Abstract
The present work investigated the spatial distribution and ecological risk assessment of total and mild acid-leachable trace elements in surface sediments (top 0–10 cm; grain size ≤ 63 μm) along the Hooghly (Ganges) River Estuary and Sundarban Mangrove Wetland, India. The trace elements, analyzed by ICPMS, showed wide range of variations with the following descending order (mean values expressed in milligrams per kilogram): Fe (25,050 ± 4918) > Al (16,992 ± 4172) > Mn (517 ± 102) > Zn (53 ± 18) > Cu (33 ± 11) > Cr (29 ± 7) > Ni (27 ± 6) > Pb (14 ± 3) > As (5 ± 1) > Se (0.37 ± 0.10) > Cd (0.17 ± 0.13) > Ag (0.16 ± 0.19) > Hg (0.05 ± 0.10). In the acid-leachable fraction, Cd (92%) is dominated followed by Pb (81%), Mn (77%), Cu (70%), and Se (58%) indicating their high mobility, imposing negative impact on the adjacent benthos. The sediment pollution indices (both enrichment factor and contamination factor) suggested severe pollution by Ag at the sampling site Sajnekhali, a wildlife sanctuary in Sundarban. The mean probable effect level quotient indicated that surface sediments in the vicinity of the studied region have 21% probability of toxicity to biota. The result of multivariate analyses affirms lithogenic sources (e.g., weathering parent rocks, dry deposition) for As, Pb, Cr, Cu, and Ni, whereas Cd and Hg originated from anthropogenic activities (such as urban and industrial activities). Both human-induced stresses and natural processes controlled trace element accumulation and distribution in the estuarine system, and remedial measures are required to mitigate the potential impacts of these hazardous trace elements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace elements (TEs) are of environmental concern due to their toxicity, wide point and non-point sources, and persistent nature and are transported through flowing water, and subsequently accumulate in the bodies of aquatic organisms which may, in turn, enter the human food chain resulting in health hazards (Fu et al. 2013; Zhang et al. 2014; Guan et al. 2016). Trace elements once released into aquatic environments are transported and distributed between the aqueous phase and sediment through the process of adsorption and desorption. Due to adsorption, hydrolysis, and co-precipitation of metal ions, a substantial quantity of free metal ions forms complexes and gets deposited in the sediment while only a small portion of ions remain dissolved in the water column (Bastami et al. 2015). As emphasized by Bartoli et al. (2012), more than 99% of the pollutants are stored in the sediments while only a negligible fraction of them remain dissolved in the aquatic phase. Trace elements are derived from a set of multiple anthropogenic activities (wastewater discharge, agricultural runoffs, vehicular emissions, sand mining, etc.) as well as natural processes (such as physical and chemical weathering of parent rocks, atmospheric deposition) in the coastal environments. The accumulation and mobility of TEs in coastal sediments are controlled cumulatively by a set of factors such as the nature of sediment particles, properties of adsorbed compounds, and characteristics of specific metal and organic matter (Bastami et al. 2014). The complex and dynamic intertidal zones play an important role in the biogeochemical cycling of elements where physicochemical and biological interactions between terrestrial and marine systems have profound influences on the transport and fate of TEs (Spencer 2002; Ding et al. 2009; Zhang et al. 2015).
The total element concentrations in sediment do not justify the actual toxicity of TEs and hence to be considered as a poor indicator of pollution. Toxicity as well as element properties is mainly related to the binding state of TEs, their bioavailable fraction and edaphic factors of sediment (Nyamangara 1998; Ma et al. 2016). Thus, for better understanding the chemical behaviors of TEs in terms of chemical interaction, mobility, biological availability, and potential toxicity, operationally defined tests for bioavailability can be carried out. A weak (1.0 M) hydrochloric acid leach for 1 h at room temperature (~ 23 °C) is usually considered to be indicative of the weakest bound elements in sediments and defined as the “exchangeable” or “bioavailable” fraction. This fraction may equilibrate with the aqueous phase and thus become the most mobile portion. The high relative abundance of an element in the bioavailable fraction is directly link to enhanced risk to the environment (Wang et al. 2015).
The Hooghly River Estuary (HRE) (87° 55′ 01″ N to 88° 48′ 04″ N latitude and 21° 29′ 02″ E to 22° 09′ 00″ E longitude), the distributary of the major Ganges River, extends over an area of approximately 6 × 104 km2 and is about 295 km long. The Hooghly River, major water source for the people living in the plains of West Bengal, India, receives huge amount of industrial and urban pollutants from point sources and large amount of pesticides, fertilizers, and organic pollutants from non-point sources along its course. A mass fraction of people is absolutely dependent for the use of the river water such as bathing, drinking, farming, riverine transport, religious ceremonies, cremation, and scattering of ashes after death. Accelerated development due to rapid urbanization and industrialization of the catchment area in the last few decades is responsible for the high-level accumulation of both organic and inorganic pollutants in the region. Sundarban Mangrove Wetland (SMW), located south of trophic of cancer, is the largest tide-dominating delta in the estuarine phase of the tidal Hooghly River with an area of 9630 km2. It is a low lying, humid, vulnerable complex delta having geo-genetic link to the tectonic Bengal basin (http://www.portal.gsi.gov.in). Protected as a UNESCO World Heritage site, it is characterized as the largest single block of tidal halophytic mangrove forest in the world. However, the region is under constant human pressures (i.e., over-exploitation of living and non-living resources, collection of prawn larvae, oil spill) which have severely degraded the mangrove ecosystem. The number of researchers has worked upon the water quality (Naha Biswas et al. 2013; Rakshit et al. 2014; Bhattacharya et al. 2015a, 2015b; Rakshit et al. 2015) and organic and inorganic sediment pollution level (Chatterjee et al. 2009; Antizar-Ladislao et al. 2011; Corsolini et al. 2012; Sarkar et al. 2012; Watts et al. 2013; Antizar-Ladislao et al. 2015; Watts et al. 2017; Sarkar et al. 2017) covering Hooghly River Estuary and Sundarban Mangrove Wetland. The main objectives of the present study are as follows: (i) to measure the spatial distribution of total and acid-leachable TEs within the sediments of the Hooghly River Estuary and Sundarban Mangrove Wetland, (ii) to evaluate the ecological risk and toxicity of sediment-bound TEs considering ecological risk indices and sediment quality guidelines (SQGs), and (iii) to identify the possible pollution sources by using multivariate statistical analyses.
Materials and methods
Collection and preservation of sediment samples
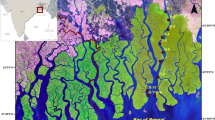
During March 2014–June 2014, surface sediment samples (top 0–10 cm) were collected during ebb tide from intertidal areas of 14 sampling sites along the HRE and SMW. The sampling sites were selected for the purpose of systematic coverage of the study region, the influence of mixing of fresh and saline water, the ecological stress experienced and distances from the sea (Bay of Bengal) (Fig. 1, Table 1). Eight sampling sites were chosen along the stretches of the HRE covering a distance of ~ 175 km [namely, Tribeni (S1), Barrackpore (S2), Babughat (S3), Budge Budge (S4), Nurpur (S5), Diamond Harbour (S6), Lot 8 (S7), and Gangasagar (S8)] and six sampling sites from the adjacent SMW [Chemaguri (S9), Chandanpiri (S10), Jharkhali (S11),Canning (S12), Sajnekhali (S13), and Jyotirampur (S14)].
