Abstract
Polycyclic aromatic hydrocarbons (PAHs) were analyzed at different altitudes of Taurus Mountains in semipermeable membrane devices (SPMD) and in half-, one-and-a-half-, and two-and-a-half-year-old pine needles. SPMDs were deployed for three different exposure periods: March to September (Summer), September to March (Winter), and March to March (whole year) at eight sites where needle samples were collected. The values of PAHs in needles were between 4.4 to 6066 pg g/fw in half-year-old, 7.2 to 111,115 pg g/fw in 1.5-year-old, and 9.7 to 85,335 pg g/fw in 2.5-year-old needles. Mass of PAHs collected by SPMDs varied from <MDL to 8060 ng/SPMD in winter, from 0.98 to 585 ng/SPMD in summer, and <MDL to 9360 ng/SPMD in whole year deployment, respectively. PAH profiles were dependent on the seasonal differences and locations. Roughly, clear decreasing trends with altitude were observed both with SPMD and needles for many individual and groups of PAHs except for the SPMD-summer short-time data. A cross-plot of Fluo/(Fluo+Pyr) vs Ant/(Ant+Phe) diagnostic ratios indicated grass/wood burning (possibly due to forest fires) in summer and petrogenic combustion in winter. Results of the study showed that SPMD and conifer needles are effective passive samplers to measure PAHs in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are well-known potent atmospheric pollutants that have a characteristic as gas-particle partitioning. Some PAHs consist of two to six benzene rings and these rings are bonded together in linear, angular, or cluster arrangements (Bohlin et al. 2010). Sixteen PAHs have classified as high-priority pollutants by US Environmental Protection Agency (EPA) due to their mutagenic and carcinogenic properties (ATSDR 2009). European Union REACH regulation Annex XVII places a limitation of using eight PAHs in certain products and concentration limits as well as restriction by US EPA in the use of 18 PAHs in consumer goods. Following signature and ratification of Stockholm Convention by Turkey in 2005, Turkish authorities and policy makers have encouraged researchers to conduct studies on the occurrence and fate of environmentally hazardous semi-volatile organic pollutants including persistent organic pollutants (POPs) and PAHs (Turgut et al. 2010a; Turgut et al. 2012; Turgut et al. 2010b; Falay et al. 2013). Combustion of materials containing carbon and hydrogen in domestic heating, industrial processes, power plants, motor vehicle, and waste incineration exhaust generate huge amount of PAHs. They are also produced as a result of forest fires or by vegetation burning in legally permitted areas. Once released to the atmosphere, PAHs can be transported over long distances before they deposit onto soils, vegetation, or waters through dry or wet deposition processes (Ravindra et al. 2008).
Traditionally, airborne PAHs are sampled using high-volume (Hi-Vol) samplers to collect particulate and vapor phases separately by employing glass fiber filters and polyurethane foam disks (PUFs). However, such equipment has relatively high costs by means of management, labor, electricity requirement, and low flexibility for their location. These disadvantages limit their use in wide-scale monitoring programs, especially when conducting sampling campaigns which require concurrent sampling at many locations to determine spatial or altitudinal variation. Passive sampling techniques enable us to overcome disadvantages of Hi-Vol samplers to assess POPs in urban areas/background. The employment of passive samplers on a large scale was aimed to fill information gaps and correlate the results obtained by existing active air surveillance programs (Shoeib and Harner 2002).
Recently, POPs studies have locked the interest using semipermeable membrane devices (SPMDs) (Piccardo et al. 2010; Söderström et al. 2005; Liu et al. 2007; Levy et al. 2009; Zhu et al. 2008; Bartkow et al. 2006; Strandberg et al. 2006; Esteve-Turrillas et al. 2008; Cranor et al. 2009; Ly-Verdu et al. 2010) and passive sampling by pine needles for air sampling of POPs including PAHs (Tremolada et al. 1996; Hwang et al. 2003; Howe et al. 2004; Piccardo et al. 2005; Wang et al. 2005; Liu et al. 2006; Wang et al. 2006; Kuang et al. 2014; van Drooge et al. 2014). Passive samplers accumulate chemicals via diffusion requiring no electricity for operation; thus, they can be employed on a large coverage area.
