Abstract
Different types of manganese ore raw materials were prepared for use as catalysts, and the effects of different manganese ore raw materials and calcination temperature on the NO conversion were analyzed. The catalysts were characterized by XRF, XRD, BET, XPS, H2-TPR, NH3-TPD, and SEM techniques. The results showed that the NO conversion of calcined manganese ore with a Mn:Fe:Al:Si ratio of 1.51:1.26:0.34:1 at 450 °C reached 80% at 120 °C and 98% at 180~240 °C. The suitable proportions and better dispersibility of active ingredients, larger BET surface area, good reductibility, a lot of acid sites, contents of Mn4+ and Fe3+, and surface-adsorbed oxygen played important roles in improving the NO conversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen oxides (NO x ) are a major source of the atmospheric pollution and readily cause acid rain and photochemical smog, which have significant influences on human health. Therefore, the removal of NO x has attracted increasing attention from researchers. At present, to satisfy the NO x emission standards of coke-oven flue gas, low-NO x combustion technology is used to reduce the formation of NO x . However, with the improvement of emission standards, the low-NO x combustion technology cannot satisfy the emission requirements.
Selective catalytic reduction (SCR) of NO x with ammonia (NH3) has been extensively used to treat flue gas (Guo et al. 2015b). The common industrial catalysts for SCR of NO are V2O5/TiO2 or V2O5-WO3/TiO2, for which the operating temperature occurs at approximately 300~400 °C. Because of the high operating temperature, the catalyst must be located upstream of the dust removal system and the desulfurizer unit to avoid a reheating process, but it suffers from deactivation due to high concentrations of dust and sulfur dioxide (Sounak et al. 2008). The temperature of coke-oven flue gas is relatively low and centers on 180~250 °C. If V2O5/TiO2 or V2O5-WO3/TiO2 catalysts are used to remove the NO x of coke-oven flue gas, the NO x conversion will be very low and the NH3 loss will be significant, so it is difficult to meet the emission standards. In addition, the V2O5/TiO2 or V2O5-WO3/TiO2 catalysts are not applicable to the low-temperature flue gas of dusting and desulfurization from power plant (Li et al. 2010). Therefore, many studies have been performed to find a low-temperature NH3-SCR catalyst with high activity.
The SCR of NO by various catalysts combined with NH3 has been frequently investigated (Chen et al. 2015). The active components of catalysts have mainly concentrated on the transition metal oxides (Mn, Fe, Ni, Cr, Co, Zr, Cu, La, etc.) and some precious metals (Pt, Ra, Au, etc.) (Zheng and Wang 2014). Stanciulescu et al. (2012) studied the low-temperature catalytic reduction of NO x for manganese-based catalysts. The results showed that bimetallic catalyst, based on Fe or Mn ion-exchanged zeolites, was active for NH3-SCR of NO x . Schill et al. (2014) prepared a mesoporous Mn0.6Fe0.4/TiO2 catalyst and found that it had good catalytic activity at low temperature. Putluru et al. (2015) prepared Mn/TiO2 and Mn-Fe/TiO2 catalysts by an impregnation method and a co-precipitation method and found that Mn0.75Fe0.25Ti catalysts, which were prepared by the co-precipitation method, showed high NO x conversion at low temperatures. Chen et al. (2011) prepared Fe-Mn mixed-oxide catalysts containing Fe3Mn3O8 which showed high NO x conversion at low temperatures. Yang et al. (2011, 2016) studied the inverse spinel structure of Mn-Fe-based catalysts, which showed high low-temperature SCR activity. Zhang et al. (2015a, b) prepared carbon nanotubes (CNT) loaded with Mn-FeO x and CNT loaded with MnO2-Fe2O3-CeO2-Ce2O3 catalysts, respectively. Their NO x conversions were 73.6~100% at 120~180 °C. Zhou et al. (2013) prepared MnO x -FeO x catalysts by different methods. The results showed that their NO x conversion had a certain difference at low temperature. Although many catalysts could obtain high NO x conversion at low temperatures, the cost of these catalysts was very high. Additionally, they failed to clearly explain why these catalysts could reduce the temperature of NO x conversion.