Sediment samples were collected from each sampling site in triplicate with a polyethylene spatula and placed in acid-rinsed polyethylene zipper bags. The collected samples were transported to the laboratory on ice where they were kept at − 20 °C until further analyses. Prior to analysis, the sediments were defrosted at room temperature, dried at 40 °C until they reached constant weight. A portion of the dried sediment sample was ground with an agate mortar and pestle, passed through 63-μm mesh sieve, and stored in sealed plastic containers before physical and chemical analysis. A fraction of fresh unsieved sample was separated for determining the sediment quality parameters such as organic carbon and grain size fraction.
Analytical procedure
The pH of the samples was determined in situ with the help of an ORP meter (model no. HI 98160) using combination glass electrode manufactured by Hanna instrument (India) Pvt. We have considered the sediment geochemical characteristics (pH, organic carbon (C org), sediment grain size fractions, and textural parameters), the details of which have been described in previous publication (Sarkar et al. 2017).
Trace element analysis
From each homogenate sample, 0.2 ± 0.01g samples were weighed into separate teflon tubes. Digestions to determine total element concentrations were completed using an aqua regia 1:3 concentrated HNO3:HCl. Samples were microwaved for 20 min using a microwave accelerated reaction system (MARSXpress®) with 1600 watts IEC and frequency of 2450 MHz and allowed to cool for 15 min before decanting. The elemental analysis was conducted using a Perkin Elmer NexION 300D inductively coupled plasma mass spectrometer (ICPMS). Optimization was performed as outlined in the NexION 300D user’s manual; in particular, the nebulizer gas flow rate and torch alignment were adjusted to yield the greatest sensitivity possible while maintaining low levels of oxides (< 2%) and doubly charged ions (< 3%). The sample solutions were then analyzed against a three-point calibration curve to determine the concentrations of each element. A calibration standard and independent soil digests were analyzed at regular intervals during analytical runs to ensure the instrument maintained acceptable linearity and sensitivity criteria. Duplicate blanks, and the Australian National Measurement Institute reference sediment, AGAL-12, were digested and analyzed with the batch of samples. The recovery rates of the certified standard for all elements ranged between 77 and 112% and were within the quality control Global Acceptance Criteria (GAC) (Table 2).
Acid-leachable fractions of elements were worked out by using the defined procedure suggested in the sediment quality guidelines such as ANZECC and ARMCANZ (2000). From each homogenate, 0.2 ± 0.01g samples were weighed into an acid-cleaned Erlenmeyer flask. Twenty milliliters of 1 M HCl was added to each flask and agitated at room temperature for 1 h. Samples were centrifuged, and the liquid was decanted for analyses by Perkin Elmer NexION 300D inductively coupled plasma mass spectrometer (ICPMS) using the same protocols described above.
Assessment method of sediment pollution
For interpretation of data, the choice of background values plays an important role (Memet 2011). The best alternative is to compare concentrations between contaminated and mineralogically and texturally comparable, uncontaminated sediments (Rubio et al. 2000). Since there were no data on background concentrations for the studied sediments of the region, the background values used in this paper were the average shale value (Turekian and Wedepohl 1961).
Enrichment factor
The enrichment factor for the TEs was calculated according to the following equation (Herut and Sandler 2006)
Enrichment factor (EF) values lower than 1.5 indicate that the metal is entirely sourced from crustal contributions and values higher than this indicate that a significant portion of the element is from non-crustal sources (Herut and Sandler 2006). In this study, iron (Fe) was used as the reference element for geochemical normalization for the following reasons: (i) Fe is associated with fine solid surfaces, (ii) its geochemistry is similar to that of many TEs, and (iii) its natural concentration tends to be uniform (Bhuiyan et al. 2010).
Contamination factor and Pollution Load Index
For the entire sampling program, Pollution Load Index (PLI) has been determined as the nth root of the product of the n contamination factor (CF):
where n = number of elements, CF = contamination factor = CSample / Cbackground value, CSample = mean metal concentration in polluted sediments, and Cbackground value = mean natural background value of that metal. CF values were interpreted as suggested by Hakanson (1980), where CF < 1 indicates low contamination; 1 < CF < 3 is moderate contamination; 3 < CF < 6 is considerable contamination; and CF > 6 is very high contamination. When PLI > 1, it means that pollution exists; otherwise, if PLI < 1, there is no metal pollution (Tomlinson et al. 1980).
Geoaccumulation index
The geoaccumulation index (I geo), another parameter to assess the TE contamination, is defined as the following equation:
where C n is the concentration of metal (n) and B n is the geochemical background concentration of metal (n). The factor of 1.5 is a background matrix correction factor that includes possible variations of the background values due to lithogenic effects. A seven-level classification of I geo is defined as follows: uncontaminated (I geo ≤ 0), uncontaminated to moderately contaminated (0 < I geo ≤ 1), moderately contaminated (1 < I geo ≤ 2), moderately to strongly contaminated (2 < I geo ≤ 3), strongly contaminated (3 < I geo ≤ 4), strongly to extremely contaminated (4 < I geo ≤ 5), and extremely contaminated (I geo > 5) (Müller 1981).
Ecological risk assessment
Sediment quality guidelines
The sediment quality guidelines (SQGs), which have been developed from biological toxicity test of benthic environment, are used to evaluate adverse biological effects of sedimentary contaminations in the study region. Two sets of sediment quality guidelines such as (i) effects range-low (ER-L) and effects range-medium (ER-M) and (ii) threshold effect level (TEL) and probable effect level (PEL) are proposed to determine whether the TEs in sediments pose a threat to aquatic ecosystem (Long et al. 1998; MacDonald et al. 2000). In this study, we have used both sets of SQGs to assess the potential adverse biological effects.
ER-L and ER-M are SQGs developed by Long and Morgan (1990) to categorize the range of TE concentrations in sediment the effects of which are scarcely observed or predicted (below the ER-L), occasionally observed (ER-L–ER-M), and frequently observed (above the ER-M) (Long et al. 1995). The TEL/PEL SQGs are also applied to assess the degree to which the sediment-associated chemical status might adversely affect aquatic organisms and are designed to assist in the interpretation of sediment quality (Macdonald et al. 1996; Long et al. 1998; Macdonald et al. 2000). The TEL was interpreted to present chemical concentrations below which adverse biological effects rarely occur, and the PEL was intended to present chemical concentrations above which adverse biological effects frequently occur (Macdonald et al. 2000).
Mean-PEL-Quotient
In order to evaluate the possible biological effects of the coupled toxicity of Ag, As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn in the surface sediments of HRE and SMW, the mean-PEL-quotient (m-PEL-Q) by Long et al. (1998) was used, and values were calculated using the following formula:
where C i is the sediment concentration of metal “i,” PELi is the PEL (probable effect level) for metal “i,” and “n” is the number of the studied elements. It was reported that the mean- PEL- quotients of < 0.1 have a 9% probability of being toxic, the mean- PEL- quotients of 0.11–0.5 have a 21% probability of being toxic, the mean- PEL- quotients of 0.51–1.5 have a 49% probability of being toxic, and the mean- PEL- quotients of > 1.50 have a 76% probability of being toxic (Long et al. 2000).
Data pretreatments and statistical analyses
Before multivariate statistical analysis, the normality of the variable’s distribution was checked by analyzing skewness and kurtosis statistical tests (Zhang et al. 2009), because most multivariate statistical methods require variables to conform to normal distributions (Sheykhi and Moore 2013). Values of standardized skewness and standardized kurtosis outside the range of − 2 to + 2 indicate significant departures from normality (Kannel et al. 2007; Sheykhi and Moore 2013).
