Abstract
We studied the effect of electron competition on chromate (Cr(VI)) reduction in a methane (CH4)-based membrane biofilm reactor (MBfR), since the reduction rate was usually limited by electron supply. A low surface loading of SO4 2− promoted Cr(VI) reduction. The Cr(VI) removal percentage increased from 60 to 70% when the SO4 2− loading increased from 0 to 4.7 mg SO4 2−/m2-d. After the SO4 2− loading decreased back to zero, the Cr(VI) removal further increased to 90%, suggesting that some sulfate-reducing bacteria (SRB) stayed in the reactor to reduce Cr(VI). However, a high surface loading of SO4 2− (26.6 mg SO4 2−/m2-d) significantly slowed down the Cr(VI) reduction to 40% removal, which was probably due to competition between Cr(VI) and SO4 2− reduction. Similarly, when 0.5 mg/L of Se(VI) was introduced into the MBfR, Cr(VI) removal percentage slightly decreased to 60% and then increased to 80% when input Se(VI) was removed again. The microbial community strongly depended on the loadings of Cr(VI) and SO4 2−. In the sulfate effect experiment, three genera were dominant. Based on the correlation between the abundances of the three genera and the loadings of Cr(VI) and SO4 2−, we conclude that Methylocystis, a type II methanotroph, reduced both Cr(VI) and sulfate, Meiothermus only reduced Cr(VI), and Ferruginibacter only reduced SO4 2−.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is associated with a variety of human activities, e.g., metallurgic industry, refractory applications, and chemicals synthesis (Barnhart 1997). The dominant oxidation states of Cr in wastewater are chromate (Cr(VI)) and chromite (Cr(IV)). Cr(VI) has high solubility and toxicity and may lead to cardiovascular shock, diarrhea, vomiting, and liver and kidney necrosis in human bodies (Barnhart 1997; Dayan and Paine 2001; Kantar et al. 2008). The US Environmental Protection Agency has set the maximum contaminant level (MCL) for Cr in drinking water at 100 μg/L (USEPA 2015). However, Cr(III), the reduced form of Cr(VI), is essential to humans (Srivastava et al. 1999) and can transform into precipitates in neutral or alkaline conditions, making it easy to be removed from wastewater (Anderson and Kozlovsky 1985; Palmer and Wittbrodt 1991). Therefore, reduction of Cr(VI) to Cr(III) is a feasible method for remediate Cr-contaminated wastewater (Cheung and Gu 2007).
Having the advantage in low cost and sustainability (Lovley and Coates 1997; Zahoor and Rehman 2009), bioreduction of Cr(VI) is deemed to be a promising method for Cr removal. Chromate-reducing bacteria reported in the literature, e.g., Staphylococcus epidermidis (Vatsouria et al. 2005), Desulfovibrio vulgaris (Lovley and Phillips 1994), Pseudomonas sp. (McLean and Beveridge 2001), and Enterobacter cloacae (Waki et al. 1989), utilize organics or hydrogen (H2) as the electron donor to mediate Cr(VI) reduction, in aerobic or anaerobic condition. Recently, Luo et al. (2015) and Lai et al. (2016a, b) reported that methane could also serve as the sole electron donor. They observed efficient bioreduction of Cr(VI) in the CH4-based membrane biofilm reactors (MBfR), in which CH4 was supplied through hollow fibers. This process has drawn great interest in recent years because CH4 is an inexpensive and widely available electron donor (Lai et al. 2016a, b; Zhong et al. 2017). Furthermore, CH4 is a potent greenhouse gas that has 25 times higher greenhouse potential than carbon dioxide (CO2) (Hu et al. 2014; Knittel and Boetius 2009); thus, consumption of CH4 can also reduce the global greenhouse effect.
A frequent co-existing electron acceptor with Cr(VI) is sulfate (SO4 2−), which is an extensively spreading oxyanion in wastewater (Huber et al. 1997). SO4 2− reduction is performed by sulfate-reducing bacteria (SRB) which are very diverse phylogenetically, spreading from ε- and δ-Proteobacteria to Clostridia (Mori et al. 2003). In the process of dissimilatory SO4 2− reduction, ATP sulfurylase activates SO4 2− by connecting it to phosphate radical of ATP, producing adenosine phosphosulfate (APS) (Peck 1959). APS was then reduced by an APS reductase to adenosine monophosphate (AMP) and sulfite (SO3 2−). SO3 2− was finally reduced to sulfide (S2−) by sulfite reductase. The interactions between Cr(VI) and SO4 2− reductions depend upon the microbial community structure in the CH4-based biofilms. SRB are versatile, and some of them are able to reduce Cr(VI) through enzymes (Michel et al. 2001; Chardin et al. 2003). On the other hand, sulfite (S2−) derived from SO4 2− reduction (Smith and Gadd 2000) can abiotically react with Cr(VI). SO4 2− also possibly inhibits Cr(VI) reduction, due to the competition for membrane space, electron donor, et al., between chromate-reducing bacteria and SRB (Tang et al. 2012a).