Manganese ore is a natural mineral raw material and mainly contains manganese and iron-based materials. The raw materials are widely available and the price is low. Using manganese ore as a de-NO x catalyst can reduce the cost. However, few studies have been conducted to investigate the de-NO x performance of manganese ore catalysts. Park et al. (2001) studied the de-NO x performance of natural manganese ore at low temperature. The results showed that the de-NO x efficiency of natural manganese ore was high at low temperature, but the sulfur resistance was poor. Zha et al. (2015) prepared de-NO x catalysts by calcining different types of iron ore. The results indicated that iron ore catalysts showed a better de-NO x performance at low temperatures. There are few studies on manganese ore as de-NO x catalysts, and many problems remain. For example, the effect of calcination temperature on the de-NO x performance of manganese ore is unknown.
Motivated by the need to develop a low-temperature SCR catalyst with high activity, low cost, and minimal pollution, we prepared de-NO x catalysts using manganese ore. The properties of catalysts were characterized by XRF measurements, X-ray diffraction (XRD), the Brunaur-Emmett-Teller (BET) method, X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). The results indicated that the catalyst showed a high activity at low temperatures due to suitable proportions of Mn, Fe, Al, and Si and the better dispersibility of the active ingredients, as well as the larger BET surface area after calcination. Apart from this, good reductibility, a lot of acid sites, the contents of Mn4+ and Fe3+, and surface-adsorbed oxygen (Oβ) also played important roles in the NO conversion.
Experimental
Catalyst preparation
Photographs of four types of manganese ore (A1, A2, B1, and B2) raw materials are shown in Fig. 1. For the manganese ore raw materials to be used as catalysts, they were treated as follows. First, the manganese ore particles were dried at 105 °C for 3 h and then ground for 35 s using an automatic grinding machine to obtain 100-mesh powders. Ground samples were dried at 105 °C for 6 h. Then, the dried samples were calcined at 350, 450, and 550 °C for 5 h to obtain the manganese ore catalysts.
Catalyst performance test
The catalyst performance tests were carried out in a fixed-bed quartz flow reactor using approximately 0.5 g of catalyst at atmospheric pressure. The reactor consisted of quartz glass (8 mm i.d. × 600 mm in length) with a thermocouple that was inserted into the reactor to control the temperature of the catalyst bed. The reactor was heated by a temperature-controlled furnace with feed gases containing 500 ppm NO, 500 ppm NH3, 5 vol% O2, and N2 as balance. The gas flow rate in this experiment was kept at 100 mL/min (the corresponding gas hourly space velocity (GHSV) was 15,000 h−1.) and the mass flow controllers were used to control the gas flow. The activity tests were examined at 120~240 °C. The concentrations of NO in the inlet and outlet gases were measured by an ECOM-J2KN multi-function flue gas analyzer (Germany).
The catalyst activity test was measured by the NO conversion according to the following equation:
[NO]in and [NO]out denote the concentration of NO import and export, respectively, and the concentration of NO2 was negligible.
Catalyst characterization
The XRF measurements were carried out on an ARL ADVANT 'X-3600* fluorescence spectrometer. The elemental contents of the catalysts can be assessed by qualitative and quantitative analysis.
The XRD measurements were carried out on an X-ray diffractometer with CuKα radiation (Bruker D8 Advance, Germany). The diffraction patterns were taken in the 2θ range of 10~80° at a scan speed of 1° min−1 and a resolution of 0.02°.
The BET surface property of catalysts was determined by a V-Sorb 2800 analyzer. The textural characteristics of these samples were measured by N2 adsorption at 77 K and then degassed under vacuum at 180 °C for 12 h. The specific surface area was determined by the Brunauer-Emmet-Teller method.
XPS analysis was conducted on an Escalab 250Xi X-ray electron spectrometer (Thermo Scientific, USA) with an Al Kα X-ray source. The observed spectra were calibrated with the C1s binding energy (BE) value of 284.6 eV.