The results from HRE and SMW demonstrated that the parameter distributions were far from normal, with a 95% significance level (calculated skewness and kurtosis were − 1.88 to 3.64 and − 1.43 to 13.45, respectively). The test confirmed that most variables were not normally distributed, with 95% significance level. Hence, log transformation was carried out to transform the data set to normal form and to eliminate the influence of different units of variance and give each determined variable an equal weight (Wang et al. 2013).
In order to decipher the interrelationship among TEs, Pearson’s correlation coefficient (PCC), hierarchical cluster analysis (HCA), and principal component analysis (PCA) were performed. PCC was performed to assess if there was significant relationship between the elements and confirm the results of multivariate analysis (Tahri et al. 2005; Facchinelli et al. 2001; Tariq et al. 2006; Wang et al. 2015). Significant difference was considered when p ≤ 0.05. Pearson’s correlation coefficient was performed as all the parameters were continuous in nature, and we like to study them on the basis of their actual values rather than relative positions. HCA is used to identify spatial variability between the sites based on physicochemical parameters or TE enrichment level based on concentrations, Euclidean distance is used as dissimilarity matrix, and Ward’s method is used as a linkage method (Zahra et al. 2014). Both the PCC and HCA were performed using the statistical software Minitab 13. PCA is used to ascertain contamination sources (natural and anthropogenic), whereby a complex data set is simplified by creating several new variables or factors, each representing a cluster of interrelated variables within the dataset (Varol 2011) and was performed using XLSTAT 14 software. Liu et al. (2003) classified the factor loading as strong, moderate, and weak depending on the loading values > 0.75, 0.75–0.50, and 0.50–0.30, respectively.
Results and discussion
Sediment geochemical characteristics
In this study, pH, C org, sediment grain size, and textural properties were determined to obtain the general characteristics of the surface sediment samples from 14 sampling sites. Coefficients of variation (CV) were used to estimate the variability of the analyzed parameters. The CV for pH was 3.59% indicating low variation (CV < 15%) while for C org, the variability of the measurement was 25.67% which indicated moderate variation (15% < CV < 36%, based on Zhang and Gao 2015) (Table 3). Values of pH showed variations from slightly acidic to slightly alkaline (6.61 to 7.63) nature. The acidic nature is mainly pronounced at Chandanpiri (S10) and Jharkhali (S11) and might originate as a result of the decomposition of mangrove litter and hydrolysis of tannins in mangrove plants releasing various kinds of organic acids (Chatterjee et al. 2007). The sediments were characterized by homogeneous concentrations of C org content that varied from 0.27% at Tribeni (S1) to 0.66% at Gangasagar (S8) with an average of 0.46%, exhibiting an irregular distribution pattern. The low C org values obtained might be the result of marine sedimentation and mixing processes at the sediment water interface where the rate of delivery as well as rates of degradation by microbial-mediated processes can be high (Canuel and Martens 1993).
The grain-size fraction of particles varied from 38.52% at Jyotirampur (S14) to 61.60% at Lot 8(S7) for clay (less than 4 μm) with an average of 51%. The fraction of silt (4–63 μm) particles varied from 14.40% at Canning (S12) to 55% at Jyotirampur (S14) with an average of 32%. Sand content (greater than 63 μm) varied from 0.54% at Chemaguri (S9) to 45.31% at Canning (S12) with an average of 16.91%. The surface sediments were predominantly composed of silt and clay, especially for Lot 8(S7), Chemaguri (S9), Jharkhali (S11), Sajnekhali (S13), and Jyotirampur (S14) with the percentages of fine fraction (clay + silt) > 90%. It is suggested that the observed enrichment of fine material was due to the low fluvial discharge and a better mixing of saline and fresh water, which could have facilitated flocculation and subsequent settling of suspended particles (Nair et al. 1982). Furthermore, the textural properties of the sediment varied from sandy clay to clayey very fine (Fig. 2). However, the grain-size distribution or the texture of sediments did not exhibit clear spatial difference among the sampling sites.
Spatial distribution of total elements along the HRE and adjacent SMW
The significant spatial variations of total element concentrations in sediments were analyzed with one-way analysis of variance (ANOVA) (F = 282.00; p < 0.05). The spatial distributions of the trace (Ag, As, Pb, Cd, Cr, Cu, Ni, Se, Zn, and Hg), minor (Mn), and major elements (Fe and Al) are presented in Table 3; Fig. 3(a-m). The CVs of Ag, Cd, and Hg were 116.51, 76.51, and 203.95%, respectively, which were characterized as high variation (CV > 36%), suggested that their distribution was uneven and might originate from different anthropogenic sources (Abuduwaili et al. 2015; Zhang et al. 2016). In contrast, the CVs for all the other elements reflected medium variation (15% < CV < 36%). The main sources of contamination of TEs in this estuarine system include aquaculture activities, smelting activities, pulp and paper manufacturing, fertilizers used in the nearby agricultural fields, manufacture of textile and plastic products, input of industrial and domestic sewage, and use of antifouling paints.
The concentrations of Ag varied from 0.05 mg kg−1 at Chemaguri (S9) to 0.80 mg kg−1 at Sajnekhali (S13) with an average of 0.16 ± 0.12 mg kg −1. Silver might originate from urban sewage and is historically linked to urban populations for its use in photo processing operations. Elevated concentrations of Ag present in coastal sediments have already been associated with anthropogenic pollution as revealed in recent studies (Cossa et al. 2014; Antizar-Ladislao et al. 2015).
Arsenic concentration in the sediments ranged from 3.11 mg kg−1 at Babughat (S3) to 9.10 mg kg−1 at Gangasagar (S8). The high concentration of this metalloid in this estuarine region might be attributed to application of fertilizers and insecticides in the nearby agricultural fields, intense exploitation of ground water, waste incineration, and burning of coal for domestic purposes. The contaminant is also derived from urbanization of the catchment area and the associate sewage and domestic water discharge (Morelli and Gasparon 2014).
The maximum concentration of the metalloid Se obtained at Barrackpore (S2) (0.56 mg kg−1) might be attributed to smelting activities, fly ash, coal-fired power stations, sewage effluents, and agricultural inputs from the nearby catchment area. The total Se content in sediment worldwide ranged from 0.05 to 1.50 mg kg−1 with an average value of 0.44 mg kg−1 (Kabata-Pendias 2011).
An overall uniform pattern of spatial distribution of Hg was pronounced ranging from 0.00 to 0.05 mg kg −1 with a mean value of 0.02 ± 0.01, except Budge Budge (S4) where many fold increase (0.39 mg kg−1) of its concentration was recorded. Previous trends of total Hg in the studied wetlands (HRE and SMW) also revealed low concentrations and oscillated within a very narrow range over the years as follows: < 0.004–0.093 mg kg−1 (Chatterjee et al. 2009), 0.032–0.196 mg kg−1 (Chatterjee et al. 2012), 0.004–0.030 mg kg−1 (Sarkar et al., 2017), and 0.009–0.035 mg kg−1 (Mondal et al. 2018). No enrichment of Hg was observed at Jyotirampur (S14) which might be attributed to minimal anthropogenic influences due to its long distance from the industrial belts of Calcutta megacity and Howrah located upstream of the HRE (Fig. 1). The potential sources of Hg pollution are atmospheric deposition, erosion, use of antifouling paints, urban discharge, agricultural materials, mining, and combustion in the metropolitan city of Calcutta along with the untreated discharges from industries (Silva-Filho et al. 2011).