Microbial reduction of CrO4 2− and SeO4 2− has drawn great attention in recent years due to the simplicity and low cost (Lai et al. 2016a, b). Both of chromate and selenate-reducing bacteria are phylogenetically diverse. Variety of chromate-reducing bacteria, e.g., Enterobacter cloacae (Wang et al. 1991), Pantoea agglomerans (Francis et al. 2000), Pseudomonas putida (Park et al. 2000), Escherichia coli (Ackerley et al. 2004), and selenate-reducing bacteria, e.g., Bacillus (Fujita et al. 1997), Sulfurospirillum (Lenz et al. 2009), Enterobacter cloacae (Ma et al. 2009), and Desulfurispirillum indicum (Rauschenbach et al. 2010), have been isolated. The objective of this study is to study the effect of SO4 2− and SeO4 2− on Cr(VI) reduction in a CH4-based MBfR by changing the loadings of SO4 2− or SeO4 2− in the influent. Overall, we also want to understand the mechanisms involved in the interaction of SO4 2−/SeO4 2− and Cr(VI) reduction by looking into the community structure change of the biofilms using electron microscope and high-throughput HiSeq sequencing technology.
Materials and methods
Startup and continuous operation
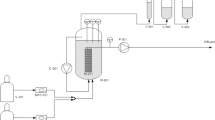
We set up the same MBfR system as described in Lai et al. (2016a, b). The core component of MBfR is the composite hollow fibers manufactured by Mitsubishi Rayon (model MHF-200TL, Mitsubishi, Ltd., Japan). The total volume of the MBfR was 65 mL, while the total membrane surface area was 58 cm2. The liquid in the system were mixed by using a peristaltic pump at 100 mL/min. We inoculated the reactor with 10 mL of a culture that had been adapted to anaerobic oxidation of methane coupled to chromate reduction. Inorganic medium was deoxygenated and prepared as influent for the MBfR (Lai et al. 2016a, b). The medium contained the following mineral salts per liter of ultrapure water: NH4Cl 0.05 g, CaCl2 1 mg, NaHCO3 0.3 g, MgCl2 2 mg, MgSO4·7H2O 2 mg, KH2PO4 0.2 g, Na2HPO4·12H2O 0.4 g, 1 mL acid trace element solution (HCl 100 mM, 1 g of FeCl·7H2O, 68 mg of ZnCl·7H2O, 14 mg of H3BO3, 120 mg of CoCl2·6H2O, 500 mg of MnCl2·4H2O, 320 mg of CuCl2, 95 mg of NiCl2·6H2O per liter), and 1 mL alkaline trace element solution (Luo et al. 2015). We stimulated the growth of the inoculum by adding Cr(VI) into the medium at 10 mg/L and recirculating the medium for 48 h. Afterwards, the influent Cr(VI) concentration was adjusted to ~ 1 mg/L, corresponding to a loading rate of 124 mg Cr(VI)/m2-d throughout the whole experiments. To evaluate the effect of SO4 2− on Cr(VI) reduction, we varied the SO4 2− concentration in the influent medium: 0, 3.5, 0, 20, and 0 mg of SO4 2−/L in stages 1, 2, 3, 4, and 5, respectively. Each stage was maintained for at least 2 weeks until the variation of the effluent concentrations of Cr(VI) and SO4 2− were < 10%. We kept the liquid recirculation rate at 100 mL/min. The CH4 pressure was constant at 10 psig, and the temperature was stable (29 ± 1 °C) throughout the experiments.
We did the selenate effect experiment separately in another methane-based MBfR. The Cr(VI) concentration in the influent was kept at 1 mg/L (124 mg Cr(VI)/m2-d) throughout the whole experiments, while Se(VI) was varied at 0, 1, 0, 0.5, 0 mg/L for stages 6–10. Each stage was ended until reaching steady state (the variation of effluent concentrations of Cr(VI) and Se(VI) was < 10%). The liquid recirculation of MBfR was maintained at 100 mL/min, while the pressure of CH4 and the temperature were kept at 10 psig and 30 °C, respectively.
Chemical analyses
We took liquid samples of the influent and effluent of MBfR using 10-mL gas-tight syringes every 2 days and centrifuged the liquid samples (15,000g, 10 min) to remove insoluble Cr(III) precipitates. We then filtered them immediately through a 0.22-μm membrane filter (LC + PVDF membrane, Shanghai Xinya, China). The Cr(VI) concentration was analyzed by diphenyl carbazide method (Method 3500-Cr D, APHA 1998), and the concentration of Se(VI) was determined using ion chromatography (Metrohm 833 Basic IC plus, Switzerland) equipped with an S-Supp-5 column. We determined the concentration of SO4 2− using ion chromatography (Metrohm 833 Basic IC plus, Switzerland) equipped with an S-Supp-5 column. The eluent was 3.2 mM NaHCO3 and 1.0 mM Na2CO3, and the flow rate was 0.7 mL/min. The concentrations of dissolved oxygen (O2) were ~ 0.2 mg/L for the influent and ≤ 0.1 mg/L for the effluent measured by dissolved oxygen probe (Starter, model 300D, Ohaus Instruments Company, Germany). The pH values throughout the experiments were 7.0–7.5 measured by a pH meter (Seven Easy, Mettler Toledo, Switzerland).