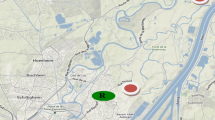
Taurus (Toros) Mountains are a mountain chain that splits the Anatolian Plateau from southern part of Turkey. These mountains are able to receive atmospheric pollutants because of the lower temperature and higher precipitation and therefore are preferred for studying the effect of atmospheric transport of pollution (Kukucka et al. 2009). Mersin, one of the metropolitan cities of Turkey, having a petroleum refinery (since 1957) and an old thermal coal power plant that was in operation from 1970 to 1993, is surrounded by industrial and agricultural areas as well as facing rapid but disordered urbanization.
The aim of the current work was to investigate latitudinal, altitudinal, and seasonal variation in PAH on a transect starting at sea level in Mersin and ending at 1881 m above sea level on Taurus Mountains by using SPMD and pine needles and to evaluate their possible sources as well as assessing usefulness of the passive sampling for sites where application of active sampling is not feasible.
Materials and methods
SPMDs were deployed and pine needles were collected on a latitudinal transect on Taurus Mountains in two sampling campaigns which were carried out between March–September 2009 and September 2009–March 2010.
Sampling site
Eight sampling points were selected in an altitudinal transect on Taurus Mountains for SPMDs and seven for pine needles (Fig. 1). Sampling location 1 was located at sea shore with typically high traffic volume. The rest of the sampling sites were away from human activities as far as possible and samplers were deployed in legally protected reserves which were particularly possible for sampling points 5, 6, 7, and 8.
Sampling
Passive sampling of needles was conducted using 0.5-, 1.5-, and 2.5-year old needles collected from the seventh branch of whorl of three well-exposed dominant needle trees in September 2009. The needle sampling was carried out from pine needles (Pinusbrutia) in locations 2, 3, 4, and 5 and juniper needles (Juniperusexcelsa) in location 6 and cedar needles (Cedruslibani) in location 7. The sampling was carried out in September 2009.
SPMDs (tubes with dimensions of 23 cm × 2.5 cm and with a membrane thickness of 67.4 μm) were filled with 0.7-mL triolein (1.2.3-tris[cis-9-octadecenoylglycerol]) and were deployed for 6 months’ intervals between March–Sept. 2009 and Sept. 2009–March 2010. Another set of SPMDs was deployed for 1 year between March 2009 to March 2010. SPMDs were transported in hermetic sealed, clean glass material to and from the sampling site to prevent possible contaminations caused by transportation. Quadruplicates of these membranes were placed in parallel on square frames. These frames were put into deployment devices (Stevenson huts) of untreated wood, which were exposed to air at 3.0 m above the ground in small forest clearings. These deployment structures allow a baffled airflow through them. The devices protect the SPMDs from direct sunlight and meteorological conditions such as precipitation, hindering the wet deposition on the SPMD. After exposure, SPMDs were stored without cleaning their surface at −20 °C until analysis. SPMD blanks were also kept under identical storage conditions as pine needle samples.
Chemical analysis
Sample preparation
After thawing, the samples were inspected visually and in cases of significant loads of particles, they were wiped with a wet paper tissue. The extraction and cleanup of the samples were performed according to the method described by Zhu et al. (2008). In brief, the sliced SPMD were extracted by shaking overnight with cyclohexane, while 10 g of intact needles were extracted with a mixture of n-hexane/dichloromethane (1:1, v/v). Before the extraction was started, a mixture of deuterated PAH (16 EPA PAH) was added to the extraction solvent. The extracts were dried with anhydrous sodium sulfate, reduced in volume, and passed with n-hexane/dichloromethane (1:1, v/v) over a glass chromatography column filled with silica and deactivated alumina (3% water). In the second step, the extracts were purified by C18-modified silica and acetonitrile as elution solvent in a SPE cartridge. Finally, the samples were reduced to 50 μL and a recovery standard was added.
