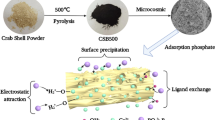
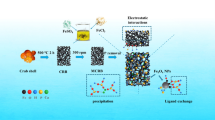
Abstract
The removal and recovery of phosphate from water by calcium-silicate composite (CSC) and alkali-treated calcium-silicate composite (ASC) was investigated. ASC had a higher specific surface area and total pore volume, and exhibited better performance of phosphate adsorption than CSC. In the batch mode adsorption studies, the isotherm adsorption experiments data fitted well the Langmuir isotherm model and the maximum adsorption capacities were 120 and 73.0 mg/g for ASC and for CSC, respectively. For the kinetic study, the experimental data fitted very well the pseudo-second-order kinetic model. The uptake of phosphate could be performed well over a wide pH range, from 3.0 to 13.0 for ASC and from 4.0 to 13.0 for CSC. The adsorption of phosphate by ASC was very selective even with 10 times higher concentration of other coexistent anions. For the adsorption of low phosphate concentration (10 mg/L), ASC could efficiently remove phosphate at the dosage of 0.8 g/L, while CSC was even difficult to remove phosphate at the dosage of 4.0 g/L. Phosphate fractionation results and FTIR spectra showed that phosphate-Ca complex was formed through phosphate adsorption process. The adsorbed phosphate could be successfully desorbed by 2% citric acid solution, indicating that the adsorbent after adsorbed phosphate could be reusable as fertilizer in the agricultural field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive application of phosphorous fertilizers has led to serious water pollution such as ecological imbalance, promoting the growth of unwanted algae and related algal toxins, which are a threat to human health and ecological environment (Paerl 2008; Smith 2003). Phosphorus is also an essential element for the growth of crops, and it is not abundant in the farmland at present. Furthermore, phosphate rock is a non-renewable resource and it is predicted that the current global reserves will be consumed in next 100 years (Cordell et al. 2009). Since the phosphorus cycle on earth does not occur in gaseous form, it is difficult to return phosphorus to land after it enters into water bodies through wastewater discharge or surface runoff. Therefore, it is necessary to remove phosphate from water to avoid serious environmental problems, and the gradual depletion of phosphorus resources also urges us to investigate the recovery methods of phosphate from water.
Several physicochemical and biological treatments have been investigated for removing phosphate from water, and adsorption is superior to other treatments in terms of economical operation, simplicity of design, and high removal efficiency (Hamoudi and Belkacemi 2013; Huang et al. 2008; Su et al. 2013; Xie et al. 2014b). More importantly, adsorbed phosphorus may be recovered by desorption process, and the desorbed phosphorus can be used as fertilizer for agriculture (De-Bashan and Bashan 2004; Sengupta and Pandit 2011). Therefore, the use of a proper adsorbent is the key to ensure removal and recovery efficiency of phosphate from water.
The utilization of industrial wastes and natural materials as adsorbent to remove phosphate in the water, has received big interest in the scientific field and several reports have described their application (Babatunde and Zhao 2010; Lǚ et al. 2013; Mezenner and Bensmaili 2009; Rout et al. 2014; Xu et al. 2009). The greatest advantages of using those adsorbents are their cost-effectiveness and waste resource utilization. However, most industrial wastes and natural materials do not have effective phosphate adsorption capacities unless they are modified by some chemical treatments (Agyei et al. 2002; Cheung and Venkitachalam 2000; Wu et al. 2006; Xie et al. 2014a).
Shells are very abundant in the world and a large number of shells are discarded as solid waste by marine product manufactures. It is widely known that CaCO3, a main component of shell, can adsorb phosphate from water (Martis et al. 1989; Millero et al. 2001). However, Yeom has used scallop shell as adsorbent to adsorb phosphate from water, the adsorption capacity was relatively low (Yeom and Jung 2009). A significant amount of waste glass is also produced every year, and the main component of glass is SiO2. Some studies have reported that calcium-silicate composites could remove phosphate from water through adsorption process (Renman and Renman 2010; Xuechu et al. 2009). Thus, waste glass and shells can be combined to make a novel adsorbent for the reduction of phosphate concentration in water.