H2-TPR measurement was carried out on Auto Chem II 2920 instrument containing 0.1 g catalyst. A 10% H2 and 90% Ar mixture with a flow rate of 30 mL/min was fed to the sample, which was heated from 80 to 800 °C with a heating rate of 10 °C/min. The H2 content of the effluent gas was analyzed by a thermal conductivity detector (TCD) (Micromeritics, USA).
NH3-TPD measurement was carried out on Auto Chem II 2920 instrument containing 0.1 g catalyst. Catalysts (0.1 g) were pretreated at 100 °C in a flow of He (30 mL/min) for 2 h and then cooled to 50 °C. A 10% NH3–90% He mixture was fed in at 50 °C until saturation, after which the catalyst was swept with He and heated at an increasing temperature up to 550 °C with a heating rate of 10 °C/min.
A SEM (HITACHI, S-4800) was used to observe the microstructural phenomena of the catalyst surface.
Results and discussion
Catalyst activity evaluation
Figure 2 illustrates the NO conversion of four manganese ore catalysts. Figure 2 shows that, with increasing temperature, the NO conversions of these catalysts increase gradually. At approximately 120 °C, the NO conversions reach 80% for the samples A1 and A2 and reach 75 and 66% for the samples B2 and B1, respectively. When the temperature is approximately 160 °C, the NO conversion of the sample A2 increases significantly and reaches over 90%. At 180~240 °C, the NO conversion remains approximately 98%. It can be concluded that the NO conversion of the four samples have some differences, which may result from the compositions, material structures, and surface properties of manganese ores. The NO conversion of the sample A2 is best at 120~240 °C, as Fig. 2 shown.
As shown in Fig. 3, the NO conversion of the sample A2 is obviously higher than that of the reference (Zhang et al. 2016a, b) under the same experimental conditions. In the study of Cao et al. (2015), the GHSV is lower than that of our experiment. However, the NO conversion is also lower than that of the sample A2.
A comparison of the NO conversion between sample A2 and those reported in literatures. Reaction conditions of sample A2: 500 ppm NO, 500 ppm NH3, 5 vol% O2, N2 as balance, and GHSV = 15,000 h−1. Reaction conditions of Cao et al. (2015): 700 ppm NO, 700 ppm NH3, 3 vol% O2, N2 as balance, and GHSV = 10,000 h−1. Reaction conditions of Zhang et al. (2016a, b): 500 ppm NO, 500 ppm NH3, 5 vol% O2, N2 as balance, and GHSV = 15,000 h−1.
Effect of calcination temperature
As discussed above, the NO conversion of the sample A2 was the best, so we chose the sample A2 for further study.
The compositions of manganese ore are very complex. In order to obtain a better dispersion and larger specific surface area of the active ingredients of manganese ore, it should be calcined. Moreover, appropriate calcination temperatures can make the active ingredients of the catalyst lose oxygen to form oxygen vacancies, which can generate active sites that are beneficial to flue gas adsorption (Wang et al. 2015).
Figure 4 shows the NO conversions of the sample A2 without calcination or calcined at different temperatures. The NO conversions of calcined samples are apparently higher than those of the sample without thermal treatment at 120~240 °C. Compared with 350 and 550 °C, the NO conversion for the sample calcined at 450 °C is the best, which is beyond 80% at 120 °C and approximately 90% at 160 °C and remains approximately 98% at 180~240 °C. If the calcination temperature is low, the sample may not achieve a good surface performance, which affects the activity of the catalysts. However, if the calcination temperature is too high, the sample is easy to sinter, so the crystal shape of active ingredients can change. At the same time, the formation of oxygen vacancies is also affected (Ruan et al. 2001). Therefore, a suitable calcination temperature is important to the catalyst activity.
Catalyst characterization
XRF
Table 1 shows the elemental analysis of the four manganese ore samples. As shown in Table 1, the main elements of the four manganese ore samples are Mn, Fe, Al, and Si. However, the NO conversions of the four samples are different due to the different contents of Mn, Fe, Al, and Si. According to the discussion above, the NO conversion of the sample A2 is the best, so it can be concluded that the synergistic effects of Mn, Fe, Al, and Si with the Mn:Fe:Al:Si ratio of 1.51:1.26:0.34:1 contribute to high NO conversion.
