Abstract
Soil amendments, such as biochar, have been used to enhance the immobilization of heavy metals in contaminated soil. A pot experiment was conducted to immobilize the available cadmium (Cd) and lead (Pb) in soil using peanut shell biochar (PBC) and wheat straw biochar (WBC), and to observe the accumulation of these heavy metals in rice (Oryza sativa L.). The application of PBC and WBC led to significantly higher pH, soil organic carbon (SOC), and cation exchange capacity (CEC) in paddy soil, while the content of MgCl2-extractable Cd and Pb was lower than that of untreated soil. MgCl2-extractable Cd and Pb showed significant negative correlations with pH, SOC, and CEC (p < 0.01). The application of 5% biochar to contaminated paddy soil led to reductions of 40.4–45.7 and 68.6–79.0%, respectively, in the content of MgCl2-extractable Cd and Pb. PBC more effectively immobilized Cd and Pb than WBC. Sequential chemical extractions revealed that biochar induced the transformation of the acid-soluble fraction of Cd to oxidizable and residual fractions, and the acid-soluble fraction of Pb to reducible and residual fractions. PBC and WBC clearly inhibited the uptake and accumulation of Cd and Pb in rice plants. Specially, when compared to the corresponding concentrations in rice grown in control soils, 5% PBC addition lowered Cd and Pb concentrations in grains by 22.9 and 12.2%, respectively, while WBC addition lowered them by 29.1 and 15.0%, respectively. Compared to Pb content, Cd content was reduced to a greater extent in grain by PBC and WBC. These results suggest that biochar application is effective for immobilizing Cd and Pb in contaminated paddy soil, and reduces their bioavailability in rice. Biochar could be used as a soil amendment for the remediation of soils contaminated with heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil heavy metal contamination is a global environmental problem, with this problem being extremely even more serious in China. The results of national soil pollution surveys showed that 19.4% of the total agricultural soil was contaminated by heavy metals (approximately 2.0 × 107 ha of agricultural soil) in China, with cadmium (Cd) and lead (Pb) being the major pollutants of heavy metals in the soil (Zhao et al. 2015). Cd and Pb are one of the most harmful heavy metals for human health. The accumulation of Cd and Pb in crop grains harvested in these agricultural soils is of great concern because of the enhanced dietary exposure through food chain transfer, affecting human beings who consume the grain (Khan et al. 2015). The Cd and Pb concentrations in food crops that exceed the national standard for food safety is more prevalent in southern China compared to other regions because of the acidity of the soils, which increases the phytoavailability of Cd and Pb in the soil (Zhao et al. 2015; Zhu et al. 2016). Therefore, it is necessary to reduce the bioavailability of Cd and Pb in acidic agricultural soils to ensure food security and human health (Mahar et al. 2015; Zhao et al. 2015).
Biochars are porous, low-density, and rich carbon materials produced by biological residues under low-oxygen combustion (Beesley et al. 2011). Recent studies have examined the applicability of biochar for the remediation of soil contaminated with heavy metals via the reduction of the mobility and availability of heavy metals, such as Cd and Pb, thereby reducing their accumulation within plants (Park et al. 2011; Puga et al. 2015; Zheng et al. 2015; Cui et al. 2016). The application of chicken manure- and green waste-derived biochars significantly reduces Cd and Pb accumulation by Indian mustard (Brassica juncea) through the immobilization of the metals (Park et al. 2011). Similarly, sugarcane straw biochar decreases the available concentrations of Cd and Pb in soil contaminated by mine waste and reduces their uptake by two leguminous plant species (Puga et al. 2015). However, the effect of biochar on heavy metal bioavailability is related to the plant source, application rate, and the type of heavy metal (Fellet et al. 2014; Lu et al. 2014; Yin et al. 2016). Bean stalk biochar and rice straw biochar significantly decrease Cd concentrations in rice grains, but the two biochars do not significantly affect the uptake of Pb (Zheng et al. 2015). In contrast, Lu et al. (2014) reported that rice straw biochar is effective in decreasing the shoot Pb concentration of Sedum plumbizincicola, while bamboo biochar is effective in decreasing Cd in the same plant. However, few studies have examined the influence of biochar feedstock on the bioavailability of Cd and Pb in contaminated agricultural soil–crop systems.