Quantitative analysis
Instrumental analysis of PAHs was performed by capillary gas chromatography-mass spectrometry (Agilent 5890 Series II GC-Thermo MAT95 MS). Ionization was performed by electron impact (EI. 47 eV) and the MS was operated in SIM mode. Analysis was done on an Rtx-CLPesticides2 capillary column (30 m, 0.25 mm ID, 0.2 μm film thickness, Restek, Germany) with helium as the carrier gas. Injection volume was 0.5 μL and injection was done in splitless mode through a temperature programmable cold on column system KAS 3 (Gerstel, Germany). GC oven temperature program was 60 °C held for 1.5 min ramped to 160 °C at 5 °C min−1 to 260 °C at 20 °C/min to 310 °C at 5 °C/min and held for 15 min. Injector temperature program was 120 °C held for 0 min, ramped to 280 °C at 12 °C s−1 held for 5 min. Ion source and transfer line temperatures were 260 and 300 °C. Compounds targeted for the study are given in Table 1.
QA/QC
The identification of the analytes was performed by their exact mass (HRMS) and the relative retention to the mass-labeled analog. Quantification was carried out by comparing the peak heights of unlabeled and labeled compound.
Several blank samples were analyzed during the series of samples. When blank values were detected, the method detection limits (MDL) were calculated on the basis of three times the standard deviation of the mean blank values. A result is valid when the margin between the sample value and the average blank value is higher than the MDL and is reported as a result after subtraction of the average blank. Otherwise, the result is reported as not detectable. If no blank value was measured, the limit of detection was defined as three times the average noise level on the correspondent mass trace used for quantification.
Results and discussion
PAHs were measured in half-, 1.5-, and 2.5-year-old pine needles of seven locations and SPMDs at eight locations in different seasons, in Taurus Mountains, Turkey. PAH concentrations in pine needles are given in Tables 2 and 3, respectively. Detectable concentrations of individual PAHs in half-year-old needles and SPMDs ranged between 4.40 and 5180 pg/g fw and in 1.5-year-old needles ranged between 7.20 and 111,115 pg/g fw. The concentration of PAH in 2.5-year-old needles were between 9.70 and 85,300 pg/g fw. The PAH concentrations in needles are really close from previous studies reported 50–410 ng/g dw in Germany (Lehndorff and Schwark 2004) because we found that fresh weight to dry weight ratio is generally 2.5–3.0 in needles.
The mostly found PAHs were 3 ring followed by 4- and 5 ring in SPMD and needles. Some researchers suggested that the uptake of particle deposition in pine needles higher than SPMDs and/or some lighter PAHs needs longer exposure time to reach equilibrium in the air (Liu et al. 2006). In addition, the particles were wiped of the SPMD prior analysis. Similarly, Yang et al. (2007) stated that the deposition on the needles makes it difficult to stay attached to the surface. The interaction of lighter PAHs in gaseous phase with the wax layer in needles enhances the uptake levels (Lehndorff and Schwark 2004). Ratio between concentrations of target chemicals in SPMD (CSPMD) and in needle (Cneedle) ranged between 1.8 and 5.9 except for Flu (27) and Phe (34) for 6-month old needles and first set of SPMDs. CSPMD/Cneedle ranged between 2 and 9.2 except for Phe (61), Ant (72), Flu (92), Pyr (41), B[a]A (14), and Chr (14) for one-and-a-half year old needles and second set of SPMDs. The ratio was lower than 10 only for Ace (2.7), Flo (5.3), B[a]P (1.6), B[g.h.i]P (2.1), and D[a.h]A (0.4) for two-and-a-half-year-old needles and third set of SPMDs. Although there are exceptions, it can be concluded that pine needles can be used as a passive sampling medium for PAHs monitoring in ambient air.
The concentration of the analyzed compounds in 1.5-year-old needle samples are higher compared to 0.5- and 2.5-year-old needle samples showing that 1.5-year-old needles accumulated more PAHs over time. However, total PAH concentration was slightly higher in 2.5-year-old needle samples in locations 5 and 7 than that of 1.5-year-old needle samples. Old needles seem to reach equilibrium between PAH deposition and elimination.