Flux calculations
We calculated the removal fluxes of Cr(VI), O2, and SO4 2− (mg m−2 day−1) according to
in which S 0 and S are the influent and effluent Cr(VI), O2, or SO4 2− concentration (mg/L), Q is the influent flow rate to the MBfR system (L/day), and A is the membrane surface area (m2). The CH4 flux was calculated based on reaction stoichiometry shown in reaction 2 (for Cr(VI)), reaction 3 (for O2), and reaction 4 (for SO4 2−) (Rittmann and McCarty 2001).
The maximum CH4 flux (e− meq/m2 day) was calculated according to Tang et al. (2012c).
Biofilm sampling and imagining, DNA extraction, and Illumina sequencing
We cut off two ∼ 5-cm-long sections from a coupon fiber at the end of each stage. One section was used for scanning electron microscope (SEM) and energy dispersive X-ray (EDS) analysis, while the other was used for DNA extraction (Lai et al. 2016a, b). We extracted DNA by using DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD) (Lai et al. 2014) and measured the DNA concentration by Nanodrop spectrophotometer.
We used primers 515F (5-GTGCCAGCMGCCGCGG-3′ and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′) to amplify the conserved V4–V5 regions of the bacterial 16S rRNA gene and purified the PCR products using the QIAquick PCR Purification Kit (QIAGEN, Germany). The purified amplicons were sent to Novogene Technology (Beijing, China) to process Illumina MiSeq sequencing. The data were analyzed using QIIME (version 1.9.1) pipeline as described by Lai et al. (2014). We analyzed the phylogenetic sequences against the silva (SSU115)16S rRNA database using confidence threshold of 70%, analyzed the relationship of community composition in different stages by using the principal coordinate analysis (PCoA) (Shannon et al. 2003), and unweighted UniFrac distance matrix (Lozupone et al. 2006). The alpha diversity parameters was acquired by using QIIME (version 1.9.1) pipeline and presented the results in Table S2.
Results and discussion
Effect of sulfate on chromate reduction
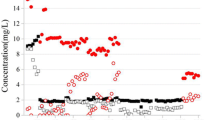
Figure 1a shows the concentrations of Cr(VI) and SO4 2− in the influent and effluent of CH4-based MBfR, while Fig. 1b shows the removal percentages of Cr(VI) and SO4 2−. The fluxes of Cr(VI), SO4 2−, and O2 (calculated from Eqs. 2, 3, and 4) are presented in Table 1 for each stage at steady state. The maximum CH4 flux that can be delivered through the hollow fiber at the constant CH4 pressure (10 psig) was much larger than the actual CH4 flux, meaning that the CH4 supply was sufficient (Luo et al. 2015).
In stage 1 (days 0–36), when Cr(VI) was supplied as the only electron acceptor in the influent (1 mg/L of Cr(VI)), the Cr(VI) removal percentage reached ~ 25% after 48 h enrichment, steadily increased to ∼ 60% at day 25, and remained stable for the rest of the stage. The EDS spectrum demonstrated the occurrence of Cr(III) precipitates, the final product of Cr(VI) reduction (Fig. 4e). The Cr(VI) removal was very similar to the pattern as in our previous research (Lai et al. 2016a, b), showing very good repeatability. However, in stage 2 (days 38–66), when ~ 3.5 mg/L of SO4 2− was introduced to the MBfR, the Cr(VI) removal percentage did not drop but continuously increased up to ∼ 70% and remained stable afterwards. Generally when a second electron acceptor is introduced, the reduction of the first fed electron acceptor (Cr(VI) in this study) will decrease due to competition for electrons and other resources. For example, Zhong et al. (2017) reported that the introduction of 280 mg NO3 −-N m2-d dramatically suppressed Cr(VI) reduction, and Zhao et al. (2011) reported that the input of NO3 − substantially lowered ClO4 − percent removals. No SO4 2− reduction was detected in this stage, which might be due to the higher redox potential for Cr(VI)/Cr(III) (− 0.13 V) compared with that for SO4 2−/S2− (− 0.22 V) (Rittmann and McCarty 2001).