Rice (Oryza sativa L.) is a major staple crop in eastern and southern China; however, industrial production, mining operations, and metal processing have led to the contamination of paddy soils (Zhao et al. 2015). Some studies have demonstrated that biochar significantly reduces the mobility of Cd and Pb in contaminated paddy soil (Bian et al. 2014; Zheng et al. 2015; Li et al. 2016; Yang et al. 2016; Yin et al. 2016). However, studies investigating how different biochars affect the uptake of Cd and Pb by rice plants remain equivocal (Khan et al. 2013; Bian et al. 2014; Zheng et al. 2015; Li et al. 2016). Sewage sludge biochar significantly reduced the accumulation of Pb, but increased that of Cd in rice (Khan et al. 2013). Wheat straw biochar significantly reduced Cd concentration in rice roots, shoots, and grain, whereas Pb concentration was only reduced in rice roots, and no differences have been observed in rice grain and shoot Pb concentrations between amended and control plots (Bian et al. 2014). Cd concentrations in rice roots, shoots, and grain were significantly reduced after applying rice straw biochar and bean stalk biochar. However, only rice straw biochar significantly reduced Pb concentrations in the roots, and neither biochar significantly changed Pb concentrations in the shoots and grains (Zheng et al. 2015). In contrast, Pb concentrations in the stems, leaves, husks, and grains were significantly reduced by the addition of 5% (w/w) rice straw biochar (Li et al. 2016). Thus, a reduction in Cd and Pb has not been consistently demonstrated in response to soil biochar amendment. Furthermore, information is still limited regarding the effectiveness of biochar for Cd and Pb immobilization and accumulation in rice tissues. Therefore, detailed studies on how different types of biochar affect the bioavailability of Cd and Pb in contaminated paddy soils are required. The objectives of this study were to (1) study the effects of biochar addition on the mobility of Cd and Pb in contaminated paddy soil and the accumulation of these metals in rice plants, (2) compare the effects of different biochar sources (wheat straw and peanut shell) on Cd and Pb accumulation, and (3) determine the correlation between Cd and Pb concentrations in rice plants and their transformation in paddy soil after biochar application.
Materials and methods
Experimental materials
Soil
Surface soil (0–15 cm) was collected from a paddy field in Shangba Village, Xinjiang Town, which is located 6 km from the Dabao Mountain mining area in Shaoguan City, Guangdong Province, South China. The Dabao Mountain mine is a polymetallic sulfide deposit. Large quantities of mine waste materials and acidic mine drainage (AMD) have been produced by the mining process for more than 40 years, which are discharged into the Hengshi River. Large areas of agricultural soils are irrigated with the AMD-contaminated water, which has low pH and high heavy metal concentrations (Cd, Pb, Cu, and Zn) (Gu et al. 2011). The soil was collected using spades, and was subsequently stored in polyvinylchloride bags. Soil samples were thoroughly mixed, air-dried, crushed, and passed through a 2-mm nylon mesh before the pot experiment. Soil properties are presented in Table 1.
Biochar
Two types of biochar—peanut shell biochar (PBC) and wheat straw biochar (WBC)—were obtained from Sanli New Energy Company, Henan Province, China; these were produced at a pyrolysis temperature of 350–500 °C. The black carbon content was 30–35%. The biochars were ground and passed through a 1-mm sieve before the pot experiment. The pH of biochar was measured in water at a 1:25 (w/v) ratio after mixing for 5 min and equilibration for 1 h (Gaskin et al. 2008). The pH was then measured using a PHS-3C pH meter (Shanghai INESA Analytical Instrument Co., Ltd., Shanghai, China). The total carbon content of the biochar was analyzed using a previously described method to measure soil organic matter (Liu 1996). The surface functional groups in the biochar were analyzed using Boehm titration (Boehm 1994). Total N concentrations were determined using the semi-micro Kjeldahl method, and total P was determined using Mo-Sb antispetrophotography method (DSH-UV755B UV-Vis Spectrophotometer, Guangzhou SH Biological Technology Co., Ltd., Guangzhou, China), after digestion with H2SO4–H2O2 (Lu 2000). The total heavy metal concentration in the biochar was measured using flame atomic absorption spectrophotometry (FAAS) (Hitachi Z-2300, Japan) after digestion with HCl–HNO3–HClO4 (v/v/v = 15:5:1) (Lu 2000). The basic properties of PBC and WBC are presented in Table 1.
Rice plants
The rice cultivar Boyou 998 was used in the experiment. Seeds were obtained from the Rice Research Institution, Guangdong Academy of Agricultural Sciences. This cultivar is widely cultivated in Guangdong Province, China.