The concentrations of PAHs in SPMD were detected the highest from location 1 which was located in the city center of Mersin and thus close by the emission sources such as home heating coal usage, oil combustion and road traffic are the main sources in the cities (Lehndorff and Schwark 2009; Miguel et al. 2004). Unfortunately, spruce was not available close to that measurement location at the Mersin meteorological station. Total concentrations of PAHs in 0.5-year-old samples appear in the following order: location 2 > 3 > 6 > 5 > 4 > 7 > 8, and in 1.5-year-old needle samples as follows: location 2 > 5 > 4 > 6 > 7 > 8 and in 2.5-year-old needle samples as follows: 2 > 3 > 4 > 5 > 6 > 7 > 8. Location 6 is an extraordinary sampling station for SPMD and needles in this study and other previous results from soil samples in DDT and PCDD/F (Turgut et al. 2012). However, authors could not identify any particular source of POPs around this location, but, various meteorological conditions like lower atmospheric mixing height, decreased sunlight intensity as well as temperature inversions (thus limiting vertical mixing and trap pollutants) would deteriorate PAH pollution at this particular location (Liu et al. 2014); therefore, we assume that sampling sites were partly contaminated by long-range atmospheric transport (Turgut et al. 2012).
Total PAH concentrations did not increase with higher altitude in 0.5- and 1.5-year-old needle samples but there was a correlation in 1.5 and 6 month-old needle samples. Wang et al. (2006) identified that the total concentrations increased with increased latitude in Himalayan spruce needles but Liu et al. (2004) have found a negative correlation between PAHs and altitude. Additionally, Zhao et al. (2014) reported a correlation between altitude and PAH concentrations on the southern and western part of the study mountain but no concentration gradient on northern side of the same mountain. Although, Zhao et al. (2014) conducted the study on soil samples, still this might be a similar case for air (thus for SPMD and pine needles). In summary, on the studied part of Taurus Mountains, mean annual temperature changes might not be one of the main controlling factors on the deposition of PAHs. Unfortunately, we do not have temperature measurement data during the study at the sampling sites and we cannot further comment on this issue. The heavier PAHs that have relatively low volatility and strong sorption to particles may be deposited from the air. They may be expected to store to at higher latitude but this was not the case in this study.
In SPMDs, detectable concentrations of 3-ring PAH in summer ranged from 512 to 585,770 pg/SPMD (Table 3) and the highest concentrations were found at location 1 which is close to the city of Mersin.
For SPMD, PAH concentration depends on their vapor phase abundance and mainly 3-ring PAHs were predominant in the total amount of PAHs followed by 4-ring and 5-ring PAHs. The values of 4-ring PAHs were between 109 and 55,946 pg/SPMD with fluoranthene as the predominant compound. Similar PAH profiles were detected in Australia, Czech Republic, Poland, Sweden, Slovakia, (Söderström et al. 2005), but Phenanthrene was the most abundant individual PAH in other studies (Piccardo et al. 2010; Liu et al. 2006; Zhu et al. 2008).
The lowest concentration of PAHs quantified were the 5-ring PAHs in summer. Most of them were below the MDL and detectable concentrations varied between 98.2 and 2181 pg/SPMD. The order of total PAH concentrations in summer was as follows: location 1 ˃ 3 ˃ 2 ˃ 4 ˃ 5 ˃ 6 ˃ 8 ˃ 7. PAH concentrations were detected generally higher in winter than in summer. The concentrations of 3-ring PAHs in winter ranged from 346 to 8,061,688 pg/SPMD, which was higher than 4-ring and 5-ring PAHs. The levels of 5-ring PAHs reached up to 30,665 pg/SPMDin the case of benzo(b)fluoranthene. As stated by Lehndorff and Schwark (2009), such a result might be expected due to the winter activities which cause an increase in PAH by domestic heating and heavier road traffic. Consequently, the atmospheric PAH levels were mostly found 2–10 times higher in winter. The source of the higher PAHs in New Delhi and Lahore was the accumulation of PAHs in Himalayan Spruce needles from Zhangmu-Nyalam region (Wang et al. 2006). Total PAH concentrations in SPMDs were found in winter in the following order: location 2 ˃ 3 ˃ 1 ˃ 4 ˃ 6 ˃ 5 ˃ 7 ˃ 8.
The concentrations of PAHs in SPMDs after 1-year exposure ranged from not detectable to 9,360,448 pg/SMPD for individual PAH and were between 72,044 and 11,213,326 pg/SPMD for total PAH concentrations (Table 3). In general, PAHs accumulated to a lower amount after half-year deployment, compared to the half-year exposure in winter season (Table 3). There was a negative correlation between concentrations of target PAHs in SPMD and altitude showing decreasing effect of Mersin City urban sources along with the altitude. A similar negative correlation was reported by Choi et al. (2009) for Canadian mountain air. The increased exposure time did not yield in a significant increase in PAHs, because the accumulation of pollutants in the sampler is a balance between uptake and elimination processes (Zhu et al. 2008).