In this study, we introduced a new calcium-silicate composite adsorbent (CSC), which was made from waste glass and shells. CSC shows a certain performance of phosphate adsorption in water while its efficiency is not very high, so it is necessary to improve its phosphate adsorption performance by its modification. The aim of this study was to obtain an efficient adsorbent from CSC for the removal and recovery of phosphate from water. For this purpose, alkali-treated calcium-silicate composite (ASC), prepared by CSC, was investigated for its phosphate adsorption potential. For comparison, the CSC was also examined.
Materials and methods
Materials
CSC used in this study was offered by Murakami Corporation in Japan. For ASC preparation, a traditional refluxing method was used, in which 10 g CSC was added to 50 mL of 2 M NaOH at 98 °C for 24 h. After cooling the reaction solution to room temperature, modified sample was washed three times with pure water and twice with dehydrated ethanol. ASC was then dried in an oven at 60 °C overnight. CSC and ASC were ground to pass through a 150-mesh (with the diameter of 106 μm) sieve and stored in containers.
The bulk chemical composition of CSC and ASC was analyzed by X-ray fluorescence analysis (RIX 2100, RIGAKU, Japan). BET surface area and total pore volume were measured by using nitrogen adsorption-desorption method (BELSORP -mini II, MICROTRACBEL, Japan). For pH value of adsorbent, 0.1 g of the adsorbents were added to 50 mL of pure water and shaken for 120 h, and the pH values were measured by using a pH meter (D-51, HORIBA, Japan). The infrared spectra of raw material and phosphate-complexes material were measured by FTIR spectrophotometer (IRAffinity-1, SHIMADZU, Japan). The phosphate solution was made by K2HPO4 (analytical grade, Kanto Chemical Corp., Japan) with pure water. The batch adsorption experiments were conducted in triplicate and the mean data were reported in this paper.
Kinetic study
The kinetic adsorption experiment was performed in 500 mL flasks with 300 mL phosphate solution and 0.6 g of the adsorbents. The initial concentration of phosphate was 500 mg/L and the flasks were stirred at 25 °C for 168 h at 100 rpm. After each specific adsorption time, 1 mL sample was taken and centrifuged at 5000 rpm for 10 min. Phosphate concentration of supernatant was measured by UV-spectrophotometer (UV-2550, SHIMADZU, Japan) using the molybdenum-blue ascorbic method (Rice et al. 2012).
Adsorption isotherms
The adsorption isotherms experiment was performed in 100 mL flasks with 50 mL of phosphate solution with different initial phosphate concentration (20–500 mg/L) and 0.1 g of the adsorbents. Suspensions were stirred at 100 rpm for 120 h at 25 °C in a constant temperature shaker to achieve equilibrium. After 120 h, the suspension was centrifuged at 5000 rpm for 10 min. The amounts of phosphate adsorbed per unit mass of adsorbents were calculated by the following equation:
where C 0 and C e are the initial and equilibrium phosphate concentrations in milligram per liter, respectively. V is the solution volume in liter, and m is the dry mass of adsorbents in grams.
Effect of solution pH
The effect of solution pH on the phosphate adsorption experiment was studied in the same way as the kinetic adsorption experiment. The solution pH was adjusted from 2.0 to 13.0 with 0.1 M HCl and 0.1 M NaOH. After 120 h of shaking, the suspensions were centrifuged, and the equilibrium phosphate concentration as well as equilibrium solution pH was measured. Since the equilibrium time was too long in this study, the phosphate concentration at 24 h was also measured in the same way, showing the removal efficiency of phosphate over a wide pH range in a relatively short time.
Influence of coexistent anions and adsorption in lake water
Experiments were conducted in 200 mL flask with 0.2 g adsorbents and 100 mL of 1 mmol/L (95 mg/L) phosphate solution with and without other anions (SO4 2−, NO3 −, Cl−, HCO3 −). The concentration of each anion was adjusted to 2.5 mmol/L, in which the total molar concentration of coexistent anions was 10 times higher than phosphate. The flasks were shaken for 120 h in the same way as the kinetic adsorption. Phosphate concentrations at 24 and 120 h were measured after centrifugation.
In order to illustrate further performance of ASC and CSC for phosphate adsorption in the presence of natural coexisting ions, sample solution from Lake Teganuma (Abiko, Japan) was taken and filtered through 0.45 μm membrane filter to remove suspended solid. A certain amount of K2HPO4 was then spiked to the lake water sample to give an initial phosphate concentration of 10 mg/L. The solution with the same phosphate concentration was also prepared by using pure water to compare with the lake water sample. Both lake water and pure water samples were shaken for 120 h in the same way as the kinetic adsorption experiment, and phosphate concentration at 24 and 120 h were determined after centrifugation.