The improvement of Cr(VI)-reducing capability of the biofilm after SO4 2− addition might be due to the accumulation of slow growing of sulfate-reducing bacteria (SRB). Michel et al. (2001) demonstrated that some SRB were able to reduce Cr(VI), using the cytochrome c or negative redox potential hemes. Cheung and Gu (2003) found that SRB were able to reduce 88% of Cr(VI) (at the initial concentration of 500 mmol/L) in 48 h. Cetin et al. (2008) also reported that in the range of 22.7–74.9 mg/L of initial Cr(VI), 99% of Cr(VI) was reduced within 2–6 days. Besides, the measured zero SO4 2− removal might due to redox cycling of S in the MBfR: the final products of SO4 2− reduction by SRB, sulfide (S2−), might be used as the electron donor for chromate-reducing bacteria to perform Cr(VI) reduction, and S2− was reoxidized to SO4 2− (Arias and Tebo 2003; Zhao et al., 2013, 2014). Moreover, sulfite (S2−) is an very strong reductive agent that can abiotically reduce Cr(VI) (Smith and Gadd 2000).
The Cr(VI) removal percentage further increased up to ~ 90% when SO4 2− was removed out of the system in stage 3 (days 68–86). This is reasonable considering that some SRB grown in stage 2 stayed in the biofilm to reduce sulfate in stage 3.
A high concentration of SO4 2− (20 mg/L, 26.59 mmol/m2-d) gave a great impact on Cr(VI) reduction in stage 4: Cr(VI) removal dropped to ~ 45%. The sulfate removal reached ~ 40%. In this stage, the inhibition from sulfate became dominant. In stage 5, when the influent SO4 2− was again returned to zero, C(VI) reduction bounced back to ~ 70%. The fact that low loading of SO4 2− promoted Cr(VI) reduction and higher loading of SO4 2− had a negative effect on Cr(VI) reduction is very similar to Tang’s study (Tang et al. 2012a,b), in which low loading of NO3 − (≤ 100 mg/m2-d) promoted ClO4 − reduction, and high loading of NO3 − (≥ 600 mg/m2-d) significantly inhibited ClO4 − reduction.
Simultaneous Cr(VI) and Se(VI) reduction in the CH4-based MBfR
Figure 2a, b shows the performance of CH4-based MBfR to reduce Se(VI) and Cr(VI), while Table 1 shows the fluxes of Cr(VI), Se(VI), and O2 (calculated from Eqs. 2, 3, 4, and 5) for each stage at steady state. Due to the large discrepancy between maximum CH4 flux and actual CH4 flux, the CH4 supply was sufficient throughout the whole experiments (Luo et al. 2015).
In stage 6 (days 0–32), when the influent contained 1 mg/L of Cr(VI) as the sole electron acceptor, the Cr(VI) removal percentage increased slowly and achieved 53% at day 22 and remained stable in this stage. In stage 7 (days 34–58), when 1 mg/L of Se(VI) was added into the influent, the Cr(VI) removal percentage decreased to only 20%, while Se(VI) removal percentage was also ∼ 20%. However, both of the Cr(VI) and Se(VI) removal percentage increased up to ∼ 60% at steady state in this stage. The inhibition of Cr(VI) reduction at the start of this stage should be due to the suppression of chromate-reducing bacteria by Se(VI). Se(VI) has high toxic to organisms due to its ability to replace sulfur in sulfur-containing proteins, leading to the damage of normal function of these proteins (Fournier et al. 2010; Lemly et al. 1993). Furthermore, the reduced form of Se(VI), selenite (Se(IV)), has been reported to slowdown the growth rate of Rhodobacter sphaeroides (Bebien et al. 2001). The increase of Cr(VI) and Se(VI) reduction in the later phase of this stage implied the adaption of chromate-reducing bacteria to the exposure to Se(VI) and the growth of selenate-reducing bacteria in the biofilm. The similar or higher removal percentage for Cr(VI) and Se(VI) was possibly due to the higher redox potential for Se(VI)/Se(IV) (0.44 V) (Doran 1982) than that for Cr(VI)/Cr(III) (− 0.13 V) (Rittmann and McCarty 2001).
In stage 8 (days 60–82), when the influent Se(VI) returned to zero, Cr(VI) removal percentage increased to 80% at steady state. This might be because that part of selenate-reducing bacteria thriving in the biofilm in stage 7 was also able to reduce Cr(VI). Some species, e.g., Bacillus and Pseudomonas aeruginosa, have the ability to reduce Cr(VI) as well as Se(VI) (Burton et al. 1987; Lovely 1993).
In stage 9 (days 84–104), when low concentration of Se(VI) (0.5 mg/L of Se(VI)) was introduced, Cr(VI) removal percentage decreased slightly to 60% but rebounded to 80% when Se(VI) was again removed out in stage 10 (days 106–128). The Se(VI) removal percentage in stage 9 was higher than that in stage 7, as the surface loading of Se(VI) is lower in stage 9 than in stage7.
Microbial community changes in the biofilms
Figure 3a, b shows the relative abundances of phylotypes at the class and genus levels in the biofilms. α-Proteobacteria was the main class in the inoculum (22%) and became absolutely dominant in all biofilm samples (58–80%).
