Abstract
Cadmium (Cd) is a biologically non-essential heavy metal while the cultivation of Cd-tolerant varieties/hybrids (V) seems the most promising strategy for remediation of Cd-contaminated soils. For this, 24-day-old seedlings of seven maize hybrids, DKC 65-25, DKC 61-25, DKC 919, 23-T-16, 32-B-33, 31-P-41, and Syn hybrid, were grown in hydroponic conditions for 21 additional days in various Cd concentrations (0, 5, 10, and 15 μM). Effects of variety, Cd, and their interaction were highly significant (p ≤ 0.05) for studied plant agronomic and physiological traits except the V × Cd interaction for leaf chlorophyll content, root-shoot length, and root dry weight. The Cd accumulation in root and shoot increased gradually with increasing Cd treatments while copper (Cu), zinc (Zn), and manganese (Mn) uptake was decreased in all hybrids. The reduction in root and shoot biomass and Cd uptake was lower in 32-B-33 and 23-T-16 compared to other hybrids. The highest accumulation of Cu, Zn, and Mn was observed in 32-B-33, DK C65-25, and 31-P-41, respectively. The differential uptake and accumulation of Cd by maize hybrids may be useful in selection and breeding for Cd-tolerant genotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accumulation of heavy metals in agricultural soils mainly results due to anthropogenic activities such as application of contaminated effluents, industrial waste, phosphate fertilizers, herbicides and pesticides, agrochemicals, and sewage sludge (Adrees et al. 2015; Rehman et al. 2015; Rizwan et al. 2016a). Coupled with these anthropogenