to Korean adults by the consumption of two Chionoecetes crabs caught from the EJS as another criterion. Here, we assessed the potential health risk for only two metals (Cd and Zn) in this study since RfD values for As (total), Co, Cu, Fe, Hg (total), and Pb were not assessed or estimated by USEPA in the present.
The RfD values (USEPA 2016a) and the calculated THQ results are shown in Table 4. The THQ values of Cd and Zn in the muscle of C. japonicus are estimated to be approximately 0.02 × 10−3 and 0.01 × 10−3, respectively. The THQ values of Cd and Zn in the muscle of C. opilio are estimated to be approximately 0.55 × 10−3 and 0.25 × 10−3, respectively. The THQ values of Cd and Zn in muscles of two Chionoecetes crabs were much less than 1.0. This implies that Cd and Zn intakes through the consumption of two Chionoecetes crabs caught from the EJS do not pose an appreciable hazard to Korean adults. Although this result has a high uncertainty because of a small amount of sample, the metal concentrations in two Chionoecetes crabs of this study are similar to or lower than those in Chionoecetes crab previously reported around the eastern sea of Korea (Mok et al. 2014).
As above shown in the results of health risk assessments, Chionoecetes crabs caught from the EJS do not have an adverse impact on the Korean health. However, Korean intake of Chionoecetes crabs (maximum ~460 g/day) is large at one time during the catch period although the daily average consumption of Chionoecetes crabs (0.01–0.39 g/day) is very small (KMOHW 2013). In addition, as demands for Chionoecetes crabs increase recently, a large amount of Chionoecetes crabs are imported from other countries (mainly Russia) and potential exposure opportunities of Koreans for metals by consumption of Chionoecetes crabs are gradually increasing. Therefore, in this study, we calculated the daily maximum permissible intake for Chionoecetes crabs using the average concentrations of trace metals in muscle of C. japonicus and C. opilio. Based on the average concentrations in the muscles of two Chionoecetes crabs, PMTDI, and THQ, the daily maximum permissible intakes of C. japonicus and C. opilio were estimated to be approximately 240 and 410 g/day, respectively (Table 4). The estimated values were relatively lower than the maximum intake of Korean (~ 460 g/day) for Chionoecetes crabs (KMOHW 2013). Thus, the appropriate consumption for Chionoecetes crabs is necessary to protect the Korean health in the future.
Conclusion
The concentrations of trace metals (As, Cd, Co, Cu, Fe, Hg, Pb, and Zn) were measured in muscle and hepatopancreas of two Chionoecetes crabs (C. japonicus and C. opilio) caught from the EJS. The highest metal concentrations were As in muscle and Fe in hepatopancreas, while the lowest concentration was Pb in both tissues. Inter-tissues and inter-species difference for the metal concentrations in two Chionoecetes crabs showed a statistically significant difference (p < 0.05). The concentrations of Cd, Co, Cu, Fe, and Pb in two Chionoecetes crabs were significantly higher in hepatopancreas than in muscle, and the concentrations of As, Cu, and Hg in both tissues were relatively higher in C. japonicus than in C. opilio. Based on the evaluation results of the potential health risk using regulatory limits, EDI, and PTDI, Chionoecetes crabs caught from the EJS do not have an adverse impact on the Korean health, and the daily maximum permissible intakes of C. japonicus and C. opilio were estimated to be approximately 240 and 410 g/day, respectively. For the Korean health protection, the appropriate regulation for intakes of Chionoecetes crabs is necessary in the future.
References
Bilandžić N, Đokić M, Sedak M (2011) Metal content determination in four fish species from the Adriatic Sea. Food Chem 124:1005–1010
Bryan GW (1979) Bioaccumulation of marine pollutants. Phil Trans R Soc Lond B 286:483–505
Burger J, Dixon C, Shukla T, Tsipoura N, Gochfeld M (2002) Metal levels in horseshoe crabs (Limulus polyphemus) from Maine to Florida. Environ Res 90:227–236
Chen MH, Chen CY, Chou HY, Wen TC (2005) Gender and size effects of metal bioaccumulation on the rock crab, Thalamita crenata, in Dapeng Bay, southwestern Taiwan. Mar Pollut Bull 50:463–484
Chiarelli R, Roccheri MC (2014) Marine invertebrates as bioindicators of heavy metal pollution. Open J Metal 4:93–106
Chun YY, Lee SI, Yoon SC, Cha HK, Kim JB (2009) Molting and growth of the snow crab Chionoecetes opilio in the East Sea of Korea. Kor J Fish Aquat Sci 42:380–386
Copat C, Arena G, Fiore M, Ledda C, Fallico R, Sciacca S, Ferrante M (2013) Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: consumption advisories. Food Chem Toxicol 53:33–37
Jadamec LS, Donaldson WE, Cullenberg P (1999) Biological field techniques for Chionoecetes crabs. University of Alaska Sea Grant College Program (AK–SG–99–02), Fairbanks, Alaska, p 80
Jewett SC, Naidu AS (2000) Assessment of heavy metals in red king crabs following offshore placer gold mining. Mar Pollut Bull 40:478–490
Julshamn K, Valdersnes S, Duinker A, Nedreaas K, Sundet JH, Maage A (2015) Heavy metals and POPs in red king crab from the Barents Sea. Food Chem 167:409–417
Kim JK, Han T (1999) Effects of inorganic nutrients and heavy metals on grouth and pigmentation of the green alga, Ulva Pertusa kjellman. Kor J Environ Biol 17:427–438
Kim CR, Yoon YY (2011) A study on trace–metals in Korean Yeongdeok crab and Russian snow crab. J Korean Soc Mar Environ Engin 14:147–153