Among the major elements, Fe (25,050 ± 4919 mg kg−1) was the most abundant at all the sampling sites with a maximum concentration of 32,200 mg kg−1 at Chemaguri (S9), followed by Al (16,992 ± 4172 mg kg−1). The elevated Fe concentration was mainly attributed to the presence of floating old rusty and stranded barges. These barges are major sources for particulate Fe, which settle down and mix with the bottom sediments in these regions. The precipitated Fe in the form of oxy-hydroxide has the affinity to scavenge other elements such as Cu, Zn, and Pb as they pass through the water en route to the sediments (Chatterjee et al. 2007).
The maximum concentrations of four hazardous TEs (As, Pb, Cr, and Ni) and the major element Al were found at Gangasagar (S8), located at the mouth of the estuary experiencing the severity of the wave climate of the coastal Bay of Bengal and a macrotidal setup. The prevalent high concentrations of these elements are linked to the fact that this sampling site receives the discharge from the whole catchment area and experiences lot of churning by the wave and tidal effects due to its location at the confluence of Hooghly River and Bay of Bengal along with the operation of mechanized boats, dredging activities, etc. Flocculation due to mixing of salt and freshwater leading to greater sedimentation might also be one of the potential factors that results in this site being a depositional environment (Chatterjee et al. 2007).
Majority of the elements showed their minimum concentration at Babughat (S3), located in the upper reaches of the study area, characterized by sandy clay sediment texture. This site experiences greater flushing activities, suspension-resuspension, and downstream transportation of the elements by ebb flow together with downstream sediment movement. The plain of the river essentially represents a shallow asymmetrical depression with a gentle easterly slope (0.00004) towards the sea (Singh 2007), which also favors low concentration of the elements.
It was observed that the trace and major element concentrations were higher in the sampling sites belonging to HRE in comparison to SMW. This heterogeneous distribution pattern of the elements might be due to the development of economic activities, discharge of untreated industrial and domestic sewages, idol immersion, agricultural runoff, dredging activities, urbanization of the catchment area, and huge siltation. Indiscriminate and unscientific sand mining by local people has also become a serious ecological threat to the Hooghly River basin, resulting in channel degradation and erosion which ruin its flow regimes and total sedimentary environment.
When comparing the average concentrations of the TEs with the average shale concentrations reported in Turekian and Wedepohl (1961), the results indicated that all the TEs had concentrations lower than their background values. However, Ag concentrations at 10 sampling sites out of the 14 have exceeded the background concentration followed by Cd (S3, S4, and S13) and Cu (S2, S7, S9, and S11), indicating the direct impact of anthropogenic activities of these elements.
Distribution of acid-leachable elements in sediments
This specific fraction is favored by edaphic factors (such as, pH, and redox conditions) which when taken up by aquatic organisms result in environmental toxicity (Sundaray et al. 2011; Bastami et al. 2017). The spatial distribution of acid-leachable elements in the intertidal surface sediments along HRE and SMW is presented in Fig. 3(a-m). The acid-leachable elements exhibited concentration much lower than the prescribed ER-L and TEL values, indicating that the biota in the area under investigation would be scarcely affected with the studied elements.
The concentration of Pb, Cd, Cu, Mn, and Se in this fraction was generally high (percentage bioavailability greater than 50%), indicating high mobility of these TEs in the sediment. The bioavailability percentage of TEs is presented in Table 4. Aluminum and Cr were the least mobile TEs with a mean bioavailability percentage of 24.0 ± 3.0 and 29.0 ± 3.0%, respectively. The acid-leachable concentration of As, Ni, and Fe varied from 1.32–2.48 mg kg−1 (1.97 ± 0.37 mg kg−1), 7.42–15.30 mg kg−1 (11.65 ± 2.24 mg kg−1), and 5400–11,400 mg kg−1 (8992.86 ± 1802.76 mg kg−1), respectively, with a mean bioavailability percentage of 35, 42, and 36%, respectively, indicating that they had some degree of mobility. Cadmium was the most mobile element with concentration ranging from 0.03 mg kg−1 at Jharkhali (S11) to 0.36 mg kg−1 at Babughat (S3) and Budge Budge (S4) simultaneously, representing 60 to 95% of the total concentration. The high Cd proportion in this fraction may be attributed to the fact that it is a typical anthropogenic element and mostly enters the aquatic environment through the discharge of industrial effluents (Wang et al. 2015). The possible sources of Cd in this estuarine environment include the use of Ni-Cd batteries, pigments, plating, and plastics, the use of phosphate fertilizers, and industrial and municipal runoff (ATSDR 1999). The acid-leachable fraction of Se was 58% of the total concentration, and distinct spatial variations were evident with a range of 0.03 mg kg−1 at Canning (S12) to 0.44 mg kg−1 at Gangasagar (S8). The concentrations of Pb, Cu, Mn, and Zn from different sampling sites was relatively constant, with the range of 8.24–16.20 mg kg−1 (11.56 ± 2.55 mg kg−1), 12.00–38.30 mg kg−1 (23.99 ± 8.67 mg kg−1), 254.00–562.00 mg kg−1 (401.21 ± 83.12 mg kg−1), and 22.80–59.30 mg kg−1 (32.13 ± 9.03 mg kg−1), respectively.
The concentration of Hg and Ag ranged from 0.00 to 0.04 mg kg−1 (0.01 ± 0.01 mg kg−1) and 0.01 to 0.22 mg kg−1 (0.08 ± 0.07 mg kg−1), respectively. Fe and Mn oxides and hydroxides are more stable in surface sediments because of their higher redox potential and the enrichment of these constituents is likely to reduce the mobility of Hg due to the tendency of Hg and Hg-bound organic complexes, which are absorbed onto these surfaces (Rasmussen 1994; Silva-Filho et al. 2011).
The results demonstrated that toxic TEs (Pb, Cu, Cd, and Se) are of potential hazards to the surrounding environments because of their higher susceptibility, bioavailability, and potential mobility. This also likely reflects a strong anthropogenic source in studied regions since the incoming pollutant from external sources initially exists in unstable chemical fractions like acid-leachable fractions (Rodriguez et al. 2009; Ma et al. 2016). However, comparing the previous data on acid-leachable TEs from this estuarine environment by Jonathan et al. (2010), it is revealed that there is multifold increase of Cd and slight increase for many other elements (Fe, Mn, Ni, and Zn). This is of concern for the long-term health of the estuary because of the high-toxic nature of Cd (Sadiq 1992).
Sediment contamination assessment
The assessment of sediment pollution by the studied TEs was calculated considering the total concentrations of the TEs.
Enrichment factor
The EF of individual element is illustrated in Fig. 4a. In this study, it was observed that the EF values of As, Cr, Mn, Ni, Zn, Hg, and Al were less than 1.5, revealing that these elements might originate entirely from crustal materials or natural weathering processes. In contrast, relatively high EF values (EF > 1.5) of Pb (S3), Cd (S2,S3,S4 and S13), Cu (S2,S7,S11), and Se (S2 and S12) indicated significant contamination of these TEs derived from paper and pulp industries, jute and textile factories, tanneries, and thermal power plant located along the upper and middle stretch of the estuary. Silver exhibited “moderate enrichment” at 50% of the sampling sites (3 ≤ EF ≤ 5) while “severe enrichment” (EF = 21.32) was encountered at Sajnekhali (S13), a popular tourist destination in Sundarban region.