Anthracene is readily photooxidized in the atmosphere (Yunker et al. 2002); however, diagnostic ratio using anthracene is commonly used in source identification in literature (Tobiszewski and Namiesnik 2012). Fluo/(Fluo+Pyr) values greater than 0.5 indicate that the major PAH input is from diesel or coal-wood combustion. Low values (<0.1) of Ant/Ant+Phe are attributed to petrogenic sources whereas it is attributed to combustion when this ratio is greater than 0.1 (Galarneau 2008). The average of Ant/(Ant+Phe) ratio for all locations in summer, winter, and half-year exposure of SPMDs was below 0.1 except for location 5. Similar results were observed for all ages of needles (Table 4). The ratio BaA/(BaA+Chr) was calculated close to 0.2 in all sampling seasons and locations. However, although pine needles and SPMDs are excellent samplers for gas phase PAH, but less for 5 and 6 rings (benzofluoranthenes until benzo[ghi]perylene), so using these later compounds for the source apportionment of PAH in the present study can be biased. If the ratios of Flu/(Flu+Pyr) are <0.4, a petrogenic source is expected while ratios between 0.4 and 0.5 indicate liquid fossil fuel combustion, and ratios >0.5 are characteristics of coal, grass, wood combustion (Budzinski et al. 1997; Yunker et al. 2002). Values of Flu/(Flu+Pyr) ratio were above 0.5 in SPMDs and needles (Tables 4, 5). Obviously, combustion of coal and/or plants (grass and wood) may be also one main source of PAHs. Inp/(Inp+B[ghi]P) ratios <0.2 are possibly indicative of petrogenic sources and when the values are between 0.2 and 0.5, that indicate the source from liquid fossil fuel (vehicle and crude oil) combustion (Yunker et al. 2002; Wang et al. 2009). The main PAH source probably is combustion of coal and/or plants (grass and wood) in Taurus Mountains, because Inp/(Inp+BghiP) ratios were mostly higher than 0.5 (Galarneau 2008) in most locations and sampling periods in SPMDs (Table 4). A cross-plot of Fluo/(Fluo+Pyr) versus Ant/(Ant+Phe) showed that diagnostic ratios in SPMDs and needles in all seasons mainly resulted from diesel, coal, and wood combustion (probably home heating and forest fires) (Fig. 2).
The ratio of diagnostic ratios in SPMD and needle for Phe/(Phe+Ant), Fluo/(Fluo+Pyr) and BaA/(BaA+CHR) are 1.02 ± 0.03, 1.35 ± 0.07, and 0.93 ± 0.05, respectively. This indicates that both media can be used PAH monitoring despite of different performance in accumulation of particulate matter. WHO (1998) reported mean profiles of individual PAHs in ambient air relative to B[a]P. Principal component analysis (PCA) (XLSTAT Free Trial Version,https://www.xlstat.com) was applied to assess potential sources of PAHs using these mean profiles. Figure 3 depicts results of PCA between mean profiles of Ant, Phe, Flu, Pyr, B[a]A, I[c.d]Prelative to B[a]P, and mean profiles of these compounds in ambient air for different sources (WHO 1998) for different sampling season in SPMDs and needles. In Fig. 3, X and Y axis show F1 and F2. F1 explain 50 to 80% of the total variance while F2 explain 14 and 29% of the total variance. As it is seen from the figures, mean profiles of individual PAHs relative to B[a]P up to approx. 1000 m altitude is generally grouped together while profiles at sea level ambient air and profiles at altitudes higher than approximately 1000 m grouped together.
Wang et al. (2006) stated that the pollutant transport and distribution in mountain areas help to understand the mechanisms to operate on a larger scale and the influence of various environmental parameters (climate, latitude, etc.); thus, some studies demonstrate clear concentration gradients but others do not. Occasionally, a certain altitudinal pattern is detected for one group of compounds, but not another.