XRD
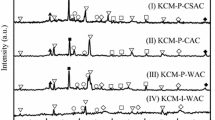
Figure 5 shows the X-ray diffraction pattern of the sample A2 without calcination or calcined at different temperatures. As shown in Fig. 5, only SiO2 peaks (PDF#46-1045) appear and are observed at 20.86°, 26.68°, 28.70°, 36.56°, 42.46°, 50.14°, 60.06°, and 68.19°. Other metal oxide peaks are not detected, indicating that Mn, Fe, or Al possibly exists in highly dispersed or an amorphous phase on the surface of the catalysts (Min et al. 2007), which may be the reason for the excellent catalytic activity at low temperatures. Figure 6 shows a partial magnification of the X-ray diffraction pattern of the sample A2 without calcination or calcined at different temperatures. Figure 6(a) shows that the sample that is not calcined has two small peaks at 18.78° and 21.29°, which correspond to the H2Si6O13 crystal phase. The calcined samples do not exhibit the H2Si6O13 peak, but the peaks of Al3Fe5O12 at 35.8° are detected (shown in Fig. 6(b)). These results show that H2Si6O13 is decomposed to SiO2 and Al3Fe5O12 is formed after the calcination, and the formation of Al3Fe5O12 may result in the high catalytic activity of the sample A2.
BET surface area
Catalytic activity is affected by the specific surface area of the catalyst (Wan et al. 2014). The BET surface areas of these catalysts measured from N2 physisorption are listed in Tables 2 and 3. The BET surface areas of the calcined samples of A2 are much higher than those of the samples without calcination (shown in Table 2). Moreover, the surface area decreases with increasing calcination temperature. The B2 sample calcined at 450 °C shows the high surface area among these samples. Combined with Figs. 1 and 2, the NO conversion of the sample A2 is the best when it is calcined at 450 °C. Although usually the high surface area of catalyst has high activity, the BET results indicate that the surface area is not the determining factor to improve catalytic activity (shown in Table 3). This is consistent with previous findings that the catalytic activity of catalyst is more dependent on surface chemistry than surface area (Li et al. 2015).
XPS analysis
XPS analysis was performed to identify the nature of the surface and the concentrations of the main elements. The XPS spectra of Mn2p, Fe2p, and O1s of the samples A2 and B1 were obtained. The results are given in Tables 4, 5, and 6 and Figs. 7, 8, and 9.
Figure 7 shows the Mn2p XPS spectra of the samples A2 and B1. The two main peaks at 642 and 654 eV are assigned to Mn2p3/2 and Mn2p1/2, respectively. The measured binding energies of Mn2p3/2 in the samples A2 and B1 (642–645 eV) are slightly higher than those reported for MnO, Mn2O3, and MnO2 (Strohmeier et al. 1985). According to previous studies (Guo et al. 2015a; Wang et al. 2012a, b), the Mn2p3/2 XPS spectra can be divided into two peaks corresponding to Mn3+ (642 ± 0.5 eV) and Mn4+ (640.5 ± 0.5 eV), respectively. The binding energy of various valence states and the valence state ratio of Mn are shown in Table 4. The results of the XPS analysis from Table 4 demonstrate that the ratio of AMn 4+/AMn 3+ for the sample A2 is slightly higher than that for the sample B1. It has been proven that Mn4+ plays a significant role in the redox cycle of the SCR reaction (Thirupathi and Smirniotis 2012), and the high Mn4+ concentration enhances the low-temperature SCR activity of the catalysts (Zhang et al. 2016a, b). In addition, Mn4+ can promote the oxidation of NO to NO2, which is favorable to promote low-temperature NO conversion (Fang et al. 2013).
The Fe2p XPS spectra of the samples A2 and B1 displayed in Fig. 8. The Fe 2p3/2 peaks are composed of two peaks near 710.4 and 712.3 eV, respectively, which indicates that the Fe in these samples is present as both Fe3+ and Fe2+ (Roosendaal et al. 1999; Zhang et al. 2014; Allen et al. 1974). The ratio of Fe3+/Fe2+ is 1.70 for the sample A2, which is higher than that for the sample B1 (shown in Table 5). According to the literature (Zhang et al. 2016a, b; Devadas et al. 2007; Delahay et al. 2005), Fe3+ facilitates NO conversion at low temperature, so the activity of the sample A2 is higher than that of the sample B1.
