Abstract
Intensive aquaculture needs to adopt techniques that are able to contribute towards sustainability. Closed systems that employ water recirculation can combine intensive production with environmental sustainability, since there is no exchange of water or discharge of effluents into the environment. In order to achieve this, effective filtration systems are required to ensure that the water quality is satisfactory for the cultivation of aquatic organisms. Chitosan, an industrial waste material derived from crustacean farming, is a renewable natural material that is biodegradable and possesses adsorbent characteristics. In this work, chitosan foam was incorporated in filters and was evaluated as an adsorbent of aquaculture pollutants, adding value to the material and at the same time providing a use for industrial waste. The foam was characterized by scanning electron microscopy and energy dispersive spectroscopy, apparent density, and water absorption capacity. It was used to remove ammonia, nitrite, orthophosphate, and turbidity from aquaculture effluents. The foam consisted of a bilayer with smooth and porous sides, which presented low density, flexibility, and high water absorption capacity. The best proportion of the foam, in terms of the mass of foam per volume of solution (% m v−1), was 0.10, which resulted in removal of 32.8, 57.2, 89.5, and 99.9% of ammonia, nitrite, orthophosphate, and turbidity, respectively. This biopolymer produced is biodegradable, and when saturated with organic compounds from aquaculture, and no longer suitable for reuse as a filter material, it can be employed as a fertilizer, hence closing the sustainability cycle of the aquaculture production chain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Management of the waste generated from the cultivation of aquatic organisms is a key factor affecting the sustainability of aquaculture today and in future years. The intensification of aquaculture systems has led to significant increases in the loads of waste materials in the cultivation water and discharged into rivers. Nitrogen and phosphorus compounds excreted by fish can affect not only the cultivation water, but also the environment as a whole (Lazzari and Baldisserotto 2008). It is therefore essential to adopt cultivation techniques that ensure the maintenance of water quality, minimize discharges of aquaculture effluents, and at the same time enable intensive production.
Closed systems with water recirculation can make intensive production compatible with environmental sustainability (Martins et al. 2010). In these systems, water passes through the fish tanks and is then treated using mechanical and biological filters, before being pumped back to the tanks. In this way, it is possible to maintain a high flow of water through the system, without the discharge of pollutants into the environment. Such systems are safer, economically viable, and ecologically correct (Bregnballe 2015).
The production of aquatic organisms using water recirculation requires efficient filtration systems that maintain water quality at satisfactory levels for the organisms concerned. The wastes generated in aquaculture include unconsumed food and products of fish metabolism released in the aquatic environment, leading to increases in the concentrations of nitrogen and phosphorus in the water (Mallasen et al. 2008). When the concentration of ammonia in the water increases, there is a decrease in its excretion by aquatic organisms, hence increasing the internal ammonia concentration, which can exceed tolerable levels and lead to mortality (Arana 2010). For fish, lethal values of total ammonia (NH3 + NH4 +) are in the range 2.0–3.0 mg L−1, while for toxic ammonia (NH3), the lethal value is 0.20 mg L−1 (Moro et al. 2013).
Aquatic organisms can also be exposed to toxic levels of nitrite originating from the oxidation of ammonia, in oxidizing environments, and the reduction of nitrate, in reducing environments. In closed systems with high stocking densities, nitrite can rapidly reach lethal levels exceeding 11.65 mg L−1 (Yanbo et al. 2006).
High concentrations of nitrogen and phosphorus in the water favor the proliferation of algae and aquatic plants, leading to eutrophication, deterioration of water quality, and profound changes in the structure of aquatic communities, which can compromise the stability of the ecosystem (Mallasen et al. 2008).
Eutrophic environments favor the development of cyanophytic algae, many of which release harmful toxins, while others produce metabolites such as geosmin and 2-methylisoborneol, which can cause earthy tastes or odors in fish products. High concentrations of these algae in cultivation water reduce the quality of fish meat, decreasing its consumption and causing negative impacts on the marketing of farmed fish, which can render such enterprises unfeasible (Mallasen et al. 2008). It is therefore necessary to maintain nutrient concentrations in the water at low levels, both during the cultivation of aquatic organisms and in the effluents released to the environment.
Biopolymers are a promising class of bioadsorbents that can be used for the removal of organic and inorganic pollutants from the aquatic environment. Chitosan, a biopolymer derived from chitin, has been widely used as a bioadsorbent in aqueous media (Favere et al. 2010). Crustacean shells, which are abundant as waste products from the fishery industry, are rich in the chitin, from which chitosan can be obtained. The use of these shells can help to reduce environmental impacts associated with their accumulation (Azevedo et al. 2007).