Contamination factor and Pollution Load Index
Silver exhibited “low to moderate contamination” (1 ≤ CF ≤ 3) at all the sampling sites except at Sajnekhali (S13) where it exhibited “severe contamination” (CF = 11.43) (Table 5). The severe contamination of Ag at this sampling site might be due to the anthropogenic activities derived from various point and non-point sources. Moderate contamination of the sediments was exhibited by Cd and Cu at 4 out of the 14 sampling sites. All the other elements exhibited CF less than 1 indicating “low contamination.”
The PLI calculated for all the sampling sites was less than 1, indicating that the region was not polluted with the elements investigated in the present study (Table 5). The PLI of estuarine sediments encountered by Dhanakumar et al. (2013) was similar to the present study, but the PLI of sediments from the Narmada and Tapti Rivers (Sharma and Subramanian, 2010) and Ulhas Estuary (Rokade 2009) showed the existence of TE pollution and contamination of the sediments by anthropogenic activities.
The PLI is a site-specific index which is calculated taking into consideration the concentration of all the TEs at that particular site whereas EF is element-specific index.
Geoaccumulation index
The I geo values revealed that 50% of the sampling sites were “uncontaminated to moderately contaminated” with Ag while Sajnekhali (S13) was strongly contaminated by the element (Fig. 4b). The calculated I geo values for all the other elements were ≤ 0. The distribution of Ag in this estuarine environment might be attributed to both natural (storm events, turbulence, perturbation by wildlife, etc.) and sourced from human-derived activities (industrial and municipal sewage discharges, tourist activities, sand mining, dredging, etc.). There is every possibility for the contaminants discharged to the river system to be transported to other adjacent coastal regions as a result of suspension and resuspension of the sediments (e.g., Reichelt and Jones 1994). The chemical and physical attributes of the sampling sites also affect the ultimate fate of the Ag in the environment.
Risk associated with TEs
Sediment quality guidelines
The concentrations of As, Cu, and Ni have exceeded the ER-L and TEL values in most of the sampling sites (Fig. 3; Table 3). The element Ag has exceeded the TEL value at Sajnekhali (S13), indicating that adverse biological effects would affect the aquatic organisms (Table 3). Overall, the mean concentrations of all the elements (except Ni) were below the prescribed ER-M guidelines. Mercury exceeded both the ER-M and TEL values at Budge Budge (S4), indicating that adverse impact on the biota would be frequently observed. However, none of the elements have exceeded the prescribed PEL guidelines.
Mean-PEL-quotient
The m-PEL-Q (Table 5) varied from 0.14 at Babughat (S3) to 0.26 at Gangasagar (S8) with a mean of 0.20 ± 0.04, indicating that the combined effects of the studied elements in the sediments had 21% probability of being toxic to the biota. The sediment-dwelling organisms are more likely to be exposed to sediment contamination due to limited mobility, indirectly affecting also higher trophic organisms in the food chain (Gati et al. 2016).
Statistical analysis
Pearson’s correlation coefficient
To distinguish the sources of the studied major, minor, and trace elements in surface sediments, Pearson’s correlation coefficient matrix was carried out. All the TEs (except for Ag, Se, Cd, Zn, and Hg) showed significant positive correlation with each other (r = 0.60–0.98; p ≤ 0.05) suggesting that they have a common point of origin, interdependence, and identical behavior during transport in the estuarine environment. The good positive correlation of Fe with Mn, Cr, Cu, Ni, Pb, and As was established (Table 6), implying that the Fe oxides are “binding” elements (Veerasingam et al. 2012). Furthermore, there was a high significant positive correlation between Mn (r = 0.85; p ≤ 0.001), Cr (r = 0.95; p ≤ 0.001), Cu (r = 0.68; p ≤ 0.01), Ni (r = 0.98; p ≤ 0.001), and Pb (r = 0.89; p ≤ 0.001) with Al, suggesting that these TEs were mainly derived from terrigenous materials along with the anthropogenic origin. Highly significant positive correlation was found between Al and As (r = 0.92; p ≤ 0.001), indicating that As was probably affected by siliciclastic and anthropogenic inputs (Xu et al. 2014). Similarly, significant positive correlations (r = 0.54–0.95; p ≤ 0.05) between the acid-leachable TEs (except Ag, Se, Cd, and Hg) were also established (Table 6), revealing their similar behavior.
Significant positive correlations between C org and acid-leachable TEs were revealed (Table 6), implying that these TEs either had strong tendency to be accumulated by plants and microbes (Han et al. 1990) and/or the direct sorption of these elements onto the surface of sediment organic matter (McComb et al. 2015). Du Laing et al. (2009) suggested that decaying plant material caused litter to accumulate and contribute to the binding of elements by adsorption, complexation, and chelation. However, no significant correlation was obtained between C org and total TEs.
Due to surface adsorption and ionic attraction, fine-grained sediments tend to have relatively high TE concentrations. In this study, only the acid-leachable fraction of As was positively correlated with silt content (r = 0.58; p ≤ 0.05), suggesting that the abundance and distribution of As were influenced by grain size to a certain extent.
Hierarchical cluster analysis
Hierarchical cluster analysis was performed to assess the spatial variability and identify similarities between concentrations of total TE and group the analyzed parameters. The result of cluster analysis revealed three distinct groups as revealed in Fig. 5, namely, (C1) Al-Fe; (C2) Zn-Cu-Ni-Cr-Pb-As, and (C3) Se-Hg-Cd-Ag. Iron and Al, due to their high natural abundance, are joined together by high similarity level, while no close similarity is shown with the rest of the groups.
Cluster 2 contains TEs which are mainly derived from anthropogenic sources while cluster 3 represents a homogeneous class comprising TEs with lowest concentration levels and small standard deviations. The association of cluster 2 with cluster 3 at a later clustering stage indicates that these TEs share familiar properties with the rest of the elements, although having a specific distribution pattern.
Principal component analysis/factor analysis
PCA was performed on the variables to establish possible relationships and input sources among the pollutant elements. Five principal components (PCs) with an eigenvalue exceeding 1 accounted for 84.59% of the total variance (Table 7; Fig. 6), which indicated that different controlling factors or sources (natural or human-induced) are responsible for the distribution of TEs in the intertidal surface sediments of the HRE and SMW.
The first principal component (PC1) explained 45.64% of the total variance and had strong loadings (> 0.75) on As, Pb, Cr, Cu, Mn, Ni, Fe, and Al and weak loadings on Se and silt content. Strong loadings of Fe and Mn with PC1 revealed that Fe-Mn oxides play an important role in the distribution of TEs in river sediments as endorsed by Nazeer et al. (2016). Fe-oxides due to their high specific surface area serve as important sorbents for TEs (Issa 2008). Aluminum is a structural element of terrigenous aluminosilicates and is a primary lithogenic component (Xu et al. 2016), which implies that this component represented TEs predominantly affected by crustal materials or natural weathering processes. This result coincides with the findings of cluster analysis and correlation coefficient. Since Fe, Mn, and Al are primarily derived from natural sources, PC1 can be termed as “lithogenic factor.”
The second component (PC2) which is dominated by Zn, Mn, and pH accounted for 11.78% of the total variance. The negative loading of Se in this factor suggested a different chemical behavior of the element with respect to Zn and Mn. The third component (PC3) had moderate loadings of Ag and weak loadings on pH and silt content and explained 9.77% of the total variation. Considering the EF and I geo results, PC2 may be derived from both lithogenic and anthropogenic sources, whereas PC3 is derived from human activities.