Effect of adsorbent dosage
Adsorbent dosage is directly related to the economic benefits of wastewater treatment. Adsorbent dosage experiment was conducted in 200 mL flasks with 100 mL of 10 mg/L phosphate solution and adsorbents dosage varying from 0.5 to 4 g/L for CSC and from 0.2 to 2 g/L for ASC. Considering about low concentration of phosphate solution and economical benefit, we only took samples at 24 h. After 24 h shaking in the same way with kinetic adsorption, the concentration of phosphate solution was measured after centrifugation.
Phosphate desorption
Phosphate at the concentration of 500 mg/L was adsorbed by ASC and CSC for 120 h to confirm that a certain amount of phosphate could be adsorbed by adsorbents. After washing three times with pure water, the adsorbed phosphate on each sample was desorbed by 2% citric acid at room temperature for 2 h with liquid/solid ratio of 100 mL/g. After citric acid treatment, the concentration of phosphate in the citric acid solution was measured after centrifugation.
Phosphate fractionation
Phosphate adsorption by ASC and CSC was conducted using 500 mg/L phosphate solution in the same way as phosphate desorption experiment. After washing three times with pure water, the pre-treated adsorbents were used for phosphate fractionation experiments, as described by the modified phosphate fractionation method of Hieltjes and Lijklema through following steps (Hieltjes and Lijklema 1980): (i) loosely bound-phosphorus (LB-P): pre-treated adsorbents were washed with 1 mol/L NH4Cl solution twice at pH 7 for 2 h, and the extracted phosphate was denoted as LB-P; (ii) alkali-soluble phosphorus (NaOH-P): the adsorbents treated by step 1 were washed with 0.1 mol/L NaOH solution for 16 h and the extracted phosphate was represented as NaOH-P; (iii) acidic-soluble phosphorus (HCl-P): the adsorbents treated by step 2 were washed with 0.5 mol/L HCl solution twice for 2 h and the desorbed phosphate was named as HCl-P; (iv) residual phosphorus (Residual-P): the final residual phosphate was obtained after the adsorbent treated by step 3.
Results and discussion
Materials characterization
The chemical composition of ASC and CSC is given in Table 1. The main contents of adsorbents were SiO2, CaO and Na2O comprising 87% and 86.8% for ASC and CSC, respectively. The contents of other metal oxides were very low. However, the modification of CSC by NaOH resulted in an increase of CaO from 21.4% to 28.6% and Al2O3 from 2.7% to 5.6%. It also caused a decrease of SiO2 from 54.3% to 45.8%. The results also showed that the specific surface area of CSC was 25.1 m2/g, while the ASC was nearly 6.5 times higher than the CSC. Similar trends were observed for total pore volume, indicating that the value of ASC was almost 5 times larger than CSC. The results for specific surface area and total pore volume suggest that ASC has considerably more adsorption sites for phosphate and may have much more chances to adsorb phosphate than CSC (Fontes and Weed 1996). Comparing with CSC, ASC also exhibited stronger alkaline properties, which may result in some different adsorption processes.
Adsorption kinetics
The adsorption kinetics of phosphate for calcium-silicate composites are shown in Fig. 1. The results indicated that the adsorption of phosphate was relatively slow for ASC and CSC, which needed 120 h to reach adsorption equilibrium for both of them. This may be due to the small pore diameter of adsorbents (Table 1). However, the adsorption speed of ASC was much faster than CSC, and the amount of adsorbed phosphate by ASC at 24 h was 82.3 mg/g, which was even higher than equilibrium amount of adsorbed phosphate by CSC (71.2 mg/g). The kinetics data had a good agreement with the pseudo-second-order rate equation (r2 > 0.99), implying that the phosphate adsorption by calcium-silicate composites was governed by a diffusion process (Ho and McKay 1999). The pseudo-second-order model can be described as follows:
where q e and q t are the amounts of adsorbed phosphate at equilibrium time and time t (mg/g), and the k is the equilibrium rate constant for the second-order kinetic adsorption (g/mg. min). The parameters obtained by fitting data to the second-order kinetic model are summarized in Table S1.