The chitosan biopolymer consists of β-(1 → 4)-2-amino-2-deoxy-D-glucopyranose units obtained from the partial deacetylation of the chitin biopolymer, which consists of β–(1 → 4)-2-acetamide-2-deoxy-D-glucopyranose units, extracted from the exoskeletons of crustaceans and the cell walls of fungi (Silva et al. 2011; Lucena et al. 2015; Gaouar Yadi et al. 2015).
This biopolymer has several important characteristics that favor its use, including nontoxicity, biodegradability, biocompatibility, and bioactivity (Jabbar et al. 2014). Its adsorptive capacity is due to the large number of amino and hydroxyl functional groups present in its structure, with the amino groups acting as coordination sites (Silva et al. 2011). Studies have described the use of chitosan as an adsorbent, flocculant, and/or coagulant of aquaculture industry pollutants, in the treatment of waste waters from fish farming (Chung et al. 2005; Chung 2006) and the fish processing industry (Garcia et al. 2016).
Chitosan is an industrial waste that is natural, renewable, biodegradable, and possesses adsorbent characteristics. This work evaluates its use in the form of a foam included as a structural component of a filter, hence adding value to the material and at the same time helping to reduce waste generation. The presence of chitosan as a flexible foam, rather than as a powder, facilitates its use as an adsorbent for pollutants in aquaculture systems.
Materials and methods
The chitosan used in the filter was dried according to the foam layer method protected by patent application number BR1020150292597 (Muniz et al. 2015). This process provided the chitosan with a support, so that it could be used in the filtration equipment without loss of the adsorbent material into the medium to be filtered. The foam produced had one smooth surface and one porous surface (Fig. 1).
Methods for determining pollutants in the effluents
Analyses were performed to determine total ammonia by the indophenol method (Koroleff 1976), nitrite by the Griess reaction (Baumgarten 1996), and dissolved orthophosphate by the ascorbic acid method (American Public Health Association (APHA) 2005). The absorbance measurements employed a Belphotonics SP2000UV spectrophotometer. The pH was measured with a Kasvi AI03449 pH meter, and turbidity was measured with a turbidimeter (AP2000, Policontrol).
Calculation of pollutant removal efficiencies
The percentage removal of a pollutant from the solution was calculated according to Eq. 1.
Where:
- Ic:
-
Initial concentration (mg L−1)
- Fc:
-
Final concentration (mg L−1)
Pollutant adsorption capacities
The adsorption capacities (Q) of the chitosan foam for the pollutants were determined according to Eq. 2.
Where:
- Q:
-
Adsorption capacity (mg g−1)
- Ic:
-
Initial concentration (mg L−1)
- Fc:
-
Final concentration (mg L−1)
- V:
-
Volume of solution (L)
- m:
-
Adsorbent mass (g)
Characterization of the foams
Scanning electron microscopy and energy dispersive spectroscopy
Both surfaces (smooth and porous) of the chitosan foams were characterized by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS). The foams were evaluated before use in the adsorption tests, and then after their use in the dissolved orthophosphate adsorption assay.
SEM was used to obtain information concerning the structural morphology of the chitosan foam, by acquiring high-resolution three-dimensional images of the samples at high magnification. The EDS system coupled to the SEM enabled qualitative and semiquantitative determination of the chemical compositions of the samples, from the emission of characteristic X-rays.
The chitosan foam samples were attached to sample holders using double-sided carbon tape and were then dried and metalized with a thin layer of gold on the surface (sputter-coating). The analyses employed a VEGA3 instrument (TESCAN). Micrographs were obtained at different magnifications, in secondary electron (SE) mode. The chemical compositions of microregions of the samples were analyzed by EDS (X-act, Oxford Instruments).
Apparent density
Foam samples were cut out, measured with a pachymeter, and weighed using an electronic balance with a precision of 0.001 g. The bulk density of the material was expressed as the total mass (g) of the foam sample per unit area (cm3). The determinations were repeated three times.
Water absorption capacity
Samples of chitosan foam were cut out, weighed, and then immersed in 1000 mL of distilled water for 1 h. The water absorption capacity of the foam was expressed as the volume (mL) of water absorbed per gram (g) of foam. The determinations were performed in triplicate.