The fourth principal component (PC4) had strong positive loadings for sand content and negatively moderate loadings for silt and clay content, thus explaining 9.12% of total variance and could be seen as a physical parameter since no TEs were associated with it. The fifth principal component (PC5) is correlated very strongly with Cd and moderately with Hg and accounts for 8.29% of the total variance. This factor represented the anthropogenic sources.
Conclusions
The results provided integrated information pertaining to the transportation and distribution of trace elements which are influenced by a set of multiple processes such as sedimentation, hydrodynamic condition, bioturbation, precipitation and flocculation of particulate substances. The total concentration of the elements showed complex and heterogeneous spatial variations with an overall enrichment of As, Pb, Cr, Ni, and Al at Gangasagar (mouth of the estuary). It is worth to mention that an abrupt increase in Ag concentration (nearer to ER-L and above TEL value) was encountered at Sajnekhali with the sediment pollution indices (EF, CF, and I geo ) indicating moderate to severe contamination of the sediment. The elevated levels of Cd (up to 92% of the total concentration) in the acid-leachable phase confirmed anthropogenic influence and are likely to pose risk to the aquatic organisms as it could mobilize from the sediment. The authors strongly recommend the need for comprehensive consideration for developing effective and efficient management policies to control TE discharge into the estuary and the associated adverse influences on the nearby environment, ecosystem, and public health.
References
Abuduwaili J, Zhang ZY, Jiang FQ (2015) Assessment of the distribution, sources and potential ecological risk of heavy metals in the dry surface sediment of Aibi Lake in Northwest China. PLoS One 10(3):e0120001. https://doi.org/10.1371/journal.pone.0120001
ANZECC and ARMCANZ (2000). Australian and New Zealand guidelines for fresh and marine water quality. Department of Environment. Canberra 2000
Antizar-Ladislao B, Mondal P, Mitra S, Sarkar SK (2015) Assessment of trace metal contamination level and toxicity in sediments from coastal regions of West Bengal, eastern part of India. Mar Pollut Bull 101(2):886–894. https://doi.org/10.1016/j.marpolbul.2015.11.014
Antizar-Ladislao B, Sarkar SK, Anderson P, Peshku T, Bhattacharya BD, Chatterjee M, Satpathy KK (2011) Baseline of butyltin contamination in sediments of Sundarban mangrove wetland and adjacent coastal regions, India. Ecotoxicology 20(8):1975–1983. https://doi.org/10.1007/s10646-011-0739-5
ATSDR. (1999). Toxicological Profile for Cadmium. Atlanta, GA: Agency for Toxic Substances and Disease Registry. Available: http://www.atsdr.cdc.gov/toxprofiles/tp5.html [accessed 19 August 2016]
Bartoli G, Papa S, Sagnella E, Fioretto A (2012) Heavy metal content in sediments along the Calore River: relationships with physical-chemical characteristics. J Environ Manag 95:9–14
Bastami KD, Neyestani MR, Esmaeilzadeh M, Haghparast S, Alavi C, Fathi S, Nourbakhsh S, Shirzadi EA, Parhizgar R (2017) Geochemical speciation, bioavailability and source identification of selected metals in surface sediments of the Southern Caspian Sea. Mar Pollut Bull 114(2):1014–1023. https://doi.org/10.1016/j.marpolbul.2016.11.025
Bastami KD, Neyestani MR, Shemirani F, Soltani F, Haghparast S, Akbari A (2015) Heavy metal pollution assessment in relation to sediment properties in the coastal sediments of the Southern Caspian Sea. Mar Pollut Bull 93:237–243
Bastami KD, Bagheri H, Kheirabadi V, Zaferani GG, Teymori MB, Hamzehpoor A, Soltani F, Haghparast S, Harami SRM, Ghorghani NF, Ganji S (2014) Distribution and ecological risk assessment of heavy metals in surface sediments along southeast coast of the Caspian Sea. Mar Pollut Bull 81(1):262–267. https://doi.org/10.1016/j.marpolbul.2014.01.029
Bhattacharya BD, Nayak DC, Sarkar SK, Naha Biswas S, Rakshit D, Ahmed MK (2015a) Distribution of dissolved trace metals in coastal regions of Indian Sundarban mangrove wetland: a multivariate approach. J Clean Prod 96:233–243. https://doi.org/10.1016/j.jclepro.2014.04.030
Bhattacharya BD, Hwang JS, Sarkar SK, Rakshit D, Murugan K, Tseng L (2015b) Community structure of mesozooplankton in coastal waters of Sundarban mangrove wetland, India: a multivariate approach. J Mar Syst 141:112–121. https://doi.org/10.1016/j.jmarsys.2014.08.018
Bhuiyan MAH, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173(1–3):384–392. https://doi.org/10.1016/j.jhazmat.2009.08.085
Canuel EA, Martens CS (1993) Seasonal variability in the sources and alteration of organic matter associated with recently deposited sediments. Org Geochem 20(5):563–577. https://doi.org/10.1016/0146-6380(93)90024-6
Chatterjee M, Canário JC, Sarkar SK, Branco V, Godhantaraman N, Bhattacharya BD, Bhattacharya A (2012) Biogeochemistry of mercury and methylmercury in sediment cores from Sundarban mangrove wetland, India—a UNESCO World Heritage Site. Environ Monit Assess 184(9):5239–5254. https://doi.org/10.1007/s10661-011-2336-8
Chatterjee M, Canário J, Sarkar SK, Branco V, Bhattacharya AK, Satpathy KK (2009) Mercury enrichments in core sediments in Hugli–Matla–Bidyadhari estuarine complex, north-eastern part of the Bay of Bengal and their ecotoxicological significance. Environ Geol 57(5):1125–1134. https://doi.org/10.1007/s00254-008-1404-z
Chatterjee M, Silva Filho EV, Sarkar SK, Sella SM, Bhattacharya A, Satpathy KK, Prasad MVR, Chakraborty S, Bhattacharya BD (2007) Distribution and possible source of trace elements in the sediment cores of a tropical macrotidal estuary and their ecotoxicological significance. Environ Int 33(3):346–356. https://doi.org/10.1016/j.envint.2006.11.013
Corsolini S, Sarkar SK, Guerranti C, Bhattacharya BD, Rakshit D, Jonathan MP, Godhantaraman N (2012) Perfluorinated compounds in surficial sediments of the Ganges River and adjacent Sundarban mangrove wetland, India. Mar Pollut Bull 64(12):2829–2833. https://doi.org/10.1016/j.marpolbul.2012.09.019
Cossa D, Buscail R, Puig P, Chiffoleau J-F, Radakovitch O, Jeanty G, Heussner S (2014) Origin and accumulation of trace elements in sediments of the northwestern Mediterranean margin. Chem Geol 380:61–73. https://doi.org/10.1016/j.chemgeo.2014.04.015
Dhanakumar S, Murthy KR, Solaraj G, Mohanraj R (2013) Heavy-metal fractionation in surface sediments of the Cauvery River Estuarine Region, South eastern coast of India. Arch Environ Contam Toxicol 65(1):14–23. https://doi.org/10.1007/s00244-013-9886-4
Ding Z, Liu J, Li L, Lin H, Wu H, Hu Z (2009) Distribution and speciation of mercury in surficial sediments from main mangrove wetlands in China. Mar Pollut Bull 58(9):1319–1325. https://doi.org/10.1016/j.marpolbul.2009.04.029
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407(13):3972–3985. https://doi.org/10.1016/j.scitotenv.2008.07.025