Pot experiment
Plastic pots 25 cm in height and 20 cm in diameter were used for the pot trials. There were three treatment groups with three replicates each: (1) control (without biochar, designated CK), (2) soil amended with 5% (w/w) peanut shell biochar (designated PBC), and (3) soil amended with 5% (w/w) wheat straw biochar (designated WBC). Five kilograms of dried soil was placed in each pot. Urea, potassium dihydrogen phosphate, and potassium chloride were applied to each pot as basal fertilizer to achieve an N application rate of 200 mg kg−1 and P and K equivalent to 150 mg kg−1 P2O5 and 200 mg kg−1 K2O. The fertilizers were added to the soil as solids, and the soil was mixed thoroughly before watering with tap water to produce a 3-cm water layer above the soil surface. The pots were then incubated for 60 days (May 5 to July 4, 2013) in a greenhouse at room temperature with a natural day/night regimen. Tap water was added every day to maintain the 3-cm submergence layer throughout the incubation period.
Sowing
Rice seeds were surface sterilized with 0.1% H2O2 for 20 min, rinsed thoroughly with deionized water, and soaked overnight in sterile water at room temperature. The seeds were then allowed to germinate in sterilized moist sand in a greenhouse. When the seedlings reached the three-leaf stage, five uniformly grown seedlings were transplanted to each prepared pot. Tap water was added daily to maintain the 3-cm submergence layer throughout the experimental period. All pots were arranged randomly outside a greenhouse at South China Agricultural University from July 5 to October 13, 2013. Rice plants were harvested 100 days after transplanting.
Harvest and sampling
Plants
After harvest, the rice plants were separated into roots, straws, husks, and grains. The plant parts were rinsed thoroughly, first with tap water and then with deionized water, and were oven-dried at 105 °C for 30 min and then at 60 °C until a constant weight was attained, after which dry weights were recorded. All dried tissue samples were ground using an electric steel mill. The powder was passed through a 1-mm sieve and was homogenized before chemical analysis.
Soil
Soil sample was collected from each pot and air-dried at room temperature. Plant debris was removed. The soil was then ground with a wood grinder and passed through a 1-mm nylon sieve. The sieved soil samples were collected, and the bulk of each sample was stored in plastic bags for the measurement of pH, cation exchange capacity (CEC), and available heavy metals. A small subsample was ground further, passed through a 0.149-mm nylon sieve, and stored in plastic bags for the determination of soil organic carbon (SOC) and heavy metals extracted by a sequential extraction procedure for soil.
Sample analysis
Soil
Soil pH was measured in a 1:2.5 suspension of soil to water using a PHS-3C pH meter. The mixture was shaken for 2 min and then allowed to settle for 30 min before measurements. Total N and P concentrations were determined using the semi-micro Kjeldahl and molybdenum colorimetry methods. Available P was extracted using 0.5 mol L−1 NaHCO3 and estimated using colorimetry (DSH-UV755B UV-Vis spectrophotometer). SOC was determined using the potassium dichromate oxidation (K2Cr2O7–H2SO4–H2O) spectrophotometric method (DSH-UV755B UV-Vis Spectrophotometer). Soil CEC was extracted with 1 mol L−1 ammonium acetate (pH 7) and determined by FAAS. All methods described above followed Lu (2000).
Soil samples were digested with HCl–HNO3–HClO4 (v/v/v = 15:5:1), and the total heavy metal concentrations were then determined by FAAS. Available Cd and Pb in the soils were extracted with 1 mol L−1 MgCl2 (pH 7): 2.50 g of soil sample was added to 20 mL 1 mol L−1 MgCl2 (pH 7) solution and shaken at 150 rpm for 1 h (Tessier et al. 1979). The extracts were separated from soil samples by filtration through a 0.45-μm membrane. Cd and Pb concentrations in the filtrates were determined by FAAS.
The redistribution of Cd and Pb in soil was determined using the BCR sequential extraction method (Jiang et al. 2012). Cd concentrations in the acid-soluble, reducible, oxidizable, and residual fractions of soil samples were determined using a graphite furnace atomic absorption spectrophotometer (GFAAS) (Hitachi Z-2700, Japan). Pb concentrations in the acid-soluble, reducible, oxidizable, and residual fractions of soil samples were determined by FAAS.
Plant tissues
The Cd and Pb concentrations in plant tissues was determined using FAAS after digestion with HNO3–HClO4 (v/v = 20:3) (Lu 2000).
