Abstract
The effect of metals on environmental health is well documented and monitoring these and other pollutants is considered an important part of environmental management. Developing countries are yet to fully appreciate the direct impacts of pollution on aquatic ecosystems and as such, information on pollution dynamics is scant. Here, we assessed the temporal and spatial dynamics of stream sediment metal and nutrient concentrations using contaminant indices (e.g. enrichment factors, pollution load and toxic risk indices) in an arid temperate environment over the wet and dry seasons. The mean sediment nutrient, organic matter and metal concentration were highest during the dry season, with high values being observed for the urban environment. Sediment contaminant assessment scores indicated that during the wet season, the sediment quality was acceptable, but not so during the dry season. The dry season had low to moderate levels of enrichment for metals B, Cu, Cr, Fe, Mg, K and Zn. Overall, applying the sediment pollution load index highlighted poor quality river sediment along the length of the river. Toxic risk index indicated that most sites posed no toxic risk. The results of this study highlighted that river discharge plays a major role in structuring temporal differences in sediment quality. It was also evident that infrastructure degradation was likely contributing to the observed state of the river quality. The study contributes to our understanding of pollution dynamics in arid temperate landscapes where vast temporal differences in base flow characterise the riverscape. Such information is further useful for contrasting sediment pollution dynamics in aquatic environments with other climatic regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution and land degradation is increasing globally and is having a detrimental impact on the environment (Sekabira et al. 2010; Xiao et al. 2011; Clark et al. 2015). In the developing world, population growth and poor environmental practices are the main drivers of degradation processes (Bilsborrow 1992; Bulte and Van Soest 2001), with metal pollutants identified as being particularly problematic given their association with toxic risk for environmental health i.e. the aspects of natural and built environment factors that may affect animal health (Sekabira et al. 2010). Metal pollutants are capable of accumulating in organisms thereby affecting food webs and posing both direct or indirect threats to the ecosystem health (Fatoki and Awofolu 2003; Bere et al. 2016; Zhang et al. 2016). While the effects of metals on environmental health have been well documented in developed regions, developing countries are yet to fully realise the direct impacts of industrial growth and poor land use practices on aquatic ecosystems (Fatoki and Awofolu 2003; de Paula Filho et al. 2015).
River sediment provides an essential habitat for organisms and plays an important role in a healthy ecosystem (Murray et al. 1999; de Paula Filho et al. 2015; Bere et al. 2016). Under natural conditions, these environments are integral in sink and source dynamics for various biotic and abiotic processes (Burton 2002; Bai et al. 2014, Leroy et al. 2017). As such, these habitats play an important role in hydrological and ecological processes in rivers and streams. Sediment can also provide insight into the long-term pollution history and catchment activities of a given aquatic ecosystem, as they are often sinks for nutrients, organic chemicals, pathogens and metals (Burton 2002; Bai et al. 2014; de Paula Filho et al. 2015; Akele et al. 2016; Coxon et al. 2016). If the contaminant loading into the aquatic ecosystems is sufficiently large, the sediments may accumulate excessive quantities of contaminants that directly and indirectly disrupt the ecosystem, causing significant deterioration in water quality and species loss (Burton 2002; Bere et al. 2016). Water quality is heavily influenced by sediment quality and the surrounding environs (Awofolu et al. 2005). Metals are important considerations in this regard. Under certain conditions, these elements do not always dilute and flow away, but rather settle out of the water column and end up accumulating in the sediment (Murray et al. 1999, Clark et al. 2015; Bai et al. 2011, 2016). Given that sediments are not only metal sinks but also a source of organic matter from re-suspension, sediment-bound pollutants have been observed to be chemically remobilized to re-enter the water column and/or food web (Sutherland 2000). The need to assess the sediment state and quality of an entire catchment in terms of its metallic load becomes imperative, particularly when water from the river is used for domestic, irrigation and livestock purposes given the implications for human health.
The main sources of metals are weathering of minerals and soils, mining, industry, refuse incineration, domestic effluents and waste (Fatoki and Awofolu 2003; Awofolu et al. 2005; Ogola et al. 2011). Water managers, governments, health officials and other stakeholders are concerned about the presence of metals that are toxic (e.g. lead (Pb), chromium (Cr)) in the environment. Most metals can have serious health implications since they are non-essential and of no health benefit to humans and animals (Awofolu et al. 2005; Mathee et al. 2013; Naicker et al. 2013). Other metals (e.g. zinc (Zn), calcium (Ca), sodium (Na)) are required for metabolic activities and lie within a narrow range between essentiality and toxicity depending on the concentrations taken or available (Fatoki and Awofolu 2003; Förstner and Wittmann 2012). The toxicity of metals, however, demonstrates substantial variability among organisms. For example, while Zn has low toxicity to humans, it has a high toxicity to fish (Dural et al. 2007). Hence the presence of these metals in the aquatic ecosystem is often far-reaching, with direct and indirect implications for humans (Fatoki and Awofolu 2003; Awofolu et al. 2005; Förstner and Wittmann 2012).
Countries with emerging economies in arid regions are often plagued by complex issues whereby access to water is limited, yet infrastructure, policies and policing do not necessarily facilitate optimum water resource management (Morrison et al. 2001). In southern Africa, for example, water resources are increasingly becoming polluted due to rapid urbanisation, poor management, agriculture and mining practices (Morrison et al. 2001; Dalu and Froneman 2016; Dalu et al. 2016). There is, however, limited information on the extent of metal contamination within austral temperate aquatic ecosystems and on the residency time of such pollutants within sediments in these environments. Given that river flow in austral temperate environments is often less predictable than in many northern temperate regions, pollutant source-sink dynamics are likely to differ between regions. Determining spatial and temporal metal levels is, therefore, essential for an accurate assessment of the degree of pollution in a given environment (de Paula Filho et al. 2015) and to assess the potential harmful effects it may have on ecosystem functioning and human health. Hence, it is important for water managers, the general public, policy-makers and health practitioners to have sound information on the state of the environment through continuous monitoring for a better and sustainable future. These assessments provide an objective basis for decision making, for the proper use of natural resources and could be further used as a relative measure to distinguish between natural and anthropogenic metal concentrations in river sediments.
With little being known about the spatial and temporal variation of metals within austral temperate river systems, this study focussed on the Bloukrans River system in the warm temperate region of South Africa. The main land use activity within the Bloukrans River catchment is urban settlements and intensive agriculture (i.e. livestock and crop farming). Artificial, impervious surfaces in urban environment combined with heavily engineered drainage systems have transformed hydrological pathways and increased the quantity and speed at which pollutants enter the Bloukrans River system. Therefore, the study aimed to: (i) determine selected metal (i.e. boron (B), calcium (Ca), chromium (Cr), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), Sodium (Na), Lead (Pb), Zinc (Zn)) and sediment nutrients (i.e. nitrate (NO3 −), total phosphorus (TP), phosphate (PO4 3−)) concentrations in the river sediments and identify potential pollution sources; (ii) investigate spatial and temporal distributions of metals and nutrient concentrations within the catchment in relationship to land use and identify the relationships between sediment metals and selected physico-chemical variables, and (iii) assess enrichment levels of elements and determine their ecological risks using enrichment and toxicity indicators. We hypothesised that higher metal levels and nutrient concentrations would be detected in the dry–cool period in the urban environment due to the decreased river flow and, therefore, increased deposition of metals and nutrients within the sediment.
Materials and methods
Study area
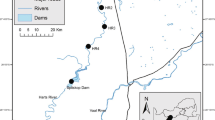
The Bloukrans River drains the town of Grahamstown (population ~ 120,000) and Belmont Valley farms, flowing in a south-east direction. The river has catchment of ~ 220 km2 and total length of ~ 40 km. The mean annual catchment region rainfall is 600–800 mm, which is evenly distributed, with mean minimum and maximum air temperatures of 1.5 and 43 °C, respectively. The Bloukrans catchment lies within the Bokkeveld Series strip (i.e. shale and sandstone; Heydorn and Grindley 1982). The river is subjected to various sources of pollution including agriculture, overflowing sewage and domestic and industrial waste (Dalu et al. 2017a; Nel et al. 2018). The study was conducted during the hot-wet (February) and cool-dry (July) periods in 2016. Twenty-two sites were selected along the length of the system to represent all the tributaries and/or streams (see Fig. 1). Sites 1–4 were below the sewage treatment works and located in intensive agricultural farms (dairy and irrigation) immediately downstream of the city of Grahamstown and were considered as agricultural sites (AS). Sites 15, 17, 19 and 21 were considered pristine sites (PS) as they were located in the upper reaches of the catchment, while the remaining sites were located within the urban area and were categorised as urban sites (US). Water flow was measured using a Flo-mate portable flowmeter Model 2000 (Marsh McBirney, Maryland (US); n = 3–6) per site for each particular season (see results in Table 1).
Sediment collection and processing
Integrated sediment samples (1.5 kg) were collected at the centre and two littoral zones of each site using a plastic hand shovel after the removal of the overlaying debris to a depth of about 5–10 cm into the sediment layer. The samples were then stored in polyethylene ziplock bags. After sampling, sediment samples were oven dried at 60 °C, before being disaggregated in a porcelain mortar and sieved through a < 0.075 mm sieve to remove plant roots and other debris.
Sediment metal analysis
All metal analysis was carried out in a South African National Accreditation System (SANAS) certified laboratory. For cation elements (B, Ca, K, Mg, Na), an acid digestion using a 1:1 mixture of 1 N nitric acid (HNO3) and hydrochloric acid at 80 °C for 30 min was carried out. Metal content of the extract was then determined using an ICP-OES optical emission spectrometer (Varian, Mulgrave, Australia). For metal (Cr, Cu, Fe, Pb, Zn) analyses, extraction was done using 5 g of dried and sieved soil to which 20 mL HNO3 (55%) and 5 mL hydrogen peroxide (30%) was added and placed on a heated sand bed (180 °C) for 8 h, before being filtered onto a Whatman filter paper. The metal content of the extract was measured using an ICP-OES optical emission spectrometer (Varian, Mulgrave, Australia) (see Clesceri et al. (1998) for detailed methodology). To estimate the accuracy of this method, a natural standard certified reference soil, namely SARM-51 (MINTEK) and SL-1 (IAEA), digested and analysed in triplicate, was used for recovery tests. The percentage recoveries of the certified values ranged between 87 and 110%.
Sediment total nutrient and organic matter concentration
Nitrate concentrations were extracted from sediment using 1 N potassium chloride (KCl). Nitrate-N (NO3 −) concentration in the extract was then determined on a SEAL Auto-Analyser 3 through reduction of NO3 − to nitrite (NO2 −) using a copper–cadmium reduction column, before the nitrate finally reacted with sulphanilamide under acidic conditions, using N-1-naphthylethylenediamine dihydrochloride (AgriLASA 2004). Total phosphorus (TP) and phosphate (PO4 3−) concentration was analysed using a Bray-2 extract as described by Bray and Kurtz (1945).
Total organic carbon (OC) was determined using a modified Walkley–Black method as described by Chan et al. (2001), whereby in a 500 mmol L−1 Erlenmeyer flask 0.5 g of sediment was placed, followed by 10 mL 0.167 M K2Cr2O7 and then 20 mL concentrated sulphuric acid. Post reaction, excess dichromate was determined by titrating against 1.0 M FeSO4 to calculate the amount of dichromate taken up by the soil (Walkley and Black 1934). Dichromate soil consumption was then used to determine oxidisable carbon levels based on the theoretical value of 1.0 mL 0.0167 M K2Cr2O7 oxidises 3 mg carbon (Walkley and Black 1934). This procedure was then repeated two more times, but instead of using 20 mL of concentrated sulphuric acid, 5 and 10 mL were respectively used, resulting in three acid-aqueous solution ratios of 0.5:1 (12 N of H2SO4), 1:1 (18 N of H2SO4) and 2:1 (24 N of H2SO4) (Walkley 1947). Along with total oxidisable carbon (TOC) concentration from the sediment, the three acid-aqueous solutions were then used to establish four fractions of decreasing oxidisability, whereby fraction 1, 2, 3 and 4 are comprised of organic carbon oxidisable under 12 N, 18 N–12 N, 24 N–18 N, and TOC–24 N H2SO4, respectively.
Sediment contamination assessment
The enrichment factor, which is a measure of the concentration of a certain metal in the sediment relative to the natural background concentration, was calculated using the formula by Buat-Menard and Chesselet (1979):
where A n is the concentration of metal n and B is the concentration of background metal (see Table S1 for the Grahamstown area). Metals which are naturally derived have an EF value of near unity, while elements of anthropogenic origin have EF values of several orders of magnitude. Six categories are recognised: < 1 background concentration, 1–2 depletion to minimal enrichment (hereafter referred to as low enrichment), 2–5 moderate enrichment, 5–20 significant enrichment (hereafter referred to as high enrichment), 20–40 very high enrichment and > 40 extremely high enrichment (Sutherland 2000).
Pollution load index (PLI) allows for the standardisation, with a high degree of accuracy, for metal concentrations found in the river sediment at each site. The PLI for each site was calculated according Tomlinson et al. (1980):
where n is the number of metals (10 in the present study) and X is the contamination factor (metal concentration in sediments divided by background values of the metal). The pollution load index value of > 1 is polluted and < 1 indicates no pollution.
Toxic risk index (TRI) based on the TEL and PEL effects for the toxic risk assessment was adapted for this study (Zhang et al. 2016). For each metal in one sediment sample, the TRI was calculated by:
The integrated toxic risk index for several metals in one sediment sample was calculated using:
where TRI i is the toxic risk index of a certain metal, C i is the measured metal content, n is the metal number and TRI is the integrated toxic risk index of one sample. The classification of the TRI: ≤ 5—no toxic risk, > 5 to ≤ 10—low toxic risk, > 10 to ≤ 15—moderate toxic risk, > 15 to ≤ 20—considerable toxic risk and > 20—very high toxic risk (Zhang et al. 2016).
To evaluate possible environmental health impact consequences of the studied metals, comparisons were made between the measured concentrations and the Canadian sediment quality guidelines (SQGs; CCME 2002) to determine the degree to which sediment-bound contaminants might adversely affect the organisms living in or near sediments (Skordas et al. 2015). We assessed two SQGs: the threshold effect level (TEL) and the probable effect level (PEL), which are a measure of concentrations below which adverse effects for sediment-dwelling organisms would be infrequently expected and chemical concentrations above which adverse effects are likely to occur frequently, respectively. These guidelines include safe levels not only for metals but also for nutrients i.e. the lowest effect level (LEL) indicating the contamination level which has no effect on the majority of sediment-dwelling organisms, and the severe effect level (SEL) that likely affects the health of sediment-dwelling organisms (Persaud et al. 1993).
Data analysis
All data was assessed for normality and homogeneity of variance and was found to conform to parametric assumptions. Differences in metal, nutrient concentrations and sediment contaminant indices between the two sampling seasons and three land types (i.e. agriculture, pristine, urban) were assessed using a two-way ANOVA analysis followed by a Tukey’s post-hoc test. Furthermore, using a Pearson correlation, we tested for relationships between metals, nutrient concentrations and organic carbon (OC). All correlations, ANOVAs and the testing of data for normality and homogeneity of variance were carried out in SPSS version 16 (SPSS Inc 2007).
Principal component analysis (PCA) with varimax rotation and cluster analysis (CA) using the average group linkage method were employed using the metal concentrations to determine natural and anthropogenic sources of pollution. Hierarchical cluster analysis (HCA) based on metal concentration data sampled across the two seasons and three land types was carried out to identify patterns in the metal concentrations for the different sampling seasons and land types. HCA were based on correlation as a distance of measure and Ward’s method as the group linkage method (Sekabira et al. 2010). All multivariate analysis were carried out in PC-ORD version 5.10 (McCune and Mefford 2006).
Results
Sediment nutrient concentrations
The mean nutrient (nitrate—NO3 −, total phosphorus—TP, phosphate—PO4 3−) concentrations and organic carbon content (OC) observed for three land types (i.e. agriculture, pristine, urban) are highlighted in Table 1. During the dry season, the mean NO3 − concentration was highest in the pristine environment while the mean TP and PO4 3− concentrations were highest in the urban environment (Table 1). High NO3 − concentrations were observed for PS 19 (3.0 mg kg−1) and 21 (2.5 mg kg−1) during the dry season, while low concentrations (0.0 mg kg−1) were observed during the wet season for certain US and PS. PO4 3− concentrations during the wet season were over 1000 mg kg−1 for most sites, with the exception of the US 14 and 18 and the PS 15, 17 and 19. High PO4 3− concentrations of 6094.1 mg kg−1 (PS 17) and 3331.3 mg kg−1 (US 16) were observed during the dry season and 4685.3 mg kg−1 (US 6) and 3217.4 mg kg−1 (US 9) PO4 3− concentrations were observed during the wet season. In the dry season, the agricultural area i.e. sites below the sewage treatment works, had low nutrient concentrations. No significant seasonal or spatial differences in PO4 3− concentrations were observed during the study (ANOVA; p > 0.05 in all cases; Table S2). However, we observed significant differences between land type (F (2, 42) = 4.07, p = 0.03), season (F (1, 43) = 10.65, p < 0.01) and land type × season (F (2, 42) = 6.37, p < 0.01) for NO3 − concentrations. Post hoc analyses, NO3 − (mean = − 0.52, p = 0.02) found significant differences between pristine and urban landscapes, with the highest concentrations being observed for the urban environment.