Conclusion
The more volatile compounds are tended to accumulate in SPMDs since they do not accumulate particulate matter and needles are tended to accumulate also particulate matter bound low volatile PAHs. The main reason for different uptake of needles and SPMD is clearly due to a partly uptake of particulate matter onto needles. Despite profile differences of sampling, the distribution of the predominant PAH compounds in air correlated well. SPMDs are easy to handle during the whole procedure, but triolein is expensive and needles are ready made, easily collectable, but might be not available in all locations.
In conclusion, SPMD and needles can be applied to estimate air concentrations of PAHs with reusable accuracy. Deployment times cannot be lengthened more than 6 months because of dissipation of PAHs on SPMDs and one-and-a-half-year-needles.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (2009) Case studies in environmental medicine, toxicity of polycyclic aromatic hydrocarbons (PAHs). Available at https://www.atsdr.cdc.gov/csem/pah/docs/pah.pdf
Bartkow ME, Jones KC, Kennedy KE, Holling N, Hawker DW, Mueller JF (2006) Evaluation of performance reference compounds in polyethylene-based passive air samplers. Environ Pollut 144(2):365–370. doi:10.1016/j.envpol.2005.12.043
Bohlin P, Jones KC, Strandberg B (2010) Field evaluation of polyurethane foam passive air samplers to assess airborne PAHs in occupational environments. Environmental Science & Technology 44(2):749–754. doi:10.1021/es902318g
Budzinski H, Jones I, Bellocq J, Pierard C, Garrigues P (1997) Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Mar Chem 58(1–2):85–97. doi:10.1016/s0304-4203(97)00028-5
Choi S-D, Shunthirasingham C, Daly GL, Xiao H, Lei YD, Wania F (2009) Levels of polycyclic aromatic hydrocarbons in Canadian mountain air and soil are controlled by proximity to roads. Env Pollut 157:3199–3206
Cranor WL, Alvarez DA, Huckins JN, Petty JD (2009) Uptake rate constants and partition coefficients for vapor phase organic chemicals using semipermeable membrane devices (SPMDs). Atmos Environ 43(20):3211–3219. doi:10.1016/j.atmosenv.2009.03.043
Esteve-Turrillas FA, Yusa V, Pastor A, de la Guardia M (2008) New perspectives in the use of semipermeable membrane devices as passive samplers. Talanta 74(4):443–457. doi:10.1016/j.talanta.2007.06.019
Falay EO, Tuna G, Altiok H, Kara M, Dumanoglu Y, Bayram A, Tolunay D, Elbir T, Odabasi M (2013) Spatial variation of polycyclic aromatic hydrocarbons (PAHs) in air, soil and tree components in Iskenderun industrial region, Turkey. International Journal of Chemical, Environmental Biological Sciences (IJCEBS) 1(2):263–267
Galarneau E (2008) Source specificity and atmospheric processing of airborne PAHs: implications for source apportionment. Atmos Environ 42(35):8139–8149
Howe TS, Billings S, Stolzberg RJ (2004) Sources of polycyclic aromatic hydrocarbons and hexachlorobenzene in spruce needles of eastern Alaska. Environmental Science & Technology 38(12):3294–3298. doi:10.1021/es034751n
Hwang H-M, Wade TL, Sericano JL (2003) Concentrations and source characterization of polycyclic aromatic hydrocarbons in pine needles from Korea, Mexico, and United States. Atmos Environ 37(16):2259–2267. doi:10.1016/S1352-2310(03)00090-6
Kuang YW, Li J, Hou EQ (2014) Lipid-content-normalized polycyclic aromatic hydrocarbons (PAHs) in the xylem of conifers can indicate historical changes in regional airborne PAHs. Environ Pollut 196:53–59. doi:10.1016/j.envpol.2014.09.018
Kukucka P, Klanova J, Sanka M, Holoubek I (2009) Soil burdens of persistent organic pollutants—their levels, fate and risk. Part II. Are there any trends in PCDD/F levels in mountain soils? Environ Pollut 157(12):3255–3263. doi:10.1016/j.envpol.2009.05.029