Adsorption of dissolved orthophosphate
Synthetic solutions containing 1.01 mg L−1 of dissolved orthophosphate with pH 7.1 were prepared from a stock solution of potassium phosphate. Chitosan foam was added to 1-L volumes of the solution at different percentages (0.10, 0.25, 0.50, and 0.75% m v−1). The solutions were kept under agitation at the temperature of 25 °C and water was collected at times of 1, 5, 10, 15, 30, 60, 90, 120, 180, and 240 min for determination of the dissolved orthophosphate concentration. The efficiency of extraction of dissolved orthophosphate by the chitosan foam was calculated using Eq. 1.
Adsorption of nitrite
Synthetic solutions containing 0.64 mg L−1 of nitrite with pH 6.8 were prepared from a stock solution of sodium nitrite. Different percentages of chitosan foam (0.10, 0.25, 0.50, and 1.00% m v−1) were added to 1-L volumes of these solutions. The solutions were kept under agitation. The solutions were kept under agitation at the temperature of 25 °C and water was collected at times and water samples were collected at 10, 15, 30, 60, 120, and 180 min for determination of the nitrite concentration. The efficiency of removal of nitrite by the chitosan foam was calculated using Eq. 1.
Adsorption of total ammonia, nitrite, and dissolved orthophosphate
Synthetic solutions were prepared containing 1.06 mg L−1 of nitrite, 1.06 mg L−1 of dissolved orthophosphate, and 0.20 mg L−1 of ammonia, with pH 6.9. Different percentages of chitosan foam (0.10, 0.25, 0.50, and 1.00% m v−1) were added to 1-L volumes of these solutions. The solutions were kept under agitation The solutions were kept under agitation at the temperature of 25 °C and water was collected at times and water samples were collected at times of 0, 10, 15, 30, 60, 120, and 180 min for determination of the concentrations of total ammonia, nitrite, and dissolved orthophosphate. The efficiency of removal of each pollutant was calculated using Eq. 1, and the adsorption capacity was calculated using Eq. 2.
Fabrication of the filters
For the removal (adsorption) of pollutants from water, filters filled with chitosan foam (Fig. 2) were prepared according to the procedure described under patent application number BR10 20170138070 (Muniz et al. 2017). The filter was constructed of tubes and sections of polyvinyl chloride (PVC). As shown in Fig. 2, 1 × 1-mm mesh plastic screens [2 and 6] were attached to the inlet and outlet ends [1 and 7] in order to retain the adsorbent material. Glass beads with diameter of 0.5 mm [3 and 5] were placed immediately after the screens in order to avoid compaction of the adsorbent, and chitosan foam sections measuring 10 × 10 × 5 mm [4] were placed in the center.
Adsorption of ammonia using the filter
Adsorption of ammonia using different percentages of chitosan foam per volume of effluent (% m v−1)
Filters filled with chitosan foam at different percentages relative to the volume of aqueous effluent contaminated with ammonia (0.05, 0.10, 0.15, and 0.20% m v−1) were coupled separately to tanks with working volumes of 8 L (Fig. 3). A submerged electric pump was used to recirculate water contaminated with ammonia at an initial concentration of 0.25 mg L−1 (pH 7.0, 26 °C) in the first use of the foam, and at an initial concentration of 0.15 mg L−1 (pH 7.0, 26 °C) when the foam was reused after drying at 45 °C.
The filters filled with foam corresponding to 0.05% (m v−1) contained a foam column 6 cm high and 4 cm in diameter, with a volume of 75 cm3, and the flow rate was 113 L h−1. In the case of the 0.10% (m v−1) filter, the column was 12 cm high and 4 cm in diameter, with a volume of 150 cm3, and the flow rate was 37 L h−1. For the 0.15% (m v−1) filter, the column was 18 cm high and 4 cm in diameter, with a volume of 225 cm3, and the flow rate was 12 L h−1. In the filter filled at 0.20% (m v−1), the column was 24 cm high and 4 cm in diameter, with a volume of 300 cm3, and the flow rate was 5 L h−1.
For drying, the foams were removed from the filters and placed in an oven at 45 °C for 24 h and were then returned to their original filters. After initiation of the recirculation, water samples were collected at 1, 5, 10, 30, 90, 120, 1140, 1200, 1440, 1560, 2160, and 2500 min for determination of the ammonia concentration. The efficiency of removal of ammonia was calculated using Eq. 1.
Adsorption of ammonia from synthetic effluents containing different initial ammonia concentrations
Synthetic effluent solutions were prepared containing different initial concentrations of ammonia (0.24, 0.37, 0.47, and 1.11 mg L−1, pH 7.0, 26 °C), and filters containing chitosan foam at a proportion of 0.1% (m v−1) were coupled to the tanks (8-L working volume). The water was recirculated using a submerged aquarium pump. After initiation of recirculation, water samples were collected at times of 1, 5, 10, 30, 90, 120, 300, 360, 420, 480, 1140, 1200, 1380, 1440, 1500, and 1560 min for determination of the ammonia concentration. The efficiency of removal of ammonia was calculated using Eq. 1.