Adsorption isotherm
Adsorption isotherm results of different phosphate concentrations on ASC and CSC are given in Fig. 2. When the initial concentration of phosphate was lower than 100 mg/L, almost all the phosphate could be adsorbed and a very low residual phosphate concentration (less than 10 mg/L) was detected in the solution, indicating a high adsorption ability of phosphate for calcium-silicate composites. It was also represented that the amount of adsorbed phosphate increased with the equilibrium phosphate concentration, but the adsorption rate gradually decreased and eventually stopped as the adsorption of phosphate leveled off. These results implied that the adsorption sites had reached saturation. The adsorption isotherm data could also be well fitted Langmuir isotherm equation (r2 > 0.99), indicating that the adsorption of phosphate by silicate-calcium composites was chemical adsorption process, and the related constants were given in Table S2. The maximum adsorptive capacity was 73.0 mg/g and 120 mg/g for CSC and ASC, respectively. The adsorption capacity of ASC was much higher than that of reported cost-effective adsorbent prepared from industries wastes and even higher than that of many functionalized adsorbents focusing on removing phosphate from wastewater (Köse and Kıvanç 2011; Liu et al. 2008).
Figure 2 clearly showed that the adsorption capacity of phosphate by ASC was much higher than CSC. The greater adsorptive capacity of ASC would be owned to the higher specific surface area and larger total pore volume. Previous studies indicated that higher specific surface area and total pore volume meant more adsorption sites on the surface and inside of adsorbents (Lowell et al. 2004), resulting in a higher phosphate adsorption capacity of ASC. CSC was mainly composed of SiO2, CaO, Na2O, and Al2O3, and those components would be activated in the process of alkaline reaction, which increased the phosphate adsorption capacity of ASC.
Influence of solution pH
The influence of solution pH on the adsorption of phosphate by ASC and CSC was investigated in a wide pH range from 2.0 to 13.0 and the results are given in Fig. 3. The values of the solution pH at 24 h and 120 h are also presented in Table 2. When the adsorption of phosphate attained equilibrium, adsorbents exhibited well adsorption capacity over a wide pH range, from 3.0 to 13.0 for ASC and from 4.0 to 13.0 for CSC. The trend of phosphate adsorption by ASC slightly decreased with the increase of pH, while the phosphate adsorption by CSC obviously increased with the increase of pH from 3.0 to 5.0 and then slightly decreased with the further increase of pH from 6.0 to 13.0. However, for the adsorption time at 24 h, the adsorption of phosphate by ASC decreased with the increase of pH from 3.0 to 12.0 and then increased again with pH from 12.0 to 13.0. For CSC, the amount of adsorbed phosphate even reached equilibrium at 24 h with pH from 3.0 to 7.0 and also increased sharply with pH from 12.0 to 13.0.
As widely recognized, pH has a significant effect on the determination of the concentration of phosphate species in the solution. The relationship between phosphate species and pH values is shown in the following equations and also the speciation diagram is illustrated in Fig. 4 (Clifford 1961).
According to Fig. 4, it is clear that HPO4 2− and PO4 3− were the main phosphate species involved in the adsorption reaction by ASC as the pHe of solution was from 9.8 to 12.8 (initial pH 3.0–13.0). A growing number of OH− could compete with phosphate during the adsorption process, resulting in a slightly decrease of phosphate adsorption when the pH value increased (Das et al. 2006). It is also shown that H2PO4 − and HPO4 2− were the main phosphate species involved in adsorption reaction by CSC when the pHe of the solution was from 7.1 to 7.6 (initial pH 3.0–6.0), while HPO4 2− and PO4 3− were the main species when the pHe of the solution was higher than 9.2 (initial pH 7.0). The different concentration of phosphate species led to the different phosphate adsorption amount under different pH by CSC. When the initial pH was from 3.0 to 7.0, phosphate adsorption was very fast and reached equilibrium in 24 h, and this might be due to the easier adsorption of H2PO4 − by CSC. At the initial pH of 13, the concentration of PO4 3− became dominant phosphate species and phosphate adsorption increased in 24 h, implying that PO4 3− was removed faster than other phosphate species by calcium-silicate composites. When the initial solution pH was 2.0, the adsorption of phosphate was nearly zero. Since the solution had a large amount of H+, those might have reacted with the alkaline adsorbents and lost their ability to adsorb phosphate.