Lehndorff E, Schwark L (2004) Biomonitoring of air quality in the cologne conurbation using pine needles as a passive sampler—part II: polycyclic aromatic hydrocarbons (PAH). Atmos Environ 38(23):3793–3808. doi:10.1016/j.atmosenv.2004.03.065
Lehndorff E, Schwark L (2009) Biomonitoring airborne parent and alkylated three-ring PAHs in the greater cologne conurbation II: regional distribution patterns. Environ Pollut 157(5):1706–1713. doi:10.1016/j.envpol.2008.12.006
Levy W, Henkelmann B, Pfister G, Bernhoeft S, Kirchner M, Jakobi G, Bassan R, Kräuchi N, Schramm K-W (2009) Long-term air monitoring of organochlorine pesticides using semi permeable membrane devices (SPMDs) in the alps. Environ Pollut 157(12):3272–3279. doi:10.1016/j.envpol.2009.05.043
Liu M, Yang Y, Xu S, Hou L, Liu Q, Ou D, Jiang H (2004) Persistent organic pollutants (POPS) in intertidal surface sediments from the Yangtze Estuarine and coastal areas, China. Journal of Coastal Research, 162–170
Liu G, Zhang G, Li J, Li X, Peng X, Qi S (2006) Spatial distribution and seasonal variations of polycyclic aromatic hydrocarbons (PAHs) using semi-permeable membrane devices (SPMD) and pine needles in the Pearl River Delta, South China. Atmos Environ 40(17):3134–3143. doi:10.1016/j.atmosenv.2006.01.027
Liu HJ, Qu JH, Dai RH, Ru J, Wang ZJ (2007) A biomimetic absorbent for removal of trace level persistent organic pollutants from water. Environ Pollut 147(2):337–342. doi:10.1016/j.envpol.2006.06.031
Liu D, Xu Y, Chaemfa C, Tian C, Li J, Luo C, Zhang G (2014) Concentrations, seasonal variations and outflow of atmospheric polycyclic aromatic hydrocarbons (PAHs) at Ningbo site, Eastern China. Atmospheric Pollution Research 5(2):203–209. doi:10.5094/APR.2014.025
Ly-Verdu S, Esteve-Turrillas FA, Pastor A, de la Guardia M (2010) Determination of volatile organic compounds in contaminated air using semipermeable membrane devices. Talanta 80(5):2041–2048. doi:10.1016/j.talanta.2009.11.001
Miguel AH, Eiguren-Fernandez A, Jaques PA, Froines JR, Grant BL, Mayo PR, Sioutas C (2004) Seasonal variation of the particle size distribution of polycyclic aromatic hydrocarbons and of major aerosol species in Claremont, California. Atmos Environ 38(20):3241–3251. doi:10.1016/j.atmosenv.2004.03.008
Piccardo MT, Pala M, Bonaccurso B, Stella A, Redaelli A, Paola G, Valerio F (2005) Pinus nigra and Pinus pinaster needles as passive samplers of polycyclic aromatic hydrocarbons. Environ Pollut 133(2):293–301. doi:10.1016/j.envpol.2004.05.034
Piccardo MT, Stella A, Pala M, Balducci D, Valerio F (2010) Field use of semipermeable membrane devices (SPMDs) for passive air sampling of polycyclic aromatic hydrocarbons: opportunities and limitations. Atmos Environ 44(16):1947–1951. doi:10.1016/j.atmosenv.2010.03.003
Ravindra K, Sokhi R, Grieken RV (2008) Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ 42(13):2895–2921. doi:10.1016/j.atmosenv.2007.12.010
Shoeib M, Harner T (2002) Characterization and comparison of three passive air samplers for persistent organic pollutants. Environmental Science & Technology 36(19):4142–4151. doi:10.1021/es020635t
Söderström H, Hajslova J, Kocourek V, Siegmund B, Kocan A, Obiedzinski W, Tysklind M, Bergqvist P (2005) PAHs and nitrated PAHs in air of five European countries determined using SPMDs as passive samplers. Atmos Environ 39(9):1627–1640. doi:10.1016/j.atmosenv.2004.11.010
Strandberg B, Gustafson P, Soderstrom H, Barregard L, Bergqvist PA, Sallsten G (2006) The use of semipermeable determine persistent membrane devices as passive samplers to organic compounds in indoor air. J Environ Monit 8(2):257–262. doi:10.1039/b513120k