Removal of turbidity
The removal of turbidity was evaluated using a sample of pisciculture water with 155.6 ± 7.4 NTU and pH 5.8 to which masses of foam equivalent to 0.05 and 0.10% (m v−1) were added, in triplicate. The removal of turbidity was evaluated after 24 and 48 h of contact, at 25 °C, with agitation at 100 rpm using a shaker (SP-223, SP Labor). The turbidity was measured using the AP2000 turbidimeter (Policontrol).
Results and discussion
Characterization of the foams
The use of adsorption is a sustainable alternative for water treatment where low-cost agroindustrial waste is readily available, adding value to the agroindustrial production chain and providing a new destination for these solid wastes (Coelho et al. 2014). Ali et al. (2013) highlighted that the main advantages of chitosan are its biodegradability, nontoxicity, rapid settling velocity, low dosage requirement, high removal efficiency, and low cost.
Scanning electron microscopy and energy dispersive spectroscopy
The SEM micrographs showed that on their porous surfaces, the chitosan foams possessed an internal region formed by interconnecting cells of different sizes (Fig. 3).
Chitosan is one of the most promising materials available for use as an adsorbent. The presence of amino and hydroxyl groups in the molecules enables many possible adsorption interactions between chitosan and pollutants (Kyzas and Bikiaris 2015). The presence of nitrogen only on the porous surface of the chitosan foam confirmed the existence of amino functional groups characteristic of the structure of the chitosan biopolymer (Table 1).
Adsorption can be classified as physical or chemical (Nascimento et al. 2014). In this study, the energy dispersive spectroscopy analyses of the surfaces of the chitosan foam confirmed that the adsorption of dissolved orthophosphate occurred on the porous surface side (Table 1), indicative of chemical adsorption. Chemical adsorption involves the exchange or sharing of electrons between the adsorbate molecules and the surface of the adsorbent, resulting in a chemical reaction. Chemical adsorption is specific and occurs due to the presence of active sites capable of chemically adsorbing the adsorbate. In physical adsorption, the binding of the adsorbate to the surface of the adsorbent involves weak nonspecific interactions that occur throughout the surface of the adsorbent (Nascimento et al. 2014).
The interaction between chitosan and the foaming agent resulted in the formation of a foam with suitable structure for use as a filtration agent, since the less porous layer retained water for a sufficiently long time for contact to occur between the adsorbates and the chitosan amino functional groups responsible for the adsorption.
Apparent density
Foams have characteristic properties resulting from the nature of the polymer, the apparent density, and the morphology. The apparent density governs mechanical properties such as resistance to compression, determined using a deformation of 10%. The polymer is responsible for resistance to high temperatures, chemicals, and fire. The morphology influences thermal and acoustic properties of the material. Both the morphology and the nature of the polymer govern the absorption and diffusion of water. Flexible foams are composed of open cells that are permeable to air and show reversible deformation and limited resistance, while rigid foams have closed cells, reduced permeability, and low resilience (Carvalho and Frollini 1999).
The chitosan foam used in the experiments had a low apparent density of 0.047 ± 0.002 g cm−3, characteristic of a flexible foam. According to Pereira et al. (2014), the mechanical properties of chitosan are weaker, compared to polymers derived from petroleum.
Water absorption capacity
The water absorption capacity of the chitosan foam was found to be 15.45 ± 1.50 mL g−1. This high value could be attributed to the capillarity mechanism whereby water could flow into the internal spaces formed during the processing of the material, favoring its application in the adsorption of pollutants present in aquaculture water.
Adsorption of ammonia
Ammonia adsorption assays using different percentages of chitosan foam mass per effluent volume (% m v−1)
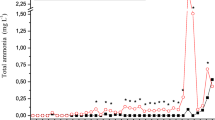
It was observed that higher percentages of chitosan foam (% m v−1) resulted in greater efficiency of removal of ammonia from the effluent (Fig. 4), due to the increased surface area of the adsorbent and the availability of more adsorption sites. The use of 0.20% chitosan foam adsorbed (removed) 67% of the ammonia from the effluent after 1800 min of recirculation, with 0.08 mg L−1 of ammonia remaining in the effluent.