Influences of coexistent anions on phosphate adsorption
In order to investigate the influence of coexistent anions on adsorption of phosphate by ASC and CSC, the amount of adsorbed phosphate with and without the presence of other common anions (SO4 2−, NO3 −, Cl−, HCO3 −) is given in Fig. 5. The results indicated that the coexistent anions had a strong effect on inhibiting phosphate adsorption by CSC, decreasing the adsorption amount from 45.2 to 28.5 mg/g. For ASC, there was no obvious effect on phosphate adsorption, indicating that ASC had a high selective adsorption ability of phosphate.
The phosphate adsorption in eutrophic lake water was also examined by CSC and ASC with the initial phosphate concentration of 10 mg/L. For comparison, the phosphate adsorption in pure water was also prepared with the same phosphate concentration and the experimental results were indicated in Fig. 6. The residual concentration of phosphate was very high after using CSC, but ASC could remove nearly 100% phosphate from both pure water and lake water, and the residual phosphate concentration was even lower than 0.05 mg/L, which could limit the growth of algae in the eutrophic water (Conley et al. 2009). Therefore, eutrophic lake water with relatively high concentration of phosphate can be treated by ASC so as to prevent algal growth.
Adsorbent dosage
The influence of adsorbent dosage on phosphate adsorption was studied by varying dose from 0.5 to 4.5 mg/L for CSC and from 0.2 to 2.0 mg/L for ASC with an initial phosphate concentration of 10 mg/L to simulate real waste water. Increased adsorbent dosage meant a greater surface area and larger numbers of adsorption sites to remove phosphate. However, compared to the high concentration of phosphate in the solution, a lower phosphate concentration may be more difficult to be removed by adsorbents due to the lower probability of collision with adsorption sites (Wu et al. 2006). As shown in Fig. 7, the removal rate of phosphate initially increased quickly with the increased dosage of ASC, and reached nearly 100% at the adsorbent dosage of 0.8 mg/L. For CSC, although the percentage of phosphate removal also increased with increasing adsorbent dosage, the removal rate was very low with much higher dose. The latest lakes environmental quality standards for phosphorus in Japan are determined to be 0.1 mg/L, namely the concentration of phosphate was nearly 0.3 mg/L (dotting line in Fig. 7). As shown in the figure, the dose needed to reach this goal was 0.8 g/L for ASC and more than 4.0 g/L for CSC. This indicates that the ASC has a better phosphate adsorptive capacity than CSC even in the low phosphate concentration solution.
Phosphate recovery
The fertilizer control act of Japan has defined the citrate solubility (soluble in 2% citric acid solution) as criteria of solubility of nutrients in the soil (Moriyama et al. 2001), which means that if the phosphate adsorbed adsorbents can be desorbed by 2% citric acid solution, phosphate can be released in the true soil and used as fertilizer. Therefore, 2% citric acid was used to desorb saturated phosphate on adsorbents and the desorption results were shown in Table 3. The results indicated that phosphate adsorbed on calcium-silicate composites could be successfully desorbed by 2% citric acid with recovery rate of more than 96%. Accordingly, phosphate adsorbed by calcium-silicate composites may be used as fertilizer for agriculture as it has high desorption rate by 2% citric acid.