Figure 9 exhibits the O1s XPS spectra of the samples A2 and B1. After a peak-fitting deconvolution, the O1s peak of the sample A2 can be separated into three peaks, and the sample B1 can be separated into two peaks. According to the literatures (Hanawa et al. 2001; Min et al. 2007; Schindler et al. 2009), the peak at the lower binding energy near 529.7 eV is attributed to lattice oxygen (Oα); the second one at higher binding energy at approximately 531.3 eV is assigned to surface-adsorbed oxygen (Oβ); the third oxygen peak near 532.1 eV originates from chemisorbed water (Oγ). Usually, Oβ is more reactive than Oα in oxidation reactions due to its higher mobility (Wu et al. 2008). As listed in Table 6, the concentration ratio of Oβ/(Oα + Oβ + Oγ) of the sample A2 is 7.7%. However, there is no Oβ present on the sample B1. Therefore, it can be concluded that the NO conversion of the sample A2 mainly depends on the existence of Oβ.
H2-TPR analysis
To investigate the redox properties of the samples A2 and B1, H2-TPR experiment was performed. The H2-TPR profiles of the samples A2 and B1 are depicted in Fig. 10. As can be seen from Fig. 10, the TPR profiles of the samples A2 and B1 had several reduction peaks, indicating that the reduction process occurred. It is difficult to estimate the precise location of reduction peak for natural manganese ore due to its complicated composition, so there is a little deviation of the reduction peak relative to the standard oxide reduction peak (Lu et al. 2000; Tae et al. 2001). The TPR curve of the sample B1 shows the peaks at 326, 357, 413, 543, 641, and 721 °C corresponding to the reduction of MnO2 to Mn2O3 (326 °C), Fe2O3 to Fe3O4 (357 °C), Mn3O4 to MnO (413 °C), Fe3O4 to FeO (543 °C), and FeO to Fe (641 and 721 °C) (Li et al. 2016), respectively. However, for the sample A2, it can be easily seen that the temperatures corresponding to reduction peak shift to lower temperatures. It is well recognized that the reduction peak temperature is an indication of reducibility; lower reduction peak means stronger reducibility (Liu et al. 2008). Accordingly, the activity of the sample A2 is higher than that of the sample B1.
NH3-TPD analysis
The adsorption and activation of NH3 on the surface acid sites of the catalysts is a key process in the low-temperature NH3-SCR reaction (Liu et al. 2015). Therefore, the NH3-TPD analyses were performed and the results are shown in Fig. 11. The similar TPD patterns are observed for the corresponding catalysts, which correspond to three desorption peaks. One strong desorption peak can be observed with a peak maxima at 103 °C followed by two weak peaks at 320 and 500 °C for the sample A2. The desorption peaks at 103 and 320 °C are attributed to the successive desorption of NH3 physisorbed on weak acid sites and the Brönsted acid sites, respectively (Wang et al. 2016), while the peak at 500 °C can be ascribed to desorption of NH3 which are coordinated to the Lewis acid sites (Shen et al. 2014). These results implied that NH3 was adsorbed on both Brönsted and Lewis acid sites on the catalyst surface. It is interesting to note that the peak areas of weak acid sites and Lewis acid sites in sample A2 are obviously larger than those of the sample B1, and the peak area stands for the adsorption amount of NH3 (Cai et al. 2016; Jiang et al. 2009), so it can be concluded that the number of acid sites of the sample A2 is more than that of the sample B1. Therefore, the SCR activity of the sample A2 was higher than that of the sample B1.