Data analysis
All data were analyzed by one-way analysis of variance. Tukey’s honest significant difference test was used to assess the statistical significance effect; the level of significance was set at p < 0.05. Bivariate correlations and Pearson’s correlation coefficients (significance set at p < 0.05 and p < 0.01) were also determined. Statistical analysis was performed using SPSS version 16.0 (SPSS Inc., USA).
Results
Plant growth and heavy metal accumulation
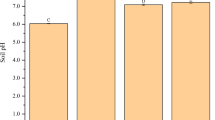
No significant difference was observed in the dry weight of grains between the two biochar treatments and the control; however, the application of WBC led to significantly lower straw dry weight (by 32.3%) than that of the control (Fig. 1).
Effect of biochar application on the weight of straw and grain dry matter of Oryza sativa L. Treatments: CK, control (no biochar applied); PBC, peanut shell biochar applied at 5% (w/w); WBC, wheat straw biochar applied at 5%. Error bars indicate the standard deviation from the means (n = 3). Different letters above the columns indicate significant differences between treatments (p < 0.05)
The Cd concentrations in the roots and grains and Pb concentrations in the roots and straw were significantly reduced by the addition of biochar; however, the type of biochar source material had no significant effect on the uptake and accumulation of Cd and Pb by rice. PBC and WBC application led to significantly lower concentrations of Cd and Pb in rice roots compared to the control (p < 0.05) (Table 2). For instance, Cd and Pb concentrations in rice roots were lower by 50.8 and 22.6% using PBC, and 48.9% and 19.4% using WBC, respectively. Biochar addition had no significant effect on Cd concentrations in the straw, but both PBC and WBC application led to significantly lower Pb concentrations in the straw (p < 0.05) (Table 2). Biochar application led to lower Cd and Pb concentrations in the grains compared to control (Table 2), with Cd concentrations being significantly lower (by 22.9% for PBC and 29.1% for WBC; p < 0.05). While Pb concentration was lower in the grains (by 12.2% for PBC and 15.0% for WBC), this difference was not significant (Table 2).
Effect of biochar on soil properties
Biochar addition led to significantly higher soil pH, SOC, and CEC (Table 3). Soil pH values in the PBC and WBC treatments were significantly higher than those in the control (p < 0.05). Soil pH was 5.96 with PBC and 5.80 with WBC, as against 5.32 in CK (Table 3). PBC and WBC treatments led to significantly higher soil SOC concentrations than those in the control (p < 0.05), but no significant difference was observed between the two treatments (Table 3). PBC and WBC treatments significantly increased soil CECs compared to the control (p < 0.05), but there was no significant difference between PBC and WBC (Table 3).
MgCl2-extractable concentrations of Cd and Pb
The application of the two types of biochar led to significantly lower Cd and Pb in both MgCl2 extracts compared to the control (Fig. 2). Cd and Pb concentrations in the MgCl2 extracts were lower by 45.7 and 79.0% for PBC and by 40.4 and 68.6% for WBC, respectively, than in the control. The addition of PBC was more effective than WBC in reducing extractable Cd and Pb. Furthermore, MgCl2–Cd and MgCl2–Pb concentrations differed significantly between PBC and WBC (Fig. 2).
Effect of biochar application on heavy metal concentrations in MgCl2 extracts in Cd- and Pb-contaminated paddy soil. Treatments: CK, control (no biochar applied); PBC, peanut shell biochar applied at 5% (w/w); WBC, wheat straw biochar applied at 5%. Error bars indicate the standard deviation from the mean (n = 3). Different letters above the columns indicate significant differences between treatments (p < 0.05)
Sequential extraction of Cd and Pb
The application of biochar altered the fraction distributions of Cd and Pb in paddy soil (Fig. 3). The acid-soluble fractions of Cd and Pb were significantly reduced by 27.76 and 48.71% for PBC, and by 21.03 and 41.68% for WBC, respectively (p < 0.05). The application of PBC and WBC significantly increased the reducible fraction of Pb by 19.04 and 24.44% (p < 0.05), respectively. PBC and WBC also led to significant increases in the oxidizable fractions of Cd, by 37.24 and 44.66% (p < 0.05), respectively. The residual fraction of Cd and Pb was increased by 37.87 and 8.68% by PBC, and by 24.88 and 2.98% by WBC, respectively. Some of the acid-soluble heavy metal fractions were transformed into reducible and residual fractions, indicating a decrease in their bioavailability.
Correlation between Cd and Pb bioavailability and soil chemical characteristics
The Cd and Pb concentrations in MgCl2 extracts showed strong and significant negative correlations (p < 0.01) with pH, SOC, and CEC (Table 4).
Correlation between Cd and Pb bioavailability in the soil and accumulation in rice
Correlation coefficients of Cd and Pb concentrations in different parts of the rice plants and the concentration of MgCl2-extractable Cd and Pb in the soil were calculated (Table 5). Concentrations in the MgCl2 extracts were significantly positively correlated with concentrations in rice roots and grains for Cd (p < 0.05) and with concentrations in roots and straw for Pb (p < 0.05) (Table 5).
Discussion
The aim of this study was to examine the effect of different biochar types on the availability and accumulation of Cd and Pb in paddy soil and rice plants. The application of both PBC and WBC led to a significant increase in the pH of the acidic soil (Table 3). Biochars have high pH and have a liming effect when applied to acidic soils (Beesley and Marmiroli 2011; Bian et al. 2014), which increases soil pH. In the process of pyrolysis to produce biochar, the basic cations (mainly Ca, Mg, K, and Na) in biomass are converted to different alkaline substances (such as oxides, hydroxides, and carbonates, such as ash) during the pyrolysis process, and are then mixed with biochar (Houben et al. 2013b). The dissolution of these alkaline substances increases soil pH and CEC. Here, the application of organic carbon-rich biochar led to significantly higher SOC in paddy soil, similar to observations by Kelly et al. (2014) and Zhang et al. (2017). We also found that the application of biochar significantly increased soil CEC (Table 3), corresponding with previous studies (Fellet et al. 2011; Houben et al. 2013a; Puga et al. 2015; Yin et al. 2016). The content of CEC of PBC and WBC was 17–19 times higher than that of the soil used in the experiment (Table 1).
Soil pH, SOC, and CEC are important factors affecting Cd and Pb availability in soil (Rieuwerts et al. 2006; Park et al. 2011; Moon et al. 2013). In the present study, we found that MgCl2-extractable Cd and Pb concentrations were significantly negatively correlated with soil pH (p < 0.01). Zheng et al. (2012) observed that NH4NO3-extractable concentrations of soil Cd and Pb were significantly negatively correlated with soil pH (p < 0.01) in a multielement-contaminated paddy soil supplemented with bean stalk and rice straw biochars. Similarly, Yang et al. (2016) reported significant negative correlations between CaCl2-extractable Cd and Pb concentrations and soil pH (p < 0.01) in a heavy metal-contaminated soil to which bamboo biochar and rice straw biochar had been added. Biochar enhanced soil pH, which led to the precipitation of Cd as CdCO3 (Xue et al. 2012) and of Pb as Pb5(PO4)3OH (Cao et al. 2011). In addition, with increasing pH, the density of cation exchange sites on the biochar surface increases, which improves metal adsorption on the biochar particles themselves (Harvey et al. 2011). Uchimiya et al. (2011) and Houben et al. (2013a) reported that cation exchange played a role in reducing the availability of Cd and Pb under biochar addition by increasing the CEC of the soil. Therefore, in our study, the decrease in extractable Cd and Pb concentrations might have also contributed to increasing soil CEC because CEC and MgCl2-extractable Cd and Pb were significantly negatively correlated (p < 0.01) (Table 5). The formation of stable organic matter complexes with heavy metals could enhance the immobilization of heavy metals with increasing SOC (Namgay et al. 2010; Cui et al. 2016; Zhang et al. 2017). The retention ability to metals enhanced with increasing SOC (Park et al. 2011). The oxygen-containing functional groups (e.g., carboxylic, alcohol, and hydroxyl groups) on the surfaces of biochars form strong complexes with Cd and Pb, thus increasing the adsorption of Cd and Pb on biochars (Uchimiya et al. 2011; Jiang et al. 2012; Xu and Zhao 2013). Moreover, biochars serve as sources of P (Table 1), which might be available to precipitate metals in soil (Ahmad et al. 2014).