Adsorption mechanism
In general, adsorbed phosphate by adsorbent is divided into four species: (i) loosely bound-phosphorus (LB-P); (ii) alkali-soluble phosphorus (NaOH-P); (iii) acidic-soluble phosphorus (HCl-P); and (iv) residual phosphorus (Residual-P). LB-P is very easy to fall off from the adsorbent and is mainly due to physical adsorption; NaOH-P and HCl-P indicate the phosphate mainly adsorbed by Fe2O3, Al2O3 and CaO, MgO, respectively (Hieltjes and Lijklema 1980). The results of phosphate fractionation were given in Table 4. Most adsorbed phosphate by adsorbents was HCl-P with 96.0% for CSC and 96.2% for ASC. Since the content of MgO was very low for calcium-silicate composites, most phosphate should be adsorbed by CaO. Other researches have also reported that phosphate would form precipitation on the surface of adsorbent as calcium phosphate when the solution has a certain amount of Ca2+ under slightly alkaline condition (Karageorgiou et al. 2007, Xuechu et al. 2009). In order to further illustrate the adsorption mechanism, FTIR spectra was used for structural analysis of adsorbents with and without phosphate complex (Fig. 8). For CSC, a strong band centered at 1450 cm−1 significantly weakened and a band at 877 cm−1 disappeared after adsorbing phosphate. The main FTIR band of CaO and CaCO3 appeared at 1430–1480 cm−1 and 877 cm−1, respectively, indicating the decrease of CaO and CaCO3 with the phosphate adsorption. Meanwhile, the main FTIR band of phosphate-Ca complex appeared at 603 cm−1 and 564 cm−1, demonstrating the precipitation of calcium phosphate formed through adsorption process (Jastrzębski et al. 2011, SUN et al. 2005). For ASC, the FTIR band at 877 cm−1 disappeared after modifying CSC with NaOH, implying that CaCO3 had been alkalified. Even though the band of CaO at 1430–1480 cm−1 did not change too much after adsorption reaction for ASC, it still weakened slightly. Most importantly, the phosphate-Ca band appeared strongly at 603 and 564 cm−1, which showed that the calcium phosphate had been formed (Jastrzębski et al. 2011). Therefore, it can be concluded that the phosphate adsorption by calcium-silicate composites is due to the generated phosphate-Ca complex and the main phosphate adsorption should be described as:
Conclusions
To develop the phosphate adsorption removal and recovery efficiency from water, a novel adsorbent ASC was modified by CSC with alkaline-thermal treatment. The performance of removing phosphate by ASC was much better than that of the CSC, and the maximum amount of adsorbed phosphate reached 120 and 73.0 mg/g, respectively. Both calcium-silicate composites exhibited a very well adsorption capacity of phosphate over a wide pH range. ASC had a great selective phosphate adsorption capacity even in the solution that the molar concentration of other anions was 10 times higher than phosphate. Besides, ASC was also more efficient in treating natural eutrophic water bodies than CSC and it could reduce phosphate concentration from 10 to 0.02 mg/L to control the eutrophication problem. The adsorbed phosphate could be recovered more than 96% by 2% citric acid solution for both adsorbents, implying that adsorbed phosphate could be used as fertilizer for agriculture. Therefore, ASC is a promising adsorbent for the removal and recovery of phosphate from water, especially for the eutrophic water bodies.
References
Agyei NM, Strydom C, Potgieter J (2002) The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary Portland cement and related blends. Cem Concr Res 32:1889–1897
Babatunde A, Zhao Y (2010) Equilibrium and kinetic analysis of phosphorus adsorption from aqueous solution using waste alum sludge. J Hazard Mater 184:746–752
Cheung K, Venkitachalam T (2000) Improving phosphate removal of sand infiltration system using alkaline fly ash. Chemosphere 41:243–249
Clifford AF (1961): Inorganic chemistry of qualitative analysis. Prentice-Hall
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Karl E, Karl E, Lancelot C, Gene E, Gene E (2009) Controlling eutrophication: nitrogen and phosphorus. Science 123:1014–1015
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305
Das J, Patra B, Baliarsingh N, Parida K (2006) Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl Clay Sci 32:252–260
De-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res 38:4222–4246
Fontes M, Weed S (1996) Phosphate adsorption by clays from Brazilian Oxisols: relationships with specific surface area and mineralogy. Geoderma 72:37–51
Hamoudi S, Belkacemi K (2013) Adsorption of nitrate and phosphate ions from aqueous solutions using organically-functionalized silica materials: Kinetic modeling. Fuel 110:107–113
Hieltjes AH, Lijklema L (1980) Fractionation of inorganic phosphates in calcareous sediments. J Environ Qual 9:405–407