At elevated concentrations, ammonia can be an environmental problem due to its toxicity, especially to aquatic organisms. Its levels in aquatic systems can be increased due to agricultural runoff and the decomposition of biological wastes. This can lead to impaired excretion of ammonia by aquatic organisms, resulting in increased internal concentrations of ammonia in the animals and a cascade of intracellular reactions that end in the death of the organism. It has been suggested that high concentrations of ammonia displace K+ and depolarize neurons, causing excessive activation of NMDA (N-methyl-D-aspartate) type receptors, leading to excessive influx of Ca2+ and subsequent death of cells in the central nervous system (Randall and Tsui 2002).
In order to reuse the chitosan foam for adsorption, it was heated for volatilization of the ammonia. Ammonia is continuously volatilized from the Earth’s surface, entering the atmosphere, where it is destroyed by photolytic reactions (Eddy 2005). Therefore, the procedure employed here did not cause any subsequent environmental harm.
When the chitosan foam was reused (Fig. 5), the removal efficiencies were lower, compared to those obtained with the fresh foam. Foams reused using a proportion of 0.05% (m v−1) provided the highest removal efficiency of 20%, with 0.12 mg L−1 of ammonia remaining in the effluent.
Since the chitosan biopolymer is biodegradable and the adsorbed ammonia is a nutrient, the saturated adsorbent could be used as an agricultural fertilizer (Haseena et al. 2016).
Adsorption assays using synthetic effluents with different initial ammonia concentrations
Higher removal efficiencies were obtained at lower initial ammonia concentrations. The use of 0.10% (m v−1) chitosan foam resulted in 43, 54, 47, and 22% removal of ammonia from synthetic effluents with initial ammonia concentrations of 0.24, 0.37, 0.47, and 1.11 mg L−1, respectively, after 1500 min of recirculation (Fig. 6).
Adsorption of dissolved orthophosphate
Faster and more efficient removal of dissolved orthophosphate was obtained using a dosage of 0.50% foam mass per volume of solution (m v−1). However, a dosage of 0.10% was equally effective after 240 min of agitation (Fig. 7). The adsorption was due to interaction of the protonated amino groups of chitosan with the phosphate ions present in the solution (Srinatha et al. 2008).
Adsorption of nitrite
Use of chitosan foam at 0.10% (m v−1) resulted in adsorption of 63% of the nitrite present in the water after 10 min of agitation, and the same efficiency was maintained up to 180 min. A 0.25% (m v−1) dosage of chitosan foam resulted in 73% adsorption of nitrite after 30 min, while a 0.50% (m v−1) dosage provided 72% removal after 180 min. Use of 1.00% (m v−1) chitosan foam resulted in adsorption physical of 78% of the nitrite only after 120 min (Fig. 8).
The dominant mechanism in the adsorption of nitrite by reticulated chitosan and the intermolecular physical adsorption caused by the van der waals force (simple electrostatic attraction). In addition to the physical adsorption, the cross-linked chitosan also has some unique adsorption mechanisms, where the crosslinked chitosan-NH2 can be protonated in crosslinked chitosan-NH3 + as coordination forms or where amino groups form the charge transfer complex with nitrite in acidic condition. Adsorption is influenced by external conditions such as pH, temperature, etc. (Jing et al. 2008).
Chitosan is a promising biopolymer for use as a biofilter for the fixation of nitrite-oxidizing bacteria (NOB). The immobilization of NOB on the surface of chitosan flakes has been shown to offer excellent potential for the removal of excess nitrite from aquaculture wastewater. NOB immobilized on chitosan with 91% deacetylation removed 0.82 ± 0.05 mg-N/(g·day) of nitrite, while NOB immobilized on chitin removed 0.44 ± 0.03 mg-N/(g·day) of nitrite (Lertsutthiwong et al. 2013).
Adsorption of ammonia, nitrite, and dissolved orthophosphate
When a dosage of 0.10% (m v−1) of chitosan foam was used, linear adsorption equilibrium was not observed for the removal of ammonia, with adsorption/desorption behavior that was probably due to competition of other pollutants present in the solution for the active sites of the material.
The removal of nitrite reached equilibrium after 30 min, while orthophosphate removal reached equilibrium after 60 min. Maximum removal percentages of 32.8, 57.2, and 89.5% were obtained for ammonia, nitrite, and orthophosphate, respectively (Table 2).
The use of 0.25% (m v−1) chitosan foam did not result in linear adsorption equilibrium for ammonia. The removal processes of nitrite and orthophosphate presented equilibria after 60 and 30 min, respectively. Use of the foam at 0.50% (m v−1) resulted in removal equilibria after 120, 30, and 60 min for ammonia, nitrite, and orthophosphate, respectively, while use of a 1.00% (m v−1) dosage of chitosan foam resulted in removal equilibria for ammonia, nitrite, and orthophosphate after 10 min.