We observed that the application of PBC and WBC led to a significantly lower acid-soluble fraction of Cd and Pb and a higher residual fraction than that observed in the control (Fig. 3). Therefore, PBC and WBC promote the conversion of easily available forms of Cd and Pb to relatively stable forms. The difference in Cd and Pb distribution between the various fractions of the control and PBC and WBC treatment groups has considerable implications for the mobility and/or bioavailability of Cd and Pb following biochar application to paddy soil. Similar to our results, Zhu et al. (2015) and Cui et al. (2016) found that biochar application led to a significantly lower acid-soluble fraction of Cd and Pb and a higher residual fraction in acidic paddy soil. The application of biochar increases the residual fraction of Cd and Pb mainly because biochar particles have strong inner-sphere complexation with these metals (Bian et al. 2014). The reducible fraction of Pb significantly increased after the addition of PBC and WBC (Fig. 3). Li et al. (2016) also found that acid-soluble Pb transformed to reducible Pb in paddy soil supplemented with rice straw biochar addition, in supporting our finding that the residual fraction of Pb was significantly higher after biochar application than in the control soil. Liu et al. (2015) also observed that adding rice straw biochar to contaminated paddy soil improves the transformation of Pb from the acid-soluble fraction to reducible and oxidizable fractions. This increase in the reducible fraction of Pb following biochar application could be attributed to the adsorption on Fe/Mn oxides (Fang et al. 2016) and bonds with the mineral phase of Fe, Al, and P on, around, and inside biochar particles (Bian et al. 2014; Ahmad et al. 2017). Fe and Mn oxides in soils are amphoteric colloids, the adsorption of which depends mainly on the surface negative charge. Biochar might improve soil pH value by decreasing H+, Fe3+, Al3+, and Mn2+ concentrations in soil solution. In turn, this action weakens the competitive adsorption of Cd and Pb and further increases the adsorption of Cd and Pb on Fe and Mn oxides (Li et al. 2013). Metals probably bound to Fe/Mn oxides in the soils, because biochars and fertilizers contain very small amounts of Fe, Mn, and Al (Shen et al. 2015; Shen et al. 2016). Furthermore, P entering the soil with biochar application might react with Pb to form hydroxypyromorphite (Cao et al. 2011). Biochars have much more P than soil and fertilizer (Table 1); therefore, Pb was probably immobilized with P in biochars. The significant increase in the oxidizable fraction of Cd after biochar addition might contribute to the increase of SOC after biochar application, which could make more metals form highly stable metal-organic complexes (Zhang et al. 2017). Furthermore, the reducing condition in the soil formed by the decomposition of organic matter helps with the formation of CdS (Covelo et al. 2007).
The Cd and Pb concentrations in different tissues of rice plants, with and without biochars addition, decreased in the order root > straw > husk > grain (Table 2), supporting previous studies (Bian et al. 2014; Zheng et al. 2015; Li et al. 2016). After PBC and WBC application, Cd and Pb concentrations in the roots, Pb concentrations in the straw, and Cd concentrations in the grains were significantly lower than those in the control (Table 3). The reduction in Cd and Pb concentrations in rice tissues might be attributed to the immobilization of Cd and Pb in the soil, as strong and significant positive correlations were noted between Cd and Pb concentrations in rice tissues and the concentrations of these metals in MgCl2 extracts (p < 0.05) (Table 5).
The effects of biochar on the immobilization and phytoavailability of Cd and Pb differed in this study. PBC and WBC reduced MgCl2-extractable metal concentrations in the order Pb > Cd (Table 3), while PBC and WBC reduced grain metal concentrations in the order Cd > Pb (Table 2). This difference could be attributed to the following: (1) the amount of Pb(II) precipitates formed on biochars was much higher than that of Cd(II) because the ability of Pb(II) to form metal-hydroxyl and metal hydroxides was greater than that of Cd(II) (Xu and Zhao 2013); or (2) the transport of Pb in plants is largely restricted because it is blocked by the Casparian strip in the endoderm, adsorbed by the negatively charged pectins in the cell wall, precipitated in intercellular spaces with insoluble oxalate and phosphate, and subjected to complexation with organic acid and phytochelatin in the vacuoles (Shahid et al. 2012).
Our study showed that both PBC and WBC significantly reduce Cd and Pb concentrations in the roots, Pb concentrations in the straw, and Cd concentrations in the grains (Table 3). Zheng et al. (2015) found that Cd concentrations in rice roots, shoots, husks, and grains significantly decreased following bean stalk biochar and rice straw biochar addition in a field experiment; however, only root Pb significantly decreased following the application of rice straw biochar. Therefore, Cd and Pb concentrations in different rice tissues respond differently to the addition of biochar from different feedstocks and to different soil environments. However, further data are needed to validate this conclusion.