The relative abundance of Methylocystis was 10% of total bacteria in the inoculum, but increased to 35% with Cr(VI) addition in stage 1 and became dominant (> 52%) with high loading of input SO4 2− in stage 4, suggesting that both Cr(VI) and sulfate can be used as electron acceptors for Methylocystis (α-Proteobacteria), a known type II methanotrophic bacterium using particulate methane monooxygenase to oxidize CH4 (Dedysh et al. 2007; Yimga et al. 2003). It is reasonable that the Methylocystis became more dominant because of more CH4 was consumed corresponding to higher loading of electron acceptors. The Pearson correlation (Table S1) proved that the abundance of Methylocystis was positively correlated with total CH4 flux.
Meiothermus (Deinococci) represented 9.7% of the total bacteria in stage 1 and increased continually to 15.4 and 28.9% in stage 2 and stage 3, respectively, consistent with the increase of chromate-reducing capability of the biofilm. The abundance of Meiothermus decreased sharply to 4.7% in stage 4, when Cr(VI) reduction was dramatically inhibited by the high loading of SO4 2−, and recovered to 18.2% in stage 5, when SO4 2− was removed. Pearson correlation (Table S1) shows that the abundance of Meiothermus had a significantly positive correlation with the Cr(VI) flux. These data suggest that Meiothermus can only reduce Cr(VI), and its growth can be inhibited by SO4 2−.
Ferruginibacter had the highest relative abundance in stages 2 and 4, when SO4 2− was introduced into the influent (Fig. S1). Ferruginibacter is a potential SRB which was found in a moving bed biofilm reactor (MBBR) with sulfate reduction (Rikmann et al. 2012). Figure 3 shows the existence of helix-shaped bacteria. Since they were only observed in the biofilm samples from the stages when SO4 2− was supplied, they are probably Ferruginibacter. SRBs are usually slow growers. The ΔG form the process of Cr(VI) reduction to Cr(III) is negative (− 21.3 KJ/e−), while that for SO4 2− reduction to H2S is positive (20.85 KJ/e−). It explains the relatively lower abundance of Ferruginibacter compared to Methylocystis and Meiothermus (Fig. 4).
The sulfate effect MBfR: SEM observations at 10000 magnification for stage 1 (a), stage 2 (b), stage 4 (c), and stage 5 (d). The white arrows indicate heliciform-shaped bacteria, which only occurred in the stages when SO4 2− was in the influent. The red arrows indicate the Cr(III) precipitates, and the precipitates were identified by EDS spectrum (e)
Figure 5 shows the unweighted PCoA based on the presence or absence of phylotypes of the biofilm samples. Stages 1, 3, and 5 were grouped together since Cr(VI) was the sole electron acceptor. Stages 2 and 4 were grouped together since both Cr(VI) and SO4 2− were supplied as the electron acceptors. However, both groups had a large distance from the inoculum. The unweighted UniFrac analysis showed a similar pattern (Fig. S2), further demonstrating that the microbial community structure in the biofilms was greatly shaped by the introduction of SO4 2−.
The microbial community structure of the selenate and chromate MBfR was addressed in the SI.
Conclusion
We found the Cr(VI)-reducing activity of biofilms in a CH4-based MBfR was greatly influenced by the introduction of SO4 2−. Low concentration of input SO4 2− promoted Cr(VI) reduction, while high concentration of input SO4 2− inhibited Cr(VI) reduction, although the CH4 supply was sufficient. The addition of 1 mg/L of Se(VI) into the influent suppressed Cr(VI) reduction at first, but promoted Cr(VI) reduction afterwards. Returning input Se(VI) to zero further improved Cr(VI) reduction. The introduction of 0.5 mg/L of Se(VI) slightly inhibited Cr(VI) reduction, although Cr(VI) removal percentage recovered when Se(VI) was removed again. Hiseq sequencing technology showed that Methylocystis, a type II methanotroph, was involved in CH4 oxidation and reduction of both Cr(VI) and SO4 2−, while the other two dominant species could only reduce one electron acceptor: Meiothermus only reduced Cr(VI) and Ferruginibacter only reduced SO4 2−. The unweighted PCoA and UniFrac analysis proved that the microbial community in the biofilms was greatly shaped by the introduction of SO4 2−. While selenate was co-existed, Meiothermus was proposed to reduce both of Cr(VI) and Se(VI), while Methylophilus implied the intermediate metabolites was involved in the electron transfer in the CH4-based biofilm. In future, we will keep working on the electron competition among different electron acceptors, the electron transportation between electron donor and acceptor through different functional microorganisms when CH4 was supplied as the sole electron donor by applying fluorescence in situ hybridization (FISH), metagenomic, metatranscriptome, metaproteomics, and other advanced technologies. Elucidating the effect of electron competition will greatly help us to explain the mechanism involved in the CH4-based MBfR and apply it for practical use.