The highest adsorbed amount was achieved for orthophosphate ions (0.96 mg g−1) (Table 2), using a foam dosage of 0.10% (m v−1). The results indicated that the chitosan foam structure had greatest affinity for dissolved orthophosphate, followed by nitrite and ammonia.
Similar results for orthophosphate removal were reported by Chung (2006), who used chitosan powder and obtained removals of 95.6% (PO4 3−), 89.2% (NH3), 69.7% (organic matter), and 61% (solids) from Anguilla japonica cultivation wastewater. The residual concentrations of PO4 3− (0.23 mg L−1) and NH3 (0.5 mg L−1) were considered acceptable for discharge into natural waters or for reuse of the water in the cultivation. The chitosan also provided selective removal of bacteria, since it did not affect nitrifying bacteria, but was effective against Edwardsiella ictaluri, hence offering an advantage over traditional bactericides.
Higher dosages of the chitosan foam resulted in lower adsorption capacities for the pollutants. Chitosan has a high charge density, resulting in destabilization of the particles at lower dosages. At a high dosage, an excess of adsorbates on the colloidal surfaces leads to colloid re-stabilization, with no sites available on the surfaces of the particles for the formation of inter-particle bonds (García et al., 2016) (Table 3).
Removal of turbidity
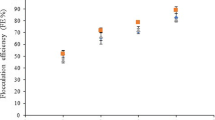
The use of 0.1% (m v−1) of chitosan foam was most effective in eliminating turbidity from the pisciculture water, with an efficiency of 99.9%.
The use of natural coagulants such as chitin and chitosan for turbidity removal is growing, as these are highly versatile, biodegradable, derived from renewable resources for the benefit and cost. The use of 100 mg L−1 of any of these coagulants is sufficient for efficient turbidity removal (96.7%) (Comiotto et al. 2014).
The use of chitosan powder or beads is efficient in the removal of aquatic pollutants (Chung 2006; Jing et al. 2008; Comiotto et al. 2014), but its recovery or withdrawal from the aquatic environment is difficult. It can be observed that even the active chitosan component is structured as a foam, it remains efficient in the treatment of effluents, it can be used in easily maintained filter being and is an advantage over other materials.
Conclusions
The chitosan composite foam consisted of a flexible low density bilayer with porous and smooth surfaces, which provided a high water absorption capacity.
The best dosage (% m v−1) of the foam in the adsorption filter was 0.10, resulting in efficient adsorption of ammonia, nitrite, orthophosphate, and turbidity, showing the potential of this material for the clean-up of water contaminated by aquaculture activities.
This biopolymer produced from a waste raw material is biodegradable, and when saturated with organic aquaculture compounds and no longer suitable for reuse as a filter material, it can be employed as a fertilizer, hence closing the sustainability cycle of the aquaculture production chain.
References
American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater. 4500-P E. Ascorbic acid method. 21ª ed. Washington, pp.4–153
Ali ZM, Laghari AJ, Ansari AK, Khuhawar MY (2013) Extraction and Characterization of Chitosan from Indian Prawn (Fenneropenaeus indicus) and its Applications on Waste Water Treatment of Local Ghee Industry. IOSR J Engineering (IOSRJEN) 3(10):28–37, e-ISSN: 2250–3021. Available in: http://www.iosrjen.org/Papers/vol3_issue10%20(part-2)/D031022837.pdf. https://doi.org/10.9790/3021-031022837
Arana, L.V., 2010. Qualidade da água em aquicultura: princípios e práticas. 3. ed. rev. e modif. Ed. da UFSC, 238 p., Florianópolis, SC, Brazil, ISBN13:9788532804891
Azevedo VVC, Chaves AS, Bezerra DC, Lia Fook MV, Costa ACFM (2007) Quitina e Quitosana: aplicações como biomateriais. Revista Eletrônica de Materiais e Processos 2(3):27–34 2007. ISSN 1809–8797. Available in: http://www2.ufcg.edu.br/revista-remap/index.php/REMAP/article/viewFile/46/81