Tobiszewski M, Namiesnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119. doi:10.1016/j.envpol.2011.10.025
Tremolada P, Burnett V, Calamari D, Jones KC (1996) Spatial distribution of PAHs in the U.K. atmosphere using pine needles. Environmental Science & Technology 30(12):3570–3577. doi:10.1021/es960269b
Turgut C, Atatanir L, Cutright TJ (2010a) Evaluation of pesticide contamination in Dilek National Park, Turkey. Environ Monit Assess 170(1–4):671–679. doi:10.1007/s10661-009-1266-1
Turgut C, Erdogan O, Ates D, Gokbulut C, Cutright TJ (2010b) Persistence and behavior of pesticides in cotton production in Turkish soils. Environ Monit Assess 162(1–4):201–208. doi:10.1007/s10661-009-0788-x
Turgut C, Atatanir L, Mazmanci B, Mazmanci MA, Henkelmann B, Schramm KW (2012) The occurrence and environmental effect of persistent organic pollutants (POPs) in Taurus Mountains soils. Environ Sci Pollut Res 19(2):325–334. doi:10.1007/s11356-011-0561-x
Van Drooge BL, Garriga G, Grimalt JO (2014) Polycyclic aromatic hydrocarbons in pine needles (Pinus halepensis) along a spatial gradient between a traffic intensive urban area (Barcelona) and a nearby natural park. Atmos Pollut Res 5:398–404. doi:10.5094/APR.2014.046
Wang D, Chen J, Xu Z, Qiao X, Huang L (2005) Disappearance of polycyclic aromatic hydrocarbons sorbed on surfaces of pine [Pinua thunbergii] needles under irradiation of sunlight: volatilization and photolysis. Atmos Environ 39(25):4583–4591. doi:10.1016/j.atmosenv.2005.04.008
Wang X-P, Yao T-D, Cong Z-Y, Yan X-L, Kang S-C, Zhang Y (2006) Gradient distribution of persistent organic contaminants along northern slope of central-Himalayas, China. Sci Total Environ 372(1):193–202. doi:10.1016/j.scitotenv.2006.09.008
Wang J, Bi Y, Pfister G, Henkelmann B, Zhu K, Schramm K-W (2009) Determination of PAH, PCB, and OCP in water from the three gorges reservoir accumulated by semipermeable membrane devices (SPMD). Chemosphere 75(8):1119–1127. doi:10.1016/j.chemosphere.2009.01.016
WHO (1998) Selected non-heterocyclic polycyclic aromatic hydrocarbons (environmental health criteria 202). World Health Organization, Geneva URI: http://www.who.int/iris/handle/10665/41958
Yang P, Chen J, Wang Z, Qiao X, Cai X, Tian F, Ge L (2007) Contributions of deposited particles to pine needle polycyclic aromatic hydrocarbons. J Environ Monit 9(11):1248–1253. doi:10.1039/b708508g
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33(4):489–515. doi:10.1016/s0146-6380(02)00002-5
Zhao X, Kim SK, Zhu W, Kannan N, Li D (2014) Long-range atmospheric transport and the distribution of polycyclic aromatic hydrocarbons in Changbai Mountain. Chemosphere 119:289–294. doi:10.1016/j.chemosphere.2014.06.005
Zhu X, Pfister G, Henkelmann B, Kotalik J, Bernhoeft S, Fiedler S, Schramm K-W (2008) Simultaneous monitoring of profiles of polycyclic aromatic hydrocarbons in contaminated air with semipermeable membrane devices and spruce needles. Environ Pollut 156(2):461–466. doi:10.1016/j.envpol.2008.01.023
Acknowledgements
This research has been supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) and International Bureau of the Federal Ministry of Education and Research, Germany, through a joint research project (project nos.109T002 in Turkey and TUR 08/002 in Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Constantini Samara
Rights and permissions
About this article
Cite this article
Turgut, C., Mazmanci, M.A., Mazmanci, B. et al. Polycyclic aromatic hydrocarbons (PAHs) determined by pine needles and semipermeable membrane devices along an altitude profile in Taurus Mountains, Turkey. Environ Sci Pollut Res 24, 7077–7087 (2017). https://doi.org/10.1007/s11356-017-8363-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8363-4