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Huang W, Wang S, Zhu Z, Li L, Yao X, Rudolph V, Haghseresht F (2008) Phosphate removal from wastewater using red mud. J Hazard Mater 158:35–42
Jastrzębski W, Sitarz M, Rokita M, Bułat K (2011) Infrared spectroscopy of different phosphates structures. Spectrochim Acta A Mol Biomol Spectrosc 79:722–727
Köse TE, Kıvanç B (2011) Adsorption of phosphate from aqueous solutions using calcined waste eggshell. Chem Eng J 178:34–39
Karageorgiou K, Paschalis M, Anastassakis GN (2007) Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J Hazard Mater 139:447–452
Liu H, Sun X, Yin C, Hu C (2008) Removal of phosphate by mesoporous ZrO2. J Hazard Mater 151:616–622
Lowell S, Shields JE, Thomas MA, Thommes M (2004) Characterization of porous solids and powders: surface area, pore size and density, vol. 16. Springer Science & Business Media, pp 11–18
Lǚ J, Liu H, Liu R, Zhao X, Sun L, Qu J (2013) Adsorptive removal of phosphate by a nanostructured Fe–Al–Mn trimetal oxide adsorbent. Powder Technol 233:146–154
Martis L, Serkes KD, Nolph KD (1989) Calcium carbonate as a phosphate binder: is there a need to adjust peritoneal dialysate calcium concentrations for patients using CaCO3? Perit Dial Int 9:325–328
Mezenner NY, Bensmaili A (2009) Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem Eng J 147:87–96
Millero F, Huang F, Zhu X, Liu X, Zhang J-Z (2001) Adsorption and desorption of phosphate on calcite and aragonite in seawater. Aquat Geochem 7:33–56
Moriyama K, Kojima T, Minawa Y, Matsumoto S, Nakamachi K (2001) Development of artificial seed crystal for crystallization of calcium phosphate. Environ Technol 22:1245–1252
Paerl H (2008) Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater–marine continuum, cyanobacterial harmful algal blooms: state of the science and research needs. Springer, pp:217–237
Renman A, Renman G (2010) Long-term phosphate removal by the calcium-silicate material Polonite in wastewater filtration systems. Chemosphere 79:659–664
Rice EW, Baird RB, Eaton AD, Clesceri L (2012) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Rout PR, Bhunia P, Dash RR (2014) Modeling isotherms, kinetics and understanding the mechanism of phosphate adsorption onto a solid waste: ground burnt patties. Journal of Environmental Chemical Engineering 2:1331–1342
Sengupta S, Pandit A (2011) Selective removal of phosphorus from wastewater combined with its recovery as a solid-phase fertilizer. Water Res 45:3318–3330
Smith VH (2003) Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ Sci Pollut Res 10:126–139
Su Y, Cui H, Li Q, Gao S, Shang JK (2013) Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res 47:5018–5026
Sun W, Li H, Wu C, Liang G (2005) Study on mineral composition of Huainan coal ash with FTIR [J]. Shaanxi Chemical Industry 10:018
Wu D, Zhang B, Li C, Zhang Z, Kong H (2006) Simultaneous removal of ammonium and phosphate by zeolite synthesized from fly ash as influenced by salt treatment. J Colloid Interface Sci 304:300–306
Xie J, Wang Z, Fang D, Li C, Wu D (2014a) Green synthesis of a novel hybrid sorbent of zeolite/lanthanum hydroxide and its application in the removal and recovery of phosphate from water. J Colloid Interface Sci 423:13–19
Xie J, Wang Z, Lu S, Wu D, Zhang Z, Kong H (2014b) Removal and recovery of phosphate from water by lanthanum hydroxide materials. Chem Eng J 254:163–170
Xu X, Gao B, Wang W, Yue Q, Wang Y, Ni S (2009) Adsorption of phosphate from aqueous solutions onto modified wheat residue: characteristics, kinetic and column studies. Colloids Surf B: Biointerfaces 70:46–52
Xuechu C, Hainan K, Deyi W, Xinze W, Yongyong L (2009) Phosphate removal and recovery through crystallization of hydroxyapatite using xonotlite as seed crystal. J Environ Sci 21:575–580
Yeom SH, Jung K-Y (2009) Recycling wasted scallop shell as an adsorbent for the removal of phosphate. J Ind Eng Chem 15:40–44
Acknowledgement
This study was funded in part by the Japan Society for the Promotion of Science (JSPS) under Grants-in-aid for Scientific Research(C) (No. 26340058) and Grant-in-Aid for Encouragement of Young Scientists (B) (No.15 K18142). Authors are thankful to Prof. Dr. Fumio Imazeki, the head of Safety and Health Organization at Chiba University, for his financial support and encouragement for this study. Authors also thank Murakami Corporation for providing Torurin product (CSC) as experimental material. Dan Jiang also acknowledges the support of the Japanese Government (MEXT) for the scholarship through the Super Global University Project.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Jiang, D., Amano, Y. & Machida, M. Removal and recovery of phosphate from water by calcium-silicate composites-novel adsorbents made from waste glass and shells. Environ Sci Pollut Res 24, 8210–8218 (2017). https://doi.org/10.1007/s11356-017-8503-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8503-x