Baumgarten, M.G.Z., 1996. Manual de análises em oceanografia química. Ed. Furg,132p., Rio Grande, Rio Grande do Sul, Brazil, ISBN: 858504246X
Bregnballe, J., 2015. A Guide to Recirculation Aquaculture: An introduction to the new environmentally friendly and highly productive closed fish farming systems. Food and Agriculture Organization of the United Nations (FAO) and EUROFISH International Organisation, 100 p., ISBN 978–92–5-108776-3. Available in: <http://www.fao.org/3/a-i4626e.pdf> Acesso em: 11/08/2016
Carvalho, G., Frollini, E., 1999. Lignina em espumas fenólicas. Polímeros, 9 (1). Available in: https://doi.org/10.1590/S0104-14281999000100009
Chung YC, Li YH, Chen CC (2005) Pollutant removal from aquaculture wastewater using the biopolymer chitosan at different molecular weights. J Environ Sci Heal A 40(9):1775–1790. https://doi.org/10.1081/ESE-200068058
Chung YC (2006) Improvement of aquaculture wastewater using chitosan of different degrees of deacetylation. Environ Technol 27(11):1199–1208. https://doi.org/10.1080/09593332708618734
Coelho GF, Gonçalves AC Jr, Sousa RFB, Schwantes D, Miola AJ, Domingues CVR (2014) Use of adsorption techniques utilizing agroindustrial waste in the removal of contaminants in Waters. J Agronomic Sci 3:291–317. Avaliable in: http://www.dca.uem.br/V3NE/21.pdf
Comiotto, C.E.G., Lopes, M.A., Dotto, G.L., Pinto, L.A.A., 2014. Remoção de turbidez e sólidos totais de efluentes do processo de obtenção de quitina. Blucher Chemical Engineering Proceedings, 1(1). https://doi.org/10.5151/chemeng-cobec-ic-01-ea-024
Eddy FB (2005) Ammonia in estuaries and effects on fish. J Fish Biol 67(6):1495–1513. https://doi.org/10.1111/j.1095-8649.2005.00930.x
Favere, V.T., Riella, H.G., Rosa, S., 2010. Cloreto de n-(2-hidroxil) propil-3-trimetil amônio quitosana como adsorvente de corantes reativos em solução aquosa. Química Nova [online]. 33(7), 1476–1481, ISSN 0100–4042. Avaliable in: https://doi.org/10.1590/S0100-40422010000700010
Gaouar Yadi, M., Benguella, B., Gaouar-Benyelles, N., Tizaoui, K., 2015. Adsorption of ammonia from wastewater using low-cost bentonite/chitosan beads. Desalination and Water Treatment, ISSN: 1944–3994 (Print) 1944–3986 (Online). https://doi.org/10.1080/19443994.2015.1119747
García, M.A., Montelongo, I., Rivero, A., de la Paz, N., Fernández, M., et al., 2016. Treatment of Wastewater from Fish Processing Industry using Chitosan Acid Salts. Int Water Wastewater Treat 2(2), Avaliable in: 10.16966/2381-5299.121
Haseena PV, Padmavathy KS, Rohit Krishnan P, Madhu G (2016) Adsorption of ammonium nitrogen from aqueous systems using chitosan-Bentonite film composite. Procedia Technol 24:733–740. https://doi.org/10.1016/j.protcy.2016.05.203
Jabbar Z, Angham A, Sami GHF (2014) Removal of azo dye from aqueous solutions using chitosan. Oriental Journal of Chemistry 30(2): 571-575. https://doi.org/10.13005/ojc/300222
Jing L, Kaixuan T, Zhipan G (2008) Study on the Adsorption of Nitrite in Water with Crosslinked Chitosan. 2nd International Conference on Bioinformatics and Biomedical Engineering ICBBE ’08, Shanghai, pp. 3673–3676. https://doi.org/10.1109/ICBBE.2008.420
Kyzas GZ, Bikiaris DN (2015) Recent modifications of chitosan for adsorption applications: a critical and systematic review. Mar Drugs 13(1):312–337. https://doi.org/10.3390/md13010312
Koroleff, F., 1976. Determination of nutrients. In Methods of seawater analysis (K. Grasshoff, ed.). Verlag Chemie Weinheim, New York, p.117–181
Lazzari R, Baldisserotto B (2008) Nitrogen and phosphorus waste in fish farming. Bol. Inst. Pesca 34(4):591–600 Avaliable in: http://www.pesca.sp.gov.br/34_4_591-600.pdf
Lertsutthiwong P, Boonpuak D, Pungrasmi W, Powtongsook S (2013) Immobilization of nitrite oxidizing bacteria using biopolymeric chitosan media. J. Environ. Sci 25(2):262–267, Avaliable in. https://doi.org/10.1016/S1001-0742(12)60059-X