Facchinelli A, Sacchi E, Mallen L (2001) Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut 114(3):313–324. https://doi.org/10.1016/S0269-7491(00)00243-8
Fu J, Hu X, Tao X, Yu H, Zhang X (2013) Risk and toxicity assessments of heavy metals in sediments and fishes from the Yangtze river and Taihu lake, China. Chemosphere 93(9):1887–1895. https://doi.org/10.1016/j.chemosphere.2013.06.061
Gati G, Pop C, Brudaşcă F, Gurzău AE, Spȋnu M (2016) The ecological risk of heavy metals in sediment from the Danube Delta. Ecotoxicology 25(4):688–696. https://doi.org/10.1007/s10646-016-1627-9
Guan Q, Wang L, Pan B, Guan W, Sun X, Cai A (2016) Distribution features and controls of heavy metals in surface sediments from the riverbed of the Ningxia-Inner Mongolian reaches, Yellow River, China. Chemosphere 144:29–42. https://doi.org/10.1016/j.chemosphere.2015.08.036
Hakanson L (1980) An ecological risk index for aquatic pollution control: a sedimentological approach. J Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Han FX, Hu AT, Qin HY (1990) Fractionation and availability of added soluble cadmium in soil environment (in Chinese, with English abstract). Environ Chem 9:49–53
Herut, B., Sandler, A. (2006). Normalization methods for pollutants in marine sediments: review and recommendations for the Mediterranean. Israel Oceanographic & Limnological Research and Geological Survey of Israel IOLR Report H, 18
Issa, I.A. (2008). Investigation of heavy metals solubility and redox properties of soils, PhD Thesis, Szent István University, Gӧdӧllő, Hungary 2008, p. 14
Jonathan MP, Sarkar SK, Roy PD, Alam MA, Chatterjee M, Bhattacharya BD, Bhattacharya A, Satpathy KK (2010) Acid leachable trace metals in sediment cores from Sunderban Mangrove Wetland, India: an approach towards regular monitoring. Ecotoxicology 19(2):405–418. https://doi.org/10.1007/s10646-009-0426-y
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Kannel PR, Lee S, Kanel SR, Khan SP (2007) Chemometric application in classification and assessment of monitoring locations of an urban river system. Anal Chim Acta 582(2):390–399. https://doi.org/10.1016/j.aca.2006.09.006
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Total Environ 313(1):77–89. https://doi.org/10.1016/S0048-9697(02)00683-6
Long, E.R., Morgan, L.G. (1990). The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trends Program. NOAA Technical Memorandum NOS OMA 52. National Oceanic and Atmospheric Administration. Seattle, Washington
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19(1):81–97. https://doi.org/10.1007/BF02472006
Long ER, Field LJ, MacDonald DD (1998) Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ Toxicol Chemi 17(4):714–727. https://doi.org/10.1002/etc.5620170428
Long ER, MacDonald DD, Severn CG, Hong CB (2000) Classifying the probabilities of acute toxicity in marine sediments with empirically-derived sediment quality guidelines. Environ Toxicol Chem 19(10):2598–2601. https://doi.org/10.1002/etc.5620191028
Ma X, Zuo H, Tian M, Zhang L, Meng J, Zhou X, Min N, Chang X, Liu Y (2016) Assessment of heavy metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere 144:264–272. https://doi.org/10.1016/j.chemosphere.2015.08.026
Macdonald DD, Carr RS, Calder FD, Long ER, Ingersoll CG (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5(4):253–278. https://doi.org/10.1007/BF00118995
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environ Contam Toxicol 39(1):20–31. https://doi.org/10.1007/s002440010075
McComb JQ, Han FX, Rogers C, Thomas C, Arslan Z, Ardeshir A, Tchounwou PB (2015) Trace elements and heavy metals in the Grand Bay National Estuarine Reserve in the northern Gulf of Mexico. Mar Pollut Bull 99(1-2):61–69. https://doi.org/10.1016/j.marpolbul.2015.07.062
Memet V (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364
Mondal, P., Mendes, R.A., Jonathan, M.P., Biswas, J.K., Murugan, K, Sarkar, S.K. (2018) Seasonal assessment of trace element contamination in intertidal sediments of the meso-macrotidal Hooghly (Ganges) River Estuary with a note on mercury speciation. Mar Pollut Bull 127:117-130.
Morelli G, Gasparon M (2014) Metal contamination of estuarine intertidal sediments of Moreton Bay, Australia. Mar Pollut Bull 89(1-2):435–443. https://doi.org/10.1016/j.marpolbul.2014.10.002
Müller G (1981) Die Schwermetallbelstung der sedimente des Neckars und seiner Nebenflusse: eine Bestandsaufnahme. Chem Zeitung 105:157–164
Naha Biswas S, Godhantaraman N, Sarangi RK, Bhattacharya BD, Sarkar SK, Satpathy KK (2013) Bloom of Hemidiscus hardmannianus (Bacillariophyceae) and its impact on water quality and plankton community structure in a mangrove wetland. Clean – Soil, Air, Water 41(4):333–339. https://doi.org/10.1002/clen.201200012
Nair RR, Hashimi NH, Rao VP (1982) On the possibility of high-velocity tidal streams as dynamic barriers to longshore sediment transport: evidence from the continental shelf off the Gulf of Kutch, India. Mar Geol 47(1-2):77–86. https://doi.org/10.1016/0025-3227(82)90020-2
Nazeer S, Hashmi MZ, Malik RN (2016) Distribution, risk assessment, and source identification of heavy metals in surface sediments of River Soan, Pakistan. Clean – Soil, Air, Water 44(9999):1–10
Nyamangara J (1998) Use of sequential extraction to evaluate zinc and copper in a soil amended with sewage sludge and inorganic metal salts. Agric Ecosyst Environ 69(2):135–141. https://doi.org/10.1016/S0167-8809(98)00101-7
Rakshit D, Sarkar SK, Bhattacharya BD, Jonathan MP, Biswas JK, Mondal P, Mitra S (2015) Human-induced ecological changes in western part of Indian Sundarban megadelta: a threat to ecosystem stability. Mar Pollut Bull 99(1-2):186–194. https://doi.org/10.1016/j.marpolbul.2015.07.027
Rakshit D, Naha Biswas S, Sarkar SK, Bhattacharya BD, Godhantaraman N, Satpathy KK (2014) Seasonal variations in species composition, abundance, biomass and production rate of tintinnids (Ciliata: Protozoa) along the Hooghly (Ganges) River Estuary, India: a multivariate approach. Environ Monit Assess 186(5):3063–3078. https://doi.org/10.1007/s10661-013-3601-9
Rasmussen PE (1994) Current methods of estimating atmospheric mercury fluxes in remote areas. Environ Sci Technol 28(13):2233–2241. https://doi.org/10.1021/es00062a006
Rodriguez L, Ruiz E, Alonso-Azcarate J, Rincon J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manag 90(2):1106–1116. https://doi.org/10.1016/j.jenvman.2008.04.007
Rokade, M.A. (2009). Heavy metal burden in coastal marine sediments of North West Coast of India related to pollution. Ph.D. thesis, University of Mumbai, India
Reichelt AJ, Jones GB (1994) Trace metals as tracers of dredging activities in Cleveland Bay—field and laboratory study. Aust J Mar Freshwat Res 45(7):1237–1257. https://doi.org/10.1071/MF9941237
Rubio B, Nombela MA, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40(11):968–980. https://doi.org/10.1016/S0025-326X(00)00039-4