References
Ackerley DF, Gonzalez CF, Blake R II, Keyhan M, Matin A (2004) Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction. Environ Microbiol 6(8):851–860. https://doi.org/10.1111/j.1462-2920.2004.00639.x
Anderson RA, Kozlovsky AS (1985) Chromium uptake, absorption, and excretion of subjects consuming self-selected diets. Am J Clin Nutr 41:1117–1183
APHA (1998) Method 3500-Cr D: Standard methods for the examination of water and waste water. American Public Health Association
Arias YM, Tebo BM (2003) Cr(VI) reduction by sulfidogenic and nonsulfidogenic microbial consortia. Appl Environ Microbiol 69(3):1847–1853. https://doi.org/10.1128/AEM.69.3.1847-1853.2003
Barnhart J (1997) Occurrences, uses and properties of chromium. Regul Toxicol Pharmacol 26(1):S3–S7. https://doi.org/10.1006/rtph.1997.1132
Bebien M, Chauvin JP, Adriano JM, Grosse S, Vermeglio A (2001) Effect of selenite on growth and protein synthesis in the phototrophic bacterium Rhodobacter sphaeroides. Appl Environ Microbiol 67:4440–4447
Burton G A, Giddings TH, Debrine P, Fall R (1987) High incidence of selenite-resistant bacteria from a site polluted with selenium. Appl Environ Microbiol 53:185–188
Cetin D, Donmez S, Donmez G (2008) The treatment of textile wastewater including chromium(VI) and reactive dye by sulfate-reducing bacterial enrichment. J Environ Manag 88(1):76–82. https://doi.org/10.1016/j.jenvman.2007.01.019
Chardin, B.; Giudici-Orticoni, M. T.; Luca, G. D.; · Guigliarelli, B.; Bruschi, M. Hydrogenases in sulfate-reducing bacteria function as chromium reductase. Appl Microbiol Biotechnol 2003, 63, 315–321, 3, DOI: https://doi.org/10.1007/s00253-003-1390-8
Cheung KH, Gu JD (2007) Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeterior Biodegrad 59(1):8–15. https://doi.org/10.1016/j.ibiod.2006.05.002
Cheung KH, Gu JD (2003) Reduction of chromate (CrO4 2–) by an enrichment consortium and an isolate of marine sulfate-reducing bacteria. Chemosphere 52(9):1523–1529. https://doi.org/10.1016/S0045-6535(03)00491-0
Dayan AD, Paine AJ (2001) Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol 20(9):439–451. https://doi.org/10.1191/096032701682693062
Dedysh SV, Belova SE, Bodelier PLE, Smirnova KV, Khmelenina VN, Chidthaisong A, Trotsenko YA, Liesack W, Dunfield PF (2007) Methylocystis heyeri sp. nov., a novel type II methanotrophic bacterium possessing ‘signature’ fatty acids of type I methanotrophs. Int J Syst Evol Microbiol 57(3):472–479. https://doi.org/10.1099/ijs.0.64623-0
Doran JW (1982) Microorganisms and the biological cycling of selenium. Adv Microb Ecol 6:1–32. https://doi.org/10.1007/978-1-4615-8318-9_1
Francis CA, Obraztsova AY, Tebo B (2000) Dissimilatory metal reduction by the facultative anaerobe Pantoea agglomerans SP1. Appl Environ Microbiol 66(2):543–548. https://doi.org/10.1128/AEM.66.2.543-548.2000
Fournier E, Adam-Guillermin C, Potin-Gautier M, Pannier F (2010) Selenate bioaccumulation and toxicity in Chlamydomonas reinhardtii: Influence of ambient sulphate ion concentration. Aquat Toxicol 97(1):51–57
Fujita M, Ike M, Nishimoto S, Takahashi K, Kashiwa M (1997) Isolation and characterization of a novel selenate-reducing bacterium, Bacillus sp. SF-1. J Ferment Bioeng 83(6):517–522. https://doi.org/10.1016/S0922-338X(97)81130-0
Hu BL, Shen LD, Lian X, Zhu Q, Liu S, Huang Q, He ZF, Geng S, Cheng DQ, Lou LP, Xu XY, Zheng P, He YF (2014) Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. PNAS 111(12):4495–4500. https://doi.org/10.1073/pnas.1318393111
Huber H, Jannasch H, Rachel R, Fuchs T, Stetter KO (1997) Archaeoglobus venef icus sp. nov., a novel facultative chemolithoautotrophic hyperthermophilic sulfite reducer, isolate from abyssal black smokers. Syst App Microbiol 20:374–380
Kantar C, Cetin Z, Demiray H (2008) In situ stabilization of chromium (VI) in polluted soils using organic ligands: the role of galacturonic, glucuronic and alginic acids. J Hazard Mater 159(2-3):287–293. https://doi.org/10.1016/j.jhazmat.2008.02.022
Knittel K, Boetius A (2009) Anaerobic oxidation of methane: progress with an unknown process. Annu Rev Microbiol 63(1):311–334. https://doi.org/10.1146/annurev.micro.61.080706.093130