The OC content of the river sediment ranged from 0.8 to 14.7% and 1.4 to 34.4% for the wet and dry seasons, respectively (Table 1). High OC concentrations at the PS 21 (14.7) in wet season and at US 16 (34.4%) and PS 21 (32.1%) in the dry season were observed. The sediment OC content was generally highest during the dry season. Similar to nutrient concentrations, no significant differences (p > 0.05) were found for OC content for the different land types and interaction (land types × season), whereas significant seasonal differences (F (1, 43) = 13.70, p < 0.01) were observed (Table S2).
Metal concentrations
Metal concentrations recorded for the three land types over the two seasons are summarised in Table 1. Urban sites generally had the highest metal concentrations for both seasons, with the exception for Cu and Mg in the wet season, which were elevated for the PS (Table 1). Zn was significantly different (F (1, 43) = 3.62, p = 0.04) across land types, with similar metal concentrations (p > 0.05) being observed for all other metals across the different land types (Table S2). Cu (F (1, 43) = 2.38, p = 0.81), Na (F (1, 43) = 3.85, p = 0.06) and Pb (F (1, 43) = 1.19, p = 0.28) were found to be similar across seasons. Using post hoc analysis, Zn (mean = − 52.91, p = 0.045) concentrations were found to differ between agriculture and urban landscapes, with high concentrations being observed for the urban environment.
Elevated metal concentrations were recorded in river sediments during the dry season, with some metals such as calcium (Ca), iron (Fe), potassium (K), magnesium (Mg) and sodium (Na) increasing > 10-fold from the wet to dry season. In the dry season, the AS below the sewage treatment works had low metal concentrations. The ranges of selected metals during the wet season were as follows: 0.2–8.9 mg kg−1 for copper (Cu), 3.3–18.0 mg kg−1 for Cr, 127.1–1860.0 mg kg−1 for Fe, 0–38.3 mg kg−1 for lead (Pb), 1.7–68.6 mg kg−1 for zinc (Zn) and 0.4–5.1 mg kg−1 for Mg. Whereas, during the dry season, the ranges of selected metals were as follows: 1.1–115.7 mg kg−1 for Cu, 6.0–26.4 mg kg−1 for Cr, 6856.4–53,101.2 mg kg−1 for Fe, 4.3–164.7 mg kg−1 for Pb, 18.2–350.1 mg kg−1 for Zn and 140.6–2083.0 mg kg−1 for Mg.
Sediment contaminant assessments
The threshold effect level (TEL), probable effect level (PEL), lowest effect level (LEL) and the severe effect level (SEL) for each metal and nutrient are presented in Table 1, with < 31% of the sites exceeding the contaminant limits. During the dry season, mostly US had metals over the PEL, TEL and LEL levels. In the dry season, the LEL for Cu (SQG level—16 mg kg−1) was over the recommended guidelines for US 6 (115.7 mg kg−1), 8 (19.6 mg kg−1), 9 (41.1 mg kg−1), 10 (28.7 mg kg−1) and 16 (27.2 mg kg−1) and PS 17 (27.7 mg kg−1) while TEL (36 mg kg−1) was over the limit at US 6 and 9. For the wet season, US 11 (38.3 mg kg−1) and PS 17 (32.2 mg kg−1) and dry season US 5 (32.3 mg kg−1), 11 (40.9 mg kg−1), and 18 (31.4 mg kg−1) and PS 17 (164.7 mg kg−1) were over the LEL for Pb (31 mg kg−1). The PEL (91 mg kg−1) for dry season PS 17, TEL (35 mg kg−1) for wet season US 11 and dry season US 11 and PS 17 were over the limit for Pb. For Cr, dry season US 16 (26.4 mg kg−1) was over limit for LEL (26 mg kg−1). Finally for Zn, the TEL (123 mg kg−1) and LEL (120 mg kg−1) were over the limit for the dry season US 5 (213.4 mg kg−1), 6 (134.1 mg kg−1), 10 (267.7 mg kg−1), 11 (125.6 mg kg−1) and 16 (175.9 mg kg−1) and PS 17 (350.1 mg kg−1), with only PS 17 being over the PEL limit (315 mg kg−1).
For OC content, all sites except for AS 3 (0.8%), US 8 (0.7%) and PS 19 (0.8%) exceeded the LEL limit, with only PS 21 (14.7%) being over the SEL limit during the wet season. During the dry season, the OC content for all sites was over the LEL limit, with almost 54.5% of sites being over SEL limit.
Enrichment factors were generally within background levels for most metals, with a few metals such as Cu, Cr, K, Fe, Mg, Na, Pb and Zn showing low to moderate enrichment in some sites (Table 3). Urban sites during the wet and dry season generally had elevated enrichment factors (EF), with EFs values being higher for the dry season (Table 3). ANOVA analysis indicated that B (F (1, 43) = 3.54, p = 0.04) and Zn (F (1, 43) = 4.11, p = 0.02) were significantly different among land types, with Ca (F (1, 43) = 3.98, p = 0.05), Cu (F (1, 43) = 3.20, p = 0.08), Na (F (1, 43) = 1.39, p = 0.25) and Pb (F (1, 43) = 1.58, p = 0.22) being similar among the two study seasons (Table S2). Post hoc analysis identified significant differences for agricultural vs urban environments for B (mean = − 0.30, p = 0.046) and Zn (mean = − 0.75, p = 0.04), with high concentrations in the urban environment.