Conclusions
We demonstrated that the application of 5% PBC and WBC significantly increased soil pH, SOC, and CEC, and effectively immobilized Cd and Pb in contaminated paddy soil, thus reducing Cd and Pb uptake by paddy. The improved soil pH, SOC, and CEC following biochar treatment played an important role in reducing the mobility and availability of Cd and Pb in the soil. Sequential extraction tests showed that biochar induced the transformation of the acid-soluble fraction of Cd to the oxidizable and residual fractions, and that of Pb to the reducible and residual fractions. Both PBC and WBC were effective in decreasing the Cd concentration in grain, but had no significant effects on Pb concentration. PBC and WBC could be used to enhance the immobilization of Cd and Pb in contaminated paddy soils. The effect of biochar application differed for Cd and Pb accumulation in different rice tissues and also varied with feedstock. Further experiments are needed to investigate the influence of biochar on the mobility and bioavailability of Cd and Pb in contaminated paddy soils under field conditions.
References
Ahmad M, Lee SS, Lee SE, Al-Wabel MI, Tsang DCW, OK YS (2017) Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J Soils Sediment 17:717–730
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, YS OK (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Beesley L, Marmiroli M (2011) The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ Pollut 159:474–480
Beesley L, Moreno-Jiménez E, Gomez-Eyles G, Harris E, Robinson B, Sizmur T (2011) A review of biochar’s potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282
Bian RJ, Joseph S, Cui LQ, Pan GX, Li LQ, Liu XY, Zhang AF, Rutlidge H, Wong SW, Chia C, Marjo C, Gong B, Munroe P, Donne S (2014) A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J Hazard Mater 272:121–128
Boehm HP (1994) Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 32(5):759–769
Cao X, Ma L, Liang Y, Gao B, Harris W (2011) Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ Sci Technol 45:4884–4889
Covelo EF, Vega FA, Andrade ML (2007) Competive sorption and desorption of heavy metals by individual soil components. J Hazard Mater 140:308–315
Cui LQ, Pan GX, Li LQ, Bian RJ, Liu XY, Yan JL, Quan GX, Ding C, Chen TM, Liu Y, Liu YM, Yin CT, Wei CP, Yang YG, Hussain Q (2016) Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: a five-year field experiment. Ecol Eng 93:1–8
Fang SE, Tsang DCW, Zhou FS, Zhang WH, Qiu RL (2016) Stabilization of cationic and anionic metal species in contaminated soils using sludge-derived biochar. Chemosphere 149:263–271
Fellet G, Marchiol L, Delle VG, Peressotti A (2011) Application of biochar on mine tailings: effects and perspectives for land reclamation. Chemosphere 83:1262–1267
Fellet G, Marmiroli M, Marchiol L (2014) Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci Total Environ 468-469:598–608
Gaskin JW, Steiner C, Harris K, Das KC, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 51(6):2061–2069
Gu HH, Qiu H, Tian T, Zhan SS, Deng THB, Chaney RL, Wang SZ, Tang YT, Morel JL, Qiu RL (2011) Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere 83:1234–1240
Harvey OR, Herbert BE, Rhue RD, Kuo LJ (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45:5550–5556
Houben D, Evrard L, Sonnet P (2013a) Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and biomass production of rapeseed (Brassica napus L.) Biomass Bioenergy 57:196–204
Houben D, Evrard L, Sonnet P (2013b) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc, and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Jiang TY, Jiang J, RK X, Li Z (2012) Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 89:249–256
Kelly CN, Peltz CD, Stanton M, Rutherford DW, Rostad CE (2014) Biochar application to Hardrock mine tailings: soil quality, microbial activity, and toxic element sorption. Appl Geochem 43:35–48
Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut R 22:13772–13799
Khan S, Chao C, Weqas M, Arp HPH, Zhu YG (2013) Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environ Sci Technol 47:8624–8632
Li HH, Liu YT, Chen YH, Wang SL, Wang MK, Xie TH, Wang G (2016) Biochar amendment immobilizes lead in rice paddy soils and reduces its phytoavailability. Sci Rep-UK DOI. https://doi.org/10.1018/srep31616
Li MY, LY D, Zhang Y, Gao YD (2013) Influence of pyrolysis temperatures of biochar obtained from the rice straw on cadmium forms. J Soil Water Conserv 27(6):261–264
Liu GS (1996) Analysis of soil physico-chemical properties and profile description. Standards Press of China, Beijing (in Chinese)