SEM analysis
To further study the reason why the sample A2 has a higher NO conversion at low temperature, the micro-morphologies of the catalyst which was without calcination and calcined at different temperatures were investigated by SEM. Additional microstructural phenomena of the catalyst samples are shown in the followed SEM images (Fig. 12). As Fig. 12 shows, for the sample without calcination, the particle size is very large. However, for the calcined sample, the particle size is relatively small. Moreover, the dispersibility of the calcined sample at 450 °C is better than that of the samples without calcination and calcined at 350 and 550 °C, so the NO conversion of the sample calcined at 450 °C is higher than that of the samples without calcination and calcined at 350 and 550 °C.
Conclusion
Manganese ore raw materials prepared for use as catalysts for NH3-SCR at low temperature were investigated. The results indicated that the NO conversion of calcined manganese ore catalyst with the Mn:Fe:Al:Si ratio of 1.51:1.26:0.34:1 at 450 °C reached 80% at 120 °C and 98% at 180~240 °C. Based on the characterization results for the catalysts, the high catalytic activity was determined to result from the following aspects. (1) The optimal synergistic effects of Mn, Fe, Al, and Si at the ratio of 1.51:1.26:0.34:1 play an important role in improving the catalytic activity. (2) The active ingredients of the catalysts, such as Mn, Fe, and Al, disperse or exist in an amorphous phase on the surface of the catalysts, which increase the surface area of the catalyst and improve the catalytic activity. (3) The good reductibility, a lot of acid sites, the presence of higher concentrations of Mn4+ and Fe3+, and O β also play important roles to improve the catalyst activity at low temperature.
References
Allen GC, Curtis MT, Hooper AJ, Tucker PM (1974) X-ray photoelectron spectroscopy of iron-oxygen systems. J Chem Soc Dalton Trans 14:1525–1530
Cai S, Hu H, Li HR, Shi L, Zhang D (2016) Design of multi-shell Fe2O3@MnO x @CNTs for the selective catalytic reduction of NO with NH3: improvement of catalytic activity and SO2 tolerance. Nano 8:3588–3598
Cao F, Su S, Xiang J, Wang PY, Hu S, Sun LS, Zhang AC (2015) The activity and mechanism study of Fe-Mn-Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 139:232–239
Chen WS, Luo J, Qin LB (2015) Selective autocatalytic reduction of NO from sintering flue gas by the hot sintered ore in the presence of NH3. J Environ Mange 164:146–150
Chen ZH, Wang FR, Li H, Yang Q, Wang LF, Li XH (2011) Low-temperature selective catalytic reduction of NO x with NH3 over Fe-Mn mixed-oxide catalysts containing Fe3Mn3O8 phase. Ind Eng Chem Res 51:202–212
Delahay G, Valade D, Guzmán-Vargas A, Coq B (2005) Selective catalytic reduction of nitric oxide with ammonia on Fe-ZSM-5 catalysts prepared by different methods. Appl Catal B Environ 55:149–155
Devadas M, Kröcher O, Elsener M, Wokaun A, Mitrikas G, Söger N, Pfeifec M, Demel Y, Mussmann L (2007) Characterization and catalytic investigation of Fe-ZSM5 for urea-SCR. Cataly Today 119:137–144
Fang C, Zhang D, Cai S, Zhang L, Huang L, Li H, Maitarad P, Shi L, Gao R, Zhang J (2013) Low-temperature selective catalytic reduction of NO with NH3 over nanoflaky MnO x on carbon nanotubes in situ prepared via a chemical bath deposition route. Nano 5:9199–9207
Guo RT, Chen QL, Ding HL, Wang QS, Pan WG, Yang NZ, Lu CZ (2015a) Preparation and characterization of CeO x @ MnO x core-shell structure catalyst for catalytic oxidation of NO. Catal Commun 69:165–169