During the wet season, most metals were within background levels for EFs (< 1), with US showing low (1–2) and moderate enrichment (2–5) for metals such as Pb and Cr in 40% of the sites. During the dry seasons, all AS (i.e. sites below sewage treatment works) had metals having background level enrichment, with a few sites having low and moderate enrichment (e.g. metals B, Cu, Cr, Fe, Mg, K, Zn). Urban sites 6 (Ca – EF 6.3), 10 (Cu – EF 9.0), 11 (K – EF 25.5, Mg– EF 25.5), 16 (Mg – EF 5.4, Na – 13.8) and 20 (Pb—EF 11.7) and PS 17 (Cu—EF 6.0) had high enrichment, with US 9 (Cu—EF 25.5) having a very high enrichment. During the dry season, PS EFs ranged between 0.3 and 2.2, with most values falling between 1 and 2, i.e. low enrichment.
Pollution load index for the wet season ranged from 3.1 to 6.7 for agriculture, from 0.8 to 17.4 for the US and from 7.0 to 22.2 for the PS. In the dry season, the ranges were 7.2 to 24.9 (AS), 8.6 to 58.4 (US) and 20.8 to 46.6 (PS) indicating high pollution levels. The US had the highest pollution loads for both seasons (Table 3). Significant pollution load indices differences were observed for land types (F (2, 42) = 4.84, p = 0.01) and seasons (F (1, 43) = 27.15, p < 0.001). Using Tukey’s post hoc analysis, differences in pollution load indices (mean = − 12.67, p = 0.02) were observed for agriculture and urban environments. Toxic risk index (TRI) indicated that most sites posed no toxic risk as they were ≤ 5 for both seasons and land types, with the exception of US 20 (TRI 6.4) which showed low toxic risk. The TRI values were moderately elevated during the dry season (mean land type TRI range 0.9–2.5) compared to the wet season (mean land type TRI range 0.3–0.8; Table 3). Significant differences were observed for TRI across land types (F (2, 42) = 4.25, p = 0.02) and between seasons (F (1, 43) = 7.65, p = 0.01). Elevated TRI values were observed in US compared to the AS (mean = − 0.99, p = 0.04).
Relationships between the measured variables
For further analysis of the general sediment characteristics of the Bloukrans River system, we employed multivariate principal component analysis (PCA; Table 2), cluster analysis (Fig. 2) and Pearson correlations (Table S3). A correlation matrix showed that with the exception of Pb, all metals in the river sediments were highly correlated with each other signifying a strong positive association (Table S3). This suggests that aside from Pb, metals in the studied sediments may have originated from a similar source. The OC content was correlated with B, Ca, Fe, Mg, Na and Zn, while nutrients P and PO4 3− being weakly positively correlated with Cr and strongly positively correlated with Pb and Zn. The NO3 − concentration was weakly positively correlated with B, Fe and K (Table S3).
Using PCA, the first two principal components (PC) explained 76.7% of the total variance, with PC1 and PC2 explaining 63.0 and 13.7% of the total variance, respectively. The Eigenvalues of the two extracted PCs were both > 1.0. Principal CA classified metals into two groups, with group 1 consisting of all metals, with the exception Pb which was the only metal in group 2 (Table 2). Hierarchical cluster analysis (HCA) results identified five distinct groups: group 1 included Pb and Zn and was clearly distinguishable from other four groups (Fig. 2). Group 2 consisted of Ca and Cu, Group 3 consisted of B, Fe, K, Mg, with Cr and Na forming their own individual separate groups.
Discussion
The study found that metal pollutants varied considerably in space and time in the Bloukrans River system in the temperate region of southern Africa. As hypothesised, pollutant concentrations were higher during the dry season. The total metal concentrations measured in the present study were in the range reported in the international literature for surface sediments (Awofolu et al. 2005; Sekabira et al. 2010; Gerber et al. 2015; Skordas et al. 2015; Bere et al. 2016) and the sediment quality was generally acceptable according to the Canadian sediment quality guidelines (Persaud et al. 1993). Metal concentrations in river sediments varied between the seasons with Fe being the dominant metals in all study sites, as it is the most abundant element in the earth’s crust (ATSDR 2008; Gerber et al. 2015). The high Fe concentrations can likely be attributed to weathering and subsequent runoff in the study areas. The total metal concentrations recorded during the dry season were similar to metal concentrations observed by Sekabira et al. (2010) along the Nakivubo River of Uganda, by Gerber et al. (2015) in the polluted Olifants, Letaba and Luvhuvhu rivers and Dahms et al. (2017) in the Limpopo River (South Africa). Significantly lower metal concentrations were observed during the wet season most likely a result of metals being re-suspended with sediments and washed further downstream by the high water flow resulting in less metal accumulation in the sediment in the study area (see Peraza-Castro et al. 2016). This is supported by the significant correlations between water flow and metals K, Fe, Cr and Mg. Similarly, sediments from the agricultural area had low metal concentrations when compared to the urban and pristine sites, likely also a result of elevated water flow and increased re-suspension of sediments in these downstream, higher stream order sites (Heydorn and Grindley 1982; Bai et al. 2015).
Sediment contaminant assessment scores indicate that for the majority of the sites during the wet season, the sediment quality was acceptable (see Table 3) but not so, during the dry season. Overall, applying the sediment pollution load index which allows for the standardisation of metal concentrations (Tomlinson et al. 1980) demonstrated the poor quality river sediment along the length of the Bloukrans River. This indicated very high levels of pollution not suitable for maintaining healthy fauna and flora populations especially during periods of very low water flow i.e. dry season (see Table 3). High metal concentrations combined together contributed toward the high pollution load index during period of low discharge/river flow. The metals responsible for decreased sediment contaminant assessment scores were B, Cu, Cr, Mg, Pb and Zn during the wet season. These metals are all associated with combustion activities (e.g. urban areas, vehicular exhaust, electroplating) and agriculture (phosphate fertilisers and pesticides; Gerber et al., 2015). All these listed activities are found in the Bloukrans River catchments.