Lai CY, Zhong L, Zhang Y, Chen JX, Wen LL, Shi LD, Sun YP, Ma F, Rittmann BE, Zhou C, Tang Y, Zheng P, Zhao HP (2016a) Bio-reduction of chromate in a methane-based membrane biofilm reactor. Environ Sci Technol 50(11):5832–5839. https://doi.org/10.1021/acs.est.5b06177
Lai CY, Wen LL, Shi LD, Zhao KK, Wang YQ, Yang XE, Rittmann BE, Zhou C, Tang Y, Zheng P, Zhao HP (2016b) Selenate and nitrate bioreductions using methane as the electron donor in a membrane biofilm reactor. Environ Sci Technol 50(18):10179–10186. https://doi.org/10.1021/acs.est.6b02807
Lai CY, Yang X, Tang Y, Rittmann BE, Zhao HP (2014) Nitrate shaped selenate reducing microbial community in a hydrogen-based biofilm reactor. Environ Sci Technol 48:3395–3402
Lemly AD (1993) Teratogenic effects of selenium in natural populations of fresh water fish. Ecotoxicol Environ Saf 26(2):181–204
Lenz M, Enright AM, Flaherty VO, Aelst ACV, Lens PNL (2009) Bioaugmentation of UASB reactors with immobilized Sulfurospirillum barnesii for simultaneous selenate and nitrate removal. Appl Microbiol Biotechnol 83(2):377–388. https://doi.org/10.1007/s00253-009-1915-x
Lovely DR (1993) Dissimilatory metal reduction. Annu Rev Microbiol 47:263–290
Lovley DR, Phillips EJP (1994) Reduction of chromate by Desulfovibrio vulgaris and its C3 cytochrome. Appl Environ Microbiol 60(2):726–728
Lovley DR, Coates JD (1997) Bioremediation of metal contamination. Curr Opin Biotechnol 8(3):285–289. https://doi.org/10.1016/S0958-1669(97)80005-5
Lozupone C, Hamady M, Knight R (2006) UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinf 7(1):371–385. https://doi.org/10.1186/1471-2105-7-371
Luo YH, Chen R, Wen LL, Meng F, Zhang Y, Lai CY, Rittmann BE, Zhao HP, Zheng P (2015) Complete perchlorate reduction using methane as the sole electron donor and carbon source. Environ Sci Technol 49:2341–2349
Ma J, Kobayashi DY, Yee N (2009) Role of menaquinone biosynthesis genes in selenate reduction by Enterobacter cloacae SLD1a-1 and Escherichia coli K12. Environ Microbiol 11(1):149–158. https://doi.org/10.1111/j.1462-2920.2008.01749.x
McLean J, Beveridge TJ (2001) Chromate reduction by a pseudomonas isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol 67(3):1076–1084. https://doi.org/10.1128/AEM.67.3.1076-1084.2001
Michel C, Brugna M, Aubert C, Bernadac A, Bruschi M (2001) Enzymatic reduction of chromate: comparative studies using sulfate-reducing bacteria. Appl Microbiol Biotechnol 55(1):95–100. https://doi.org/10.1007/s002530000467
Mori K, Kim H, Kakegawa T, Hanada S (2003) A novel lineage of sulphate-reducing microorganisms: Thermodesulfobiaceae fam. nov. Thermodesulfobium narugense, gen. nov., sp. nov. a new thermophilic isolate from a hot spring. Extremophiles 7(4):283–290. https://doi.org/10.1007/s00792-003-0320-0
Palmer CD, Wittbrodt PR (1991) Processes affecting the remediation of chromium-contaminant sites. Environ Health Persp 92:25–40. https://doi.org/10.1289/ehp.919225
Park CH, Keyhan M, Wielinga B, Fendorf S, Matin A (2000) Purification to homogeneity and characterization of a novel Pseudomonas putida chromate reductase. Appl Environ Microbiol 66(5):1788–1795. https://doi.org/10.1128/AEM.66.5.1788-1795.2000
Peck HD Jr (1959) The ATP-dependent reduction of sulfate with hydrogen in extracts of Desulfovibrio desulfuricans. Proc Natl Acad Sci U S A 45(5):701–708. https://doi.org/10.1073/pnas.45.5.701
Rauschenbach I, Narasingarao P, Haggblom MM (2010) Desulfurispirillum indicum sp. nov., a selenate- and selenite-respiring bacterium isolated from an estuarine canal. Int J Syst Evol Microbiol 61:654–658
Rikmann E, Zekker I, Tomingas M, Tenno T, Menert A, Loorits L, Tenno T (2012) Sulfate-reducing anaerobic ammonium oxidation as a potential treatment method for high nitrogen-content wastewater. Biodegradation 23(4):509–524. https://doi.org/10.1007/s10532-011-9529-2
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill Book Co, New York
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13(11):2498–2504. https://doi.org/10.1101/gr.1239303