Liu JJ, Yang X, Lu KP, Zhang XK, Huang HG, Wang HL (2015) Effect of bamboo and rice straw biochars on the transformation and bioavailability of heavy metals in soil. Acta Scien Circum 35(11):3679–3687 (in Chinese)
Lu KP, Yang X, Shen JJ, Robinson B, Huang HG, Liu D, Bolan N, Pei JC, Wang HL (2014) Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric Ecosyst Environ 191:124–132
Lu RK (2000) Assay on agro-chemical properties of soil. China Agricultural Science and Technology Press, Beijing (in Chinese)
Mahar A, Wang P, Li RH, Zhang ZQ (2015) Immobilization of lead and cadmium in contaminated soil using amendments: a review. Pedosphere 25(4):555–568
Moon DH, Park JW, Chang YY, Ok YS, Lee SS, Ahmad M, Koutsospyros A, Park JH, Baek K (2013) Immobilization of lead in contaminated firing range soil using biochar. Environ Sci Pollut R 20:8464–8471
Namgay T, Singh B, Singh BP (2010) Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.) Aust J Soil Res 48:638–647
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Puga AP, Abreu CA, Melo LCA, Paz-Ferreiro J, Beesley L (2015) Cadmium, lead, and zinc mobility and plant uptake in a mine soil amended with surgarcane straw biochar. Environ Sci Pollut R 22:17606–17614
Rieuwerts J, Ashmore M, Farago M, Thornton I (2006) The influence of soil characteristics on extractability of Cd, Pb and Zn in upland and moorland soils. Sci Total Environ 366:864–875
Shahid M, Pinelli E, Dumat C (2012) Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J Hazard Mater 219:1–12
Shen Z, Jin F, Wang F, McMillan O, Al-Tabbaa A (2015) Sorption of lead by Salisury biochar produced from British broadleaf hardwood. Bioresour Technol 193:553–556
Shen Z, Som AM, Wang F, Jin F, McMillan O, Al-Tabbaa A (2016) Long-term impact of biochar on the immobilization of nickel(II) and zinc(II) and the revegation of a contaminated site. Sci Total Environ 542:771–776
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation for particulate trace metals. Anal Chem 51(7):844–850
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190:432–441
Xu RK, Zhao AZ (2013) Effect of biochars on adsorption of Cu(II), Pb(II) and Cd(II) by three variable charge soils from southern China. Environ Sci Pollut R 20:8491–8501
Xue Y, Gao B, Yao Y, Inyang M, Zhang M, Zimmerman A, Ro K (2012) Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueos heavy metals: batch and column tests. Biochem Eng J 200-202:673–680
Yang X, Liu JJ, McGrouther K, Huang HG, Lu KP, Gao X, He LZ, Lin XM, Che L, Ye ZQ, Wang HL (2016) Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ Sci Pollut R 23:974–984
Yin DX, Wang X, Chen C, Peng B, Tang CY, Li HL (2016) Varying effect of biochar on Cd, Pb and As mobility in a multi-metal contaminated paddy soil. Chemosphere 152:196–206
Zhang RH, Li ZG, Liu XD, Wang BC, Zhou GL, Huang XX, Lin CF, Wang AH, Brooks M (2017) Immobilization and bioavailability of heavy metals in greenhouse soils amended with rice straw-derived biochar. Ecol Eng 98:183–188
Zhao FJ, Ma YB, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759
Zheng RL, Cai C, Liang JH, Huang Q, Chen Z, Huang YZ, Arp HPH, Sun GX (2012) The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere 89:856–862
Zheng RL, Chen Z, Cai C, Tie BQ, Liu XL, Reid BJ, Huang Q, Lei M, Sun GX, Baltrènaitè E (2015) Mitigating heavy metal accumulation into rice (Oryza sativa L.) using biochar amendment—a field experiment in Hunan, China. Environ Sci Pollut R 22:11097–11108
Zhu HH, Cheng C, Xu C, Zhu QH, Huang DY (2016) Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ Pollut 219:99–106
Zhu QH, Wu J, Wang LL, Yang G, Zhang XH (2015) Effect of biochar on heavy metal speciation of paddy soil. Water Air Soil Poll 226:429. https://doi.org/10.1007/s11270-015-2680-3
Acknowledgements
This project was supported by the Hunan Provincial Department of Agriculture (Hunan Agricultural Union 2015-112); the National Key Technologies R&D Program of China (2015BAD05B02); the Natural Science Foundation of Hunan Province, China (No. 2015JJ2081); the Natural Science Foundation of China (No. 41101293); and the China Postdoctoral Science Foundation (2014M562110).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Xu, C., Chen, Hx., Xiang, Q. et al. Effect of peanut shell and wheat straw biochar on the availability of Cd and Pb in a soil–rice (Oryza sativa L.) system. Environ Sci Pollut Res 25, 1147–1156 (2018). https://doi.org/10.1007/s11356-017-0495-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0495-z