Overall, the study results demonstrated a high degree of nutrient pollution in the river sediment as evident from the high loadings of organic carbon, nitrates and total phosphorus (TP and PO4 3−) but a low degree of metal pollution according to sediment contaminant assessments (i.e. Cr, Cu, Pb and Zn). We speculate that the high nutrient loads reflect increased urbanisation and poor waste water treatment within the Grahamstown area and the Bloukrans Valley agricultural activities within the catchment area. In addition to the sewage treatment works not functioning optimally, the sewage network servicing the city in general was observed to be porous, as is the case in many urban areas (Bere et al. 2016; Nhiwatiwa et al. 2017). Nutrient loading and particularly phosphorus input from sewage is, therefore, likely to play an important role with regard to the release of metals in sediments under the resultant eutrophic environment, especially during the dry season (see Chen et al. 2017). Similarly, the low metal pollution values likely reflect that of the catchment area of the river, which is characterised by the virtual absence of industry that would otherwise represent a significant source of metals.
Overall, the Bloukrans River system can be classified as marginally to highly polluted, depending on season and locality. This may have ecological implications for benthic fauna and flora because the potential risk from high metal concentrations in the river sediments does not solely depend on total concentrations but also on the determination of bioavailable fractions (Skordas et al. 2015). The total nutrient and metal concentrations showed significant seasonal fluctuations and this was linked to seasonal water flow.
A high correlation among the different metals is often indicative of a common source and mutual dependence (Singh et al. 2002; Nyangababo et al. 2005; Suresh et al. 2012; Yohannes et al. 2013). Thus, it is likely that most of the metals within the Bloukrans River sediments originated from a similar source, with the exception of Pb and Zn which could have been introduced as a result of retention from motor vehicles and industrial emissions as observed in other studies (i.e. Suresh et al. 2012; Skordas et al. 2015). Interestingly, unlike Skordas et al. (2015) who found no correlations between the OC and the metals, we found significant weak correlations for most metals with the exception for Cu, Cr, K and Pb (p < 0.05). These findings suggest that the complexation of metals with organic carbon played a significant role in metal distribution (Skordas et al. 2015). In addition, the correlation between the nutrients and selected metals (e.g. B, Cr, Fe, K, Pb, Zn) suggests that they shared common sources of enrichment. Whereas, a lack of correlation between OC, P and PO4 3− concentrations demonstrated that the individual nutrient concentrations were mainly influenced by different contamination sources (Skordas et al. 2015) and the relationship between OC and NO3 − concentrations suggests similar contamination sources (Table 3). These contamination sources are most likely linked to raw sewage spills from the Grahamstown old sewage pipeline network in the urban area and/or agriculture activities within the catchment area.
While the results of the study suggest that the sediment quality was generally acceptable, it is important to recognise that although Grahamstown is considered a small urban area, it is currently experiencing considerable urbanisation. Furthermore, agricultural activities in surrounding areas are on the increase. While conducting the survey, it was evident that sewage transport and water treatment systems in the city were compromised with burst/leaking pipes observed at several locations. Infrastructural degradation has been well observed in many municipalities of the region and is likely having direct impact on the water quality in the river in these areas. In addition, increased unregulated urban development and informal dumping are also likely contributing to the observed state of the quality of the river. These issues need to be addressed in light of growing human-induced pressure on these and other systems. It is also important to note that river discharge played a significant role in determining the seasonal variations in selected physico-chemical variables. This is important as our data suggest that during periods of low flows, metal contamination of the sediment is increased. This may pose a significant risk for human communities, river health and aquatic communities during these periods (Dalu et al. 2017b,c). Much of the Grahamstown community live below the poverty line and subsidise dietary requirements through subsistence living. For example, cattle were regularly encountered and seen utilising the rivers and streams for watering, while conducting the survey. Such animals will be more reliant on these environments during the dry period when other watering options are not available.
These results provide baseline information for general management of the Bloukrans River’s total catchment and advocates for further investigations into the seasonal variability in the river water quality so as to understand the metal and nutrient dynamics at sediment and water interface. Pollutant source-sink dynamics are pertinent for the understanding of environmental risk and such studies will be useful for the characterisation of arid temperate aquatic environments and their contrasts with ecosystems in other climatic regions.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (2008) Toxicological profile for aluminum, US Department of Health and Human Services. Public Health Service, Atlanta
Agri Laboratory Association of Southern Africa (AgriLASA) (2004) Soil handbook. Agri Laboratory Association of Southern Africa, Pretoria
Akele ML, Kelderman P, Koning CW, Irvine K (2016) Heavy metal distributions in the sediments of the Little Akaki River, Addis Ababa. Ethiopia Environ Monit Assess 188:1–13
Awofolu OR, Mbolekwa Z, Mtshemla V, Fatoki OS (2005) Levels of heavy metals in water and sediment from Tyume River and its effects on an irrigated farmland. Water SA 31:87–94
Bai J, Jia J, Zhang G, Zhao Q, Lu Q, Cui B, Liu X (2016) Spatial and temporal dynamics of heavy metal pollution and source identification in sediment cores from the short-term flooding riparian wetlands in a Chinese delta. Environ Pollut 219:379–388. https://doi.org/10.1016/j.envpol.2016.05.016
Bai J, Xiao R, Zhao Q, Lu Q, Wang J, Reddy KR (2014) Seasonal dynamics of heavy elements in tidal salt marsh soils as affected by the flow-sediment regulation regime. PLoS One 9(9):e107738. https://doi.org/10.1371/journal.pone.0107738
Bai J, Cui B, Chen B, Zhang K, Deng W, Gao H, Xiao R (2011) Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol Model 222(2):301–306. https://doi.org/10.1016/j.ecolmodel.2009.12.002
Bai J, Zhao Q, Lu Q, Wang J, Reddy KR (2015) Effects of freshwater input on trace element pollution in salt marsh soils of a typical coastal estuary, China. J Hydrol 520:186–192. https://doi.org/10.1016/j.jhydrol.2014.11.007
Bere T, Dalu T, Mwedzi T (2016) Detecting the impact of heavy metal contaminated sediment on benthic macroinvertebrate communities in tropical streams. Sci Total Environ 572:147–156. https://doi.org/10.1016/j.scitotenv.2016.07.204
Bilsborrow RE (1992) Population growth, internal migration, and environmental degradation in rural areas of developing countries. Euro J Popul 8(2):125–148. https://doi.org/10.1007/BF01797549
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59(1):39–45. https://doi.org/10.1097/00010694-194501000-00006
Buat-Menard P, Chesselet R (1979) Variable influence of atmospheric flux on the heavy metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:398–411
Bulte EH, Van Soest DP (2001) Environmental degradation in developing countries: households and the (reverse) Environmental Kuznets Curve. J Dev Econ 65:225-235.