Lucena GL, Silva AG, Honório LMC, Santos VD (2015) Avaliação da Capacidade de Adsorção da Quitosana Quaternizada na Remoção de Íons Cu2+ e Cr3+. Rev. Virtual Quim 7(6):2166–2179, ISSN: 1984–6835. https://doi.org/10.5935/1984–6835.20150128
Mallasen M, Barros HP, Yamashita EY (2008) Produção de peixes em tanques-rede e a qualidade da água. Revista Tecnologia & Inovação Agropecuária 1(1):47–51. Avaliable in: http://www.apta.sp.gov.br/publicacoes/T&IA/T&IAv1n1/Revista_Apta_Artigo_Qualidade_de_Agua.pdf
Martins CIM, Edinga EH, Verdegema MCJ, Heinsbroeka LTN, Schneiderc O, Blanchetond JP, Roque D’orbcasteld E, Verretha JAJ (2010) New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquacultural Engineering 43(3):83–93, Avaliable in. https://doi.org/10.1016/j.aquaeng.2010.09.002
Moro, G. V., Torati, L. S., Luiz, D. B., Matos, F. T., 2013. Monitoramento e manejo da qualidade da água em pisciculturas. In: LIMA, A. F. (Org). Piscicultura de água doce: multiplicando conhecimentos. Brasília, DF: Embrapa, 2013. 440p
Muniz, G. I. B., Masson, M. L., Ellendersen, L. S. N., Alves, H.J., 2015. Patente: Privilégio de Inovação. Número do registro: BR1020150292597: "Uso e obtenção de espuma seca e pó de quitosana e nanoquitosana por processo de secagem pelo método de camada de espuma", Instituição de registro: INPI - Instituto Nacional da Propriedade Industrial. Depósito: 23/11/2015, Brasil, 2015
Muniz, G. I. B.; Ellendersen, L. S. N.; Alves, H. J.; Zadinelo, I.V.; Santos, L. D. dos Milinsk, M. C.; Feroldi, M., 2017. Patente: Privilégio de Inovação. Número do registro: BR1020170138070: "Equipamento - filtro a base de espuma de quitosana e/ou nanoquitosana", Instituição de registro: INPI - Instituto Nacional da Propriedade Industrial. Depósito: 26/06/2017, Brasil, 2017
Nascimento, R.F., Lima, A.C.A., Vidal, C.B., Melo, D.Q., Raulino, G.S.C., 2014. Adsorção: aspectos teóricos e aplicações ambientais. Fortaleza: Imprensa Universitária, 2014. 256 p. ISBN: 978–85–7485-186-0
Pereira FV, Paula EL, Mesquita JP, Lucas AA, Mano V (2014) Bionanocompósitos preparados por incorporação de nanocristais de celulose em Polímeros biodegradáveis por meio de evaporação de solvente, automontagem ou Eletrofiação. Quim. Nova 37(7):1209–1219. https://doi.org/10.5935/0100-4042.20140141
Randall, D. J., Tsui, T. K. N., 2002. Ammonia toxicity in fish. Marine Pollution Bulletin 45, 17–23. Avaliable in: https://doi.org/10.1016/S0025-326X(02)00227-8
Srinatha A, Pandit JK, Singh S (2008) Ionic cross-linked chitosan beads for extended release of ciprofloxacin: In vitro characterization. Indian J Pharm Sci 70(1):16–21. https://doi.org/10.4103/0250-474X.40326
Silva APO, Melo JV, Melo JLS, Pedroza MM (2011) Remoção de íons chumbo (Pb2+) de efluentes sintéticos através de adsorção em vermiculita revestida com quitosana. Revista Liberato, Novo Hamburgo 12(17):01–106. Available in:http://www.liberato.com.br/sites/default/files/arquivos/Revista_SIER/v.%2012%2C%20n.%2017%20%282011%29/3.%20Remo%E7%E3o%20de%20%CDons.pdf
Yanbo W, Wenju Z, Weifen L, Zirong X (2006) Acute toxicity of nitrite on tilapia (Oreochromis niloticus) at different external chloride concentrations. Fish Physiol Biochem 32(1):49–54. https://doi.org/10.1007/s10695-005-5744-2
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Highlights
• The use of adsorption is a sustainable alternative for water treatment
• Filters filled with chitosan foam adsorb aquaculture pollutants
• Higher dosages of the chitosan foam resulted in lower adsorption of the pollutants
• The best dosage (% m v−1) of the foam in the adsorption filter was 0.10
Rights and permissions
About this article
Cite this article
Zadinelo, I.V., dos Santos, L.D., Cagol, L. et al. Adsorption of aquaculture pollutants using a sustainable biopolymer. Environ Sci Pollut Res 25, 4361–4370 (2018). https://doi.org/10.1007/s11356-017-0794-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0794-4