Kim SG, Kim SS, Choi HG, An YR (2011) Concentrations of trace metals in the tissues of long-beaked common dolphins (Delphinus capensis) in the East Sea, Korea. Ocean Sci J 46:55–62
KMOF (Korea Ministry of Oceans and Fisheries) (2004) Development of waste assessment framework on the sewage sludge for ocean disposal. Ministry of Oceans and Fisheries, Seoul, p 382
KMOF (Korea Ministry of Oceans and Fisheries) (2015) Statistical yearbook of oceans and fisheries. Ministry of Oceans and Fisheries, Sejong, p 496
KMOHW (Korea Ministry of Health and Welfare) (2013) Korea national health and nutrition examination survey (KNHANES) database. Ministry of Health and Welfare, Sejong, Korea. https://knhanes.cdc.go.kr/knhanes/index.do
KMOHW (Korea Ministry of Health and Welfare) (2015) Korea Health statistics 2014: Korea national health and nutrition examination survey (KNHANES IV–2). Ministry of Health and Welfare, Sejong, p 451
Kon T (1996) Overview of Tanner crab fisheries around the Japanese Archipelago. In: High latitude crabs: biology, management, and economics. University of Alaska Sea Grant College Program (AK–SG–96–02), Fairbanks, Alaska, p 13–24
Maulvault AL, Anacleto P, Lourenco HM, Carvalho ML, Nunes ML, Marques A (2012) Nutritional quality and safety of cooked edible crab (Cancer pagurus). Food Chem 133:277–283
Mok JS, Kwon JY, Son KT, Choi WS, Kang SR, Ha NY, Jo MR, Kim JH (2014) Contents and risk assessment of heavy metals in marine invertebrates from Korean coastal fish markets. J Food Protect 77:1022–1030
Mortimer MR (2000) Pesticide and trace metal concentrations in Queensland estuarine crabs. Mar Pollut Bull 41:359–366
Nissen LR, Bjerregaard P, Simonsen V (2005) Interindividual variability in metal status in the shore crab Carcinus maenas: the role of physiological condition and genetic variation. Mar Biol 146:571–580
Onsanit S, Ke C, Wang X, Wang KJ, Wang WX (2010) Trace elements in two marine fish cultured in fish cages in Fujian province, China. Environ Pollut 158:1334–1342
Perry H, Isphording W, Trigg C, Riedel R (2015) Heavy metals in red crabs, Chaceon quinquedens, from the Gulf of Mexico. Mar Pollut Bull 101:845–851
Prashanth L, Kattapagari KK, Chitturi RT, Baddam VRR, Prasad LK (2015) A review on role of essential trace elements in health and disease. J Dr NTR Univ Health Sci 4:75–85
Qiu YW, Lin D, Liu JQ, Zeng EY (2011) Bioaccumulation of trace metals in farmed fish from South China and potential risk assessment. Ecotoxicol Environ Safety 74:284–293
Rainbow PS (2007) Trace metal bioaccumulation: models, metabolic availability and toxicity. Environ Internat 33:576–582
Reed LA, Pennington PI, Wirth E (2010) A survey of trace element distribution in tissues of stone crabs (Menippe mercenaria) from South Carolina coastal waters. Mar Pollut Bull 60:2297–2302
Song KH, Choi KY, Kim CJ, Kim YI, Chung CS (2015) Assessment of the governance system for the management of the East Sea–Jung dumping site, Korea through analysis of heavy metal concentrations in bottom sediments. Ocean Sci J 50:721–740
Storelli MM (2008) Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem Toxicol 46:2782–2788
Tsuchiya A, Hinners TA, Burbacher TM, Faustman EM, Mariën K (2008) Mercury exposure from fish consumption within the Japanese and Korean communities. J Toxicol Environ Health (Part A) 71:1019–1031
Turoczy NJ, Mitchell BD, Levings AH, Rajendram VS (2001) Cadmium, copper, mercury, and zinc concentrations in tissues of the king crab (Pseudocarcinus gigas) from southeast Australian waters. Environ Internat 27:327–334
USEPA (United States Environmental Protection Agency) (2016a) Integrated Risk Information Systems assessments. United States Environmental Protection Agency. https://cfpub.epa.gov/ncea/iris2/atoz.cfm
USEPA (United States Environmental Protection Agency) (2016b) Human health risk assessment. United States Environmental Protection Agency. https://www.epa.gov/risk/human–health–risk–assessment
Wang WX (2002) Interactions of trace metals and different marine food chains. Mar Ecol Prog Ser 243:295–309
Whitford J (2003) White Rose baseline addendum—2002 biological cruise (Report No. JW Project NFS08401), Jacques whitford, p 50
WHO (World Health Organization) (2016) Evaluations of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization. http://apps.who.int/food–additives–contaminants–jecfa–database/search.aspx
Wiech M, Vik E, Duinker A, Frantzen S, Bakke S, Maage A (2017) Effects of cooking and freezing practices on the distribution of cadmium in different tissues of the brown crab (Cancer pagurus). Food Cont 75:14–20
Yosho I, Hayashi I (1994) The bathymetric distribution of Chionoecetes opilio and Chionoecetes japonicus (Majidae: Brachyura) in the western and northern areas of the Sea of Japan. Bull Japan Sea Nat Res Inst 44:59–71
Acknowledgements
We thank Y.Y. Jeon and S.H. Ju who helped with sampling and trace metal analyses. This work was supported by a research grant from the National Institute of Fisheries Science (NIFS, R2017053).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Hwang, DW., Choi, M., Lee, IS. et al. Concentrations of trace metals in tissues of Chionoecetes crabs (Chionoecetes japonicus and Chionoecetes opilio) caught from the East/Japan Sea waters and potential risk assessment. Environ Sci Pollut Res 24, 11309–11318 (2017). https://doi.org/10.1007/s11356-017-8769-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8769-z