Burton GA Jr (2002) Sediment quality criteria in use around the world. Limnology 3(2):65–75. https://doi.org/10.1007/s102010200008
Canadian Council of Ministers of the Environment (CCME) (2002) Canadian sediment quality guidelines for the protection of aquatic life. In: Canadian environmental quality guidelines. Canadian Council of Ministers of the Environment, Winnipeg
Chan KY, Bowman A, Oates A (2001) Oxidizible organic carbon fractions and soil quality changes in an Oxic Paleustalf under different pasture leys. Soil Sci Soc Am J 166(1):61–67. https://doi.org/10.1097/00010694-200101000-00009
Chen M, Ding S, Zhang L, Li Y, Sun Q, Zhang C (2017) An investigation of the effects of elevated phosphorus in water on the release of heavy metals in sediments at a high resolution. Sci Total Environ 575:330–337. https://doi.org/10.1016/j.scitotenv.2016.10.063
Clark JHA, Tredoux M, van Huyssteen CW (2015) Heavy metals in the soils of Bloemfontein, South Africa: concentration levels and possible sources. Environ Monit Assess 187(7):439–449. https://doi.org/10.1007/s10661-015-4608-1
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Coxon TM, Odhiambo BK, Giancarlo LC (2016) The impact of urban expansion and agricultural legacies on trace metal accumulation in fluvial and lacustrine sediments of the lower Chesapeake Bay basin, USA. Sci Total Environ 568:402–414. https://doi.org/10.1016/j.scitotenv.2016.06.022
Dahms S, Baker NJ, Greenfield R (2017) Ecological risk assessment of trace elements in sediment: a case study from Limpopo, South Africa. Ecotoxicol Environ Saf 135:106–114. https://doi.org/10.1016/j.ecoenv.2016.09.036
Dalu T, Froneman PW (2016) Diatom based water quality monitoring in Africa: challenges and future prospects. Water SA 42(4):551–559. https://doi.org/10.4314/wsa.v42i4.05
Dalu T, Bere T, Froneman PW (2016) Assessment of water quality based on diatom indices in a small temperate river system, Kowie River, South Africa. Water SA 42:183–193. https://doi.org/10.4314/wsa.v42i2.02
Dalu T, Wasserman RJ, Magoro M, Mwedzi T, Froneman PW, Weyl OLF (2017a) Variation partitioning of benthic diatom community matrices: effect of multiple variables on benthic diatom communities in an Austral temperate river system. Sci Total Environ 601–602:73–82. https://doi.org/10.1016/j.scitotenv.2017.05.162
Dalu T, Wasserman RJ, Tonkin JD, Alexander ME, Dalu MT, Motitsoe SN, Manungo KI, Bepe O, Dube T (2017c) Assessing drivers of benthic macroinvertebrate community structure in African highland streams: an exploration using multivariate analysis. Sci Total Environ 601:1340–1348. https://doi.org/10.1016/j.scitotenv.2017.06.023
Dalu T, Wasserman RJ, Tonkin JD, Mwedzi T, Magoro ML, Weyl OLF (2017b) Water or sediment? Partitioning the role of water column and sediment chemistry as drivers of macroinvertebrate communities in an austral South African stream. Sci Total Environ 607:317–325. https://doi.org/10.1016/j.scitotenv.2017.06.267
de Paula Filho FJ, Marins RV, de Lacerda LD, Aguiar JE, Peres TF (2015) Background values for evaluation of heavy metal contamination in sediments in the Parnaíba River Delta estuary, NE/Brazil. Mar Pollut Bull 91(2):424–428. https://doi.org/10.1016/j.marpolbul.2014.08.022
Dural M, Göksu MZL, Özak AA (2007) Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem 102(1):415–421. https://doi.org/10.1016/j.foodchem.2006.03.001
Fatoki OS, Awofolu R (2003) Levels of Cd, Hg and Zn in some surface waters from the Eastern Cape Province, South Africa. Water SA 29:375–380
Förstner U, Wittmann GT (2012) Metal pollution in the aquatic environment. Springer Berlin Heidelberg, Berlin
Gerber R, Smit NJ, van Vuren JH, Nakayama SM, Yohannes YB, Ikenaka Y, Ishizuka M, Wepener V (2015) Application of a sediment quality index for the assessment and monitoring of metals and organochlorines in a premier conservation area. Environ Sci Pollut Res 22(24):19971–19989. https://doi.org/10.1007/s11356-015-5206-z
Heydorn AEF, Grindley JR (1982) Estuaries of the Cape. Part II: Synopses of available information on individual systems. Report No.10 Kowie (CSE 10). CSIR Research Report 409. Council for Scientific and Industrial Research (CSIR), Pretoria
Leroy MC, Marcotte S, Legras M, Moncond'huy V, Le Derf F, Portet-Koltalo F (2017) Influence of the vegetative cover on the fate of trace metals in retention systems simulating roadside infiltration swales. Sci Total Environ 580:482–490. https://doi.org/10.1016/j.scitotenv.2016.11.195
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39(1):20–31. https://doi.org/10.1007/s002440010075
Mathee A, Khan T, Naicker N, Kootbodien T, Naidoo S, Becker P (2013) Lead exposure in young school children in South African subsistence fishing communities. Environ Res 126:179–183. https://doi.org/10.1016/j.envres.2013.05.009
McCune B, Mefford MJ (2006) PC-ORD:Multivariate Analysis of Ecological Data. Version 5.10. MjM Software, Gleneden Beach, Oregon, USA