Sadiq M (1992) Toxic metal chemistry in the marine environment. Marcel Dekker, New York, p 390
Sarkar SK, Mondal P, Biswas JK, Kwon EE, Ok YS, Rinklebe J (2017) Trace elements in surface sediments of the Hooghly (Ganges) estuary: distribution and contamination risk assessment. Environ Geochem Health 39:1245–1258
Sarkar SK, Binelli A, Riva C, Parolini M, Chatterjee M, Bhattacharya A, Bhattacharya BD, Jonathan MP (2012) Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons (PAHS) in core sediments of Sundarban Mangrove Wetland, India. Polycycl Aromat Compd 32(1):1–26. https://doi.org/10.1080/10406638.2011.633592
Sharma SK, Subramanian V (2010) Source and distribution of trace metals and nutrients in Narmada and Tapti river basins, India. Environ Earth Sci 61(7):1337–1352. https://doi.org/10.1007/s12665-010-0452-3
Sheykhi V, Moore F (2013) Evaluation of potentially toxic metals pollution in the sediments of the Kor river, southwest Iran. Environ Monit Assess 185(4):3219–3232. https://doi.org/10.1007/s10661-012-2785-8
Silva-Filho EV, Jonathan MP, Chatterjee M, Sarkar SK, Sella SM, Bhattacharya A, Satpathy KK (2011) Ecological consideration of trace element contamination in sediment cores from Sundarban wetland, India. Environ Earth Sci 63(6):1213–1225. https://doi.org/10.1007/s12665-010-0795-9
Singh, I.B. (2007). Large rivers: geomorphology and management. A. Gupta (Ed.) pp 347–371
Spencer KL (2002) Spatial variability of metals in the inter-tidal sediments of the Medway estuary, Kent, UK. Mar Pollut Bull 44(9):933–944. https://doi.org/10.1016/S0025-326X(02)00129-7
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi Basin, India. J Hazard Mater 186(2-3):1837–1846. https://doi.org/10.1016/j.jhazmat.2010.12.081
Tahri M, Benyaich F, Bounakhla M, Bilal E, Gruffat JJ, Moutte J, Garcia D (2005) Multivariate analysis of heavy metal contents in soils, sediments and water in the region of Meknes (central Morocco). Environ Monit Assess 102(1-3):405–417. https://doi.org/10.1007/s10661-005-6572-7
Tariq SR, Shah MH, Shaheen N, Khalique A, Manzoor S, Jaffar M (2006) Multivariate analysis of trace metal levels in tannery effluents in relation to soil and water: a case study from Peshawar. Pak J Environ Manage 79(1):20–29. https://doi.org/10.1016/j.jenvman.2005.05.009
Tomlinson DC, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy metals in estuaries and the formation pollution index. Helgoländer Meeresuntersuchungen 33(1–4):566–575. https://doi.org/10.1007/BF02414780
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Bull Geol Soc Am 72(2):175–192. https://doi.org/10.1130/0016-7606(1961)72[175:DOTEIS]2.0.CO;2
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364. https://doi.org/10.1016/j.jhazmat.2011.08.051
Veerasingam S, Venkatachalapathy R, Ramkumar T (2012) Heavy metals and ecological risk assessment in marine sediments of Chennai, India. Carpath J Earth Environ Sci 7(2):111–124
Wang ZH, Feng J, Jiang T, Gu YG (2013) Assessment of metal contamination in surface sediments from Zhelin Bay, the South China Sea. Mar Pollut Bull 76(1–2):383–388. https://doi.org/10.1016/j.marpolbul.2013.07.050
Wang Z, Wang Y, Chen L, Yan C, Yan Y, Chi Q (2015) Assessment of metal contamination in coastal sediments of the Maluan Bay (China) using geochemical indices and multivariate statistical approaches. Mar Pollut Bull 99(1-2):43–53. https://doi.org/10.1016/j.marpolbul.2015.07.064
Watts MJ, Mitra S, Marriott AL, Sarkar SK (2017) Source, distribution and ecotoxicological assessment of multielements in superficial sediments of a tropical turbid estuarine environment: a multivariate approach. Mar Pollut Bull 115(1–2):130–140. https://doi.org/10.1016/j.marpolbul.2016.11.057
Watts MJ, Barlow TS, Button M, Sarkar SK, Bhattacharya B, Alam A, Gomes A (2013) Arsenic speciation in polychaetes (Annelida) and sediments from the intertidal mudflat of Sundarban mangrove wetland, India. Environ Geochem Health 35(1):13–25. https://doi.org/10.1007/s10653-012-9471-1
Xu G, Liu J, Pei S, Kong X, Hu G (2014) Distribution and source of heavy metals in the surface sediments from the near-shore area, north Jiangsu Province, China. Mar Pollut Bull 83(1):275–281. https://doi.org/10.1016/j.marpolbul.2014.03.041
Xu F, Qiu L, Cao Y, Huang J, Liu Z, Tian X, Li A, Yin X (2016) Trace metals in the surface sediments of the intertidal Jiaozhou Bay, China: sources and contamination assessment. Mar Pollut Bull 104(1-2):371–378. https://doi.org/10.1016/j.marpolbul.2016.01.019
Zahra A, Hashmi MZ, Malik RN, Ahmed Z (2014) Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah-feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci Total Environ 470:925–933
Zhang Y, Guo F, Meng W, Wang XQ (2009) Water quality assessment and source identification of Daliao river basin using multivariate statistical methods. Environ Monit Assess 152(1-4):105–121. https://doi.org/10.1007/s10661-008-0300-z
Zhang C, Yu ZG, Zeng GM, Jiang M, Yang ZZ, Cui F, Zhu M, Shen L, Hu L (2014) Effects of sediment geochemical properties on heavy metal bioavailability. Environ Int 73:270–281. https://doi.org/10.1016/j.envint.2014.08.010
Zhang J, Gao X (2015) Heavy metals in surface sediments of the intertidal Laizhou Bay, Bohai Sea, China: distributions, sources and contamination assessment. Mar Pollut Bull 98(1–2):320–327. https://doi.org/10.1016/j.marpolbul.2015.06.035
Zhang ZY, Abuduwaili J, Jiang FQ (2015) Sources, pollution statue and potential ecological risk of heavy metals in surface sediments of Aibi Lake. Northwest China Environ Sci 36(2):490–496
Zhang Z, Juying L, Mamat Z, QingFu Y (2016) Sources, identification and pollution evaluation of heavy metals in the surface sediments of Bortala River, Northwest China. Ecotox Environ Saf 126:94–101. https://doi.org/10.1016/j.ecoenv.2015.12.025
Acknowledgements
The first author Priyanka Mondal is grateful to the DST for awarding her a research fellowship. We thank the Environmental Analytical Laboratory at Southern Cross University for the support for metal analyses. The authors extend their sincere thanks and gratitude to the anonymous reviewers who have given their valuable comments and suggestions for the upgradation of the manuscript.
Funding
The research work was financially supported jointly by two research projects bearing sanction no. DST/INSPIRE Fellowship/2014/IF140943 and INT/BLR/P-8/1, sponsored by the Department of Science and Technology (DST), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Severine Le Faucheur
Rights and permissions
About this article
Cite this article
Mondal, P., Reichelt-Brushett, A.J., Jonathan, M.P. et al. Pollution evaluation of total and acid-leachable trace elements in surface sediments of Hooghly River Estuary and Sundarban Mangrove Wetland (India). Environ Sci Pollut Res 25, 5681–5699 (2018). https://doi.org/10.1007/s11356-017-0915-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0915-0