Guo RT, Wang QS, Pan WG (2015b) The poisoning effect of heavy metals doping on catalyst for selective catalytic reduction of NO with NH3. J Mol Catal A Chem 407:1–7
Hanawa T, Hiromoto S, Asami K (2001) Characterization of the surface oxide film of a Co-Cr-Mo alloy after being located in quasi-biological environments using XPS. Appl Surf Sci 183:68–75
Jiang BQ, Liu Y, Wu ZB (2009) Low-temperature selective catalytic reduction of NO on MnO x /TiO2 prepared by different methods. J Hazard Mater 162:1249–1254
Liu J, Zhao Z, Wang JQ, Xu CM, Duan AJ, Jiang GY, Yang Q (2008) The highly active catalysts of nanometric CeO2-supported cobalt oxides for soot combustion. Appl Catal B Environ 84:185–195
Liu Y, Xu J, Li HR, Cai SX, Hu H, Fang C, Shi LY, Zhang DS (2015) Rational design and in situ fabrication of MnO2@NiCo2O4 nanowire arrays on Ni foam as high-performance monolith de-NO x catalysts. J Mater Chem A 3:11543–11553
Li JH, Zhang XL, Chen TH (2010) Characterization and ammonia adsorption-desorption of palygor-skite-supported manganese oxide as a low-temperature selective catalytic reduction catalyst. Chinese J Catal 31:454–460
Li SJ, Wang XX, Tan S, Shi Y, Li W (2015) CrO3 supported on sargassum-based activated carbon as low temperature catalysts for the selective catalytic reduction of NO with NH3. Fuel 160:35–42
Li Y, Wan Y, Li YP, Zhan SH, Guan QX, Tian Y (2016) Low-temperature selective catalytic reduction of NO with NH3 over Mn2O3-doped Fe2O3 hexagonal microsheets. Acs Appl Mater Inter 8:5224–5233
Lu WZ, Zhao XG, Wang H, Xiao WD (2000) Catalytic oxidation of NO. Chin J Catal 21:423–427
Min K, Park ED, Ji MK, Yie JE (2007) Manganese oxide catalysts for NO x reduction with NH3 at low temperatures. Appl Catal A Gen 327:261–269
Putluru SSR, Schill L, Jensen AD (2015) Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation-promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl Catal B Environ 165:628–635
Park TS, Jeong SK, Hong SH, Hong SC (2001) Selective catalytic reduction of nitrogen oxides with NH3 over natural manganese ore at low temperature. Ind Eng Chem Res 40:4491–4495
Roosendaal SJ, Asselen BV, Elsenaar JW, Vredenberg AM, Habraken FHPM (1999) The oxidation state of Fe (100) after initial oxidation in O2. Surf Sci 442:329–337
Ruan HD, Frost RL, Kloprogge JT (2001) The behavior of hydroxyl units of synthetic goethite and its dehydroxylated product hematite. Spectrochim Acta A Mol Biomol Spectrosc 57:2575–2586
Schill L, Putluru SSR, Fehrmann R, Jensen AD (2014) Low-temperature NH3-SCR of NO on mesoporous Mn0.6Fe0.4/TiO2 prepared by a hydrothermal method. Catal Lett 144:395–402
Shen BX, Ma HQ, He C, Zhang XP (2014) Low temperature NH3-SCR over Zr and Ce pillared clay based catalysts. Fuel Process Technol 119:121–129
Schindler M, Hawthorne FC, Freund MS, Burns PC (2009) XPS spectra of uranyl minerals and synthetic uranyl compounds. II: the O1s spectrum. Geochim Et Cosmochim Ac 73:2471–2487
Sounak R, Viswanath B, Hegde MS (2008) Low-temperature selective catalytic reduction of NO with NH3 over Ti0.9M0.1O2-δ (M=Gr, Mn, Fe, Co, Cu). J Phys Chem C 112:6002–6012
Stanciulescu M, Caravaggio G, Dobri A, Moir J, Burich R, Charland JP, Bulsink P (2012) Low-temperature selective catalytic reduction of NO x with NH3 over Mn-containing catalysts. Appl Catal B Environ 123:229–240
Strohmeier BR, Leyden DE, Field RS, Hercules DM (1985) Surface spectroscopic characterization of manganese/aluminum oxide catalysts. J Phys Chem 16:4922–4929
Tae SP, Soon KJ, Sung HH, Sung CH (2001) Selective catalytic reduction of nitrogen oxides with NH3 over natural manganese ore at low temperature. Ind Eng Chem Res40:4491–4495