Morrison G, Fatoki OS, Zinn E, Jacobsson D (2001) Sustainable development indicators for urban water systems: a case study evaluation of King William’s Town, South Africa, and the applied indicators. Water SA 21:219–232
Murray KS, Cauvet D, Lybeer M, Thomas JC (1999) Particle size and chemical control of heavy metals in bed sediment from the Rouge River, Southeast Michigan. Environ Sci Technol 33(7):987–992. https://doi.org/10.1021/es9807946
Naicker N, Mathee A, Barnes B (2013) Environmental lead: a public health challenge in South Africa. Epidemiology 24(4):621–622. https://doi.org/10.1097/EDE.0b013e318296c077
Nel HA, Dalu T, Wasserman RJ (2018) Sinks and sources: assessing microplastic abundance in river sediment and deposit feeders in an Austral urban river system. Sci Total Environ 612:950–956. https://doi.org/10.1016/j.scitotenv.2017.08.298
Nhiwatiwa T, Dalu T, Brendonck L (2017) Impact of irrigation based sugarcane cultivation on the Chiredzi and Runde Rivers quality, Zimbabwe. Sci Total Environ 587–588:316–325. https://doi.org/10.1016/j.scitotenv.2017.02.155
Nyangababo JT, Henry I, Omutunge E (2005) Heavy metal contamination in plants, sediments and air precipitation of Katonga, Simiyu and Nyando wetlands of Lake Victoria Basin, East Africa. Bull Environ Contam Toxicol 75(1):189–196. https://doi.org/10.1007/s00128-005-0737-5
Ogola JS, Mundalamo HR, Brandl G (2011) Investigation of the origin and distribution of heavy metals around Ebenezer Dam, Limpopo Province, South Africa. Water SA 37:173–179
Peraza-Castro M, Sauvage S, Sánchez-Pérez JM, Ruiz-Romera E (2016) Effect of flood events on transport of suspended sediments, organic matter and particulate metals in a forest watershed in the Basque Country (Northern Spain). Sci Total Environ 569:784–797. https://doi.org/10.1016/j.scitotenv.2016.06.203
Persaud D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. Ministry of the Environment and Energy, Ontario
Sekabira K, Origa HO, Basamba TA, Mutumba G, Kakudidi E (2010) Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int J Environ Sci Technol 7(3):435–446. https://doi.org/10.1007/BF03326153
Singh M, Muller G, Singh IB (2002) Heavy metals in freshly deposited stream sediments of rivers associated with urbanization of the Ganga plain, India. Water Air Soil Pollut 141(1/4):35–54. https://doi.org/10.1023/A:1021339917643
Skordas K, Kelepertzis E, Kosmidis D, Panagiotaki P, Vafidis D (2015) Assessment of nutrients and heavy metals in the surface sediments of the artificially lake water reservoir Karla, Thessaly, Greece. Environ Earth Sci 73(8):4483–4493. https://doi.org/10.1007/s12665-014-3736-1
SPSS Inc (2007) SPSS Release 16.0.0 for Windows. Polar Engineering and Consulting. SPSS Inc, Chicago
Suresh G, Sutharsan P, Ramasamy V, Venkatachalapathy R (2012) Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicol Environ Saf 84:117–124. https://doi.org/10.1016/j.ecoenv.2012.06.027
Sutherland RA (2000) Bed sediment-associated heavy metals in an urban stream, Oahu, Hawaii. Environ Geol 39(6):611–627. https://doi.org/10.1007/s002540050473
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol Meeresunters 33(1-4):566–575. https://doi.org/10.1007/BF02414780
Xiao R, Bai J, Zhang H, Gao H, Liu X, Wilkes A (2011) Changes of P, Ca, Al and Fe contents in fringe marshes along a pedogenic chronosequence in the Pearl River estuary, South China. Cont Shelf Res 31(6):739–747. https://doi.org/10.1016/j.csr.2011.01.013
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SMM, Ishizuka M (2013) Occurrence, distribution, and ecological risk assessment of DDTs and heavy metals in surface sediments from Lake Awassa-Ethiopian Rift Valley Lake. Environ Sci Pollut Res 20(12):8663–8671. https://doi.org/10.1007/s11356-013-1821-8
Zhang G, Bai J, Zhao Q, Lu Q, Jia J, Wen X (2016) Heavy metals in wetland soils along a wetland-forming chronosequence in the Yellow River Delta of China: levels, sources and toxic risks. Ecol Indic 69:331–339. https://doi.org/10.1016/j.ecolind.2016.04.042
Acknowledgements
This study was financially supported by the Claude Leon Postdoctoral Research Fellowship and Rhodes University Grant to TD, the National Research Foundation, South Africa (NRF Grant No., UID: 110507, 88746) to OLFW and RJW and Science and Technology Planning Project of Guangdong Province (2015A020215036 and 2014B030301055) to QW. Gratitude is extended to Mandla Magoro, Holy Nel and Samuel Motitsoe for assisting with field work. Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the Claude Leon Foundation and NRF do not accept any liability in this regard.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Dalu, T., Wasserman, R.J., Wu, Q. et al. River sediment metal and nutrient variations along an urban–agriculture gradient in an arid austral landscape: implications for environmental health. Environ Sci Pollut Res 25, 2842–2852 (2018). https://doi.org/10.1007/s11356-017-0728-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0728-1