Smith WL, Gadd GM (2000) Reduction and precipitation of chromate by mixed culture sulfate-reducing bacterial biofilms. J Appl Microbiol 88(6):983–991. https://doi.org/10.1046/j.1365-2672.2000.01066.x
Srivastava S, Prakash S, Srivastava MM (1999) Chromium mobilization and plant availability—the impact of organic complexing ligands. Plant Soil 212:203–208
Tang Y, Zhao HP, Marcus AK, Krajmalnik-Brown R, Rittmann BE (2012a) A steady-state biofilm model for simultaneous reduction of nitrate and perchlorate, part 1: model development and numerical solution. Environ Sci Technol 46(3):1598–1607. https://doi.org/10.1021/es203129s
Tang Y, Zhao HP, Marcus A, Krajmalnik-Brown R, Rittmann BE (2012b) A steady-state-biofilm model for simultaneous reduction of nitrate and perchlorate—part 2: parameter optimization and results and discussion. Environ Sci Technol 46(3):1608–1615. https://doi.org/10.1021/es203130r
Tang Y, Zhou C, Van Ginkel S, Ontiveros-Valencia A, Shin J, Rittmann BE (2012c) Hydrogen permeability of the hollow fibers used in H2-based membrane biofilm reactors. J Membrane Sci 407-408:176–183. https://doi.org/10.1016/j.memsci.2012.03.040
United States Environmental Protection Agency (2015) Appendix A to subpart O-regulated contaminants
Vatsouria A, Vainshtein M, Kuschk P, Wiessner ADK, Kaestner M (2005) Anaerobic co-reduction of chromate and nitrate by bacterial cultures of Staphylococcus epidermidis L-02. J Ind Microbiol Biotechnol 32:409–414
Waki M, Yasuda T, Yokoyama H, Dai Hanajima D, Ogino A, Suzuki K, Wang P-C, Mori T, Komori K, Sasatsu M, Toda K, Ohtake H (1989) Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl Environ Microbiol 55:1665–1669
Wang PC, Toda K, Ohtake H, Kusaka I, Yabe I (1991) Membrane bound respiratory system of Enterobacter cloacae strain HO1 grown anaerobically with chromate. FEMS Microbiol Lett 78(1):11–16. https://doi.org/10.1111/j.1574-6968.1991.tb04408.x
Yimga MT, Dunfield PF, Ricke P, Heyer J, Liesack W (2003) Wide distribution of a novel pmoA-like gene copy among type II methanotrophs, and its expression in Methylocystis strain SC2. Appl Environ Microbiol 69(9):5593–5602. https://doi.org/10.1128/AEM.69.9.5593-5602.2003
Zahoor A, Rehman A (2009) Isolation of Cr(VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J Environ Sci 21(6):814–820. https://doi.org/10.1016/S1001-0742(08)62346-3
Zhao HP, Van Ginkel S, Kang DW, Rittmann BE, Krajmalnik-Brown R (2011) Interactions between perchlorate and nitrate reductions in the biofilm of a hydrogen-based membrane biofilm reactor. Environ Sci Technol 45(23):10155–10162. https://doi.org/10.1021/es202569b
Zhao HP, Ilhan ZE, Ontiveros-Valencia A, Tang Y, Rittmann BE, Krajmalnik-Brown R (2013) Effects of multiple electron acceptors on microbial interactions in a hydrogen-based biofilm. Environ. Sci. Technol. 47(13):7396–7403. https://doi.org/10.1021/es401310j
Zhao HP, Ontiveros-Valencia A, Tang Y, Kim B, Van-Ginkel S, Friese D, Overstreet R, Smith J, Evens P, Krajmalnik-Brown R, Rittmann BE (2014) Removal of multiple electron acceptors by pilot-scale, two-stage membrane biofilm reactors. Water Res 54:115–122
Zhong L, Lai CY, Shi LD, Wang KD, Dai YJ, Liu YW, Ma F, Rittmann BE, Zheng P, Zhao HP (2017) Nitrate effects on chromate reduction in a methane-based biofilm. Water Res 115:130–137. https://doi.org/10.1016/j.watres.2017.03.003
Acknowledgments
The authors greatly thank the “Natural Science Funds for Distinguished Young Scholar of Zhejiang Province (LR17B070001),” the “Fundamental Research Funds for the Central Universities (2017XZZX010-03),” and the “National Natural Science Foundation of China (Grant No. 21377109, 21577123)” for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bingcai Pan
Electronic supplementary material
ESM 1
(DOCX 715 kb)
Rights and permissions
About this article
Cite this article
Lv, PL., Zhong, L., Dong, QY. et al. The effect of electron competition on chromate reduction using methane as electron donor. Environ Sci Pollut Res 25, 6609–6618 (2018). https://doi.org/10.1007/s11356-017-0937-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0937-7