Thirupathi B, Smirniotis PG (2012) Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: catalytic evaluation and characterizations. J Catal 288:74–83
Wang F, Dai H, Deng J, Bai G, Ji K, Liu Y (2012b) Manganese oxides with rod-, wire-, tube-, and flower- like morphologies: highly effective catalysts for the removal of toluene. Environ Sci Technol 46:4034–4041
Wang J, Dong X, Wang Y, Li Y (2015) Effect of the calcination temperature on the performance of a CeMoO x catalyst in the selective catalytic reduction of NO x with ammonia. Catal Today 245:10–15
Wang XB, Wu SG, Zou WX, Yu SH, Gui KT, Dong L (2016) Fe-Mn/Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3. Chinese J Catal 37:1314–1323
Wang L, Huang B, Su Y, Zhou G, Wang K, Luo H, Ye D (2012a) Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3: catalytic activity and characterization. Chem Eng J 192:232–241
Wan YP, Zhao WR, Tang Y, Li L, Wang HJ, Cui YL, Gu GL, Li YS, Shi JL (2014) Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl Catal B Environ 148:114–122
Wu Z, Jin R, Liu Y, Wang H (2008) Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal Commun 9:2217–2220
Yang SJ, Qi FH, Xiong SC, Dang H, Liao Y, Wong PK, Li JH (2016) MnOx supported on Fe-Ti spinel: a novel Mn based low temperature SCR catalyst with a high N2 selectivity. Appl Catal B Environ 181:570–580
Yang SJ, Wang CZ, Li JH, Yan NQ, Ma L, Chang HZ (2011) Low temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel: performance, mechanism and kinetic study. Appl Catal B Environ 110:71–80
Zhang R, Li Y, Zhen T (2014) Ammonia selective catalytic reduction of NO over Fe/Cu-SSZ-13. RSC Adv 4:52130–52139
Zhang W, Shi Y, Li C, Zhao QD, Li XY (2016a) Synthesis of bimetallic MOFs MIL-100(Fe-Mn) as an efficient catalyst for selective catalytic reduction of NO x with NH3. Catal Lett 146:1956–1964
Zhang W, Shi Y, Li CY, Zhao QD, Li XY (2016b) Synthesis of bimetallic MOFs MIL-100(Fe-Mn) as an efficient catalyst for selective catalytic reduction of NO x with NH3. Catal Lett 146:1956–1964
Zhang YB, Zheng YY, Wang X, Lu XL (2015b) Preparation of Mn-FeO x /CNTs catalysts by redox co-precipitation and application in low-temperature NO reduction with NH3. Catal Commun 62:57–61
Zhang YB, Zheng YY, Zou HQ, Zhang X (2015a) One-step synthesis of ternary MnO2-Fe2O3-CeO2-Ce2O3/CNT catalysts for use in low-temperature NO reduction with NH3. Catal Commun 71:46–50
Zha XB, Liang H, Gui KT, Cai S, Wang R (2015) Study on the performance of iron ore catalysts on SCR of NO x with NH3 at low-temperature. J Eng Thermophys-rus 36:811–815
Zheng YY, Wang X (2014) Research progress on Mn-based catalysts for low-temperature selective catalytic reduction of NO x . J Funct Mater 45:11008–11012
Zhou C, Zhang Y, Wang X, Xu H, Sun K, Shen K (2013) Influence of the addition of transition metals (Cr, Zr, Mo) on the properties of MnO x -FeO x catalysts for low-temperature selective catalytic reduction of NO x by ammonia. J Colloid Interf Sci 392:319–324
Acknowledgments
We greatly appreciate the financial support provided by the National Natural Science Foundation of China (Nos. 51676001, 51376007, and U1660206), the Anhui Provincial Natural Science Foundation (No. 1608085ME104), and Key Projects of Anhui Province University Outstanding Youth Talent (Nos. gxyqZD2016074 and gxyqZD2017038).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zhu, B., Yin, S., Sun, Y. et al. Natural manganese ore catalyst for low-temperature selective catalytic reduction of NO with NH3 in coke-oven flue gas. Environ Sci Pollut Res 24, 24584–24592 (2017). https://doi.org/10.1007/s11356-017-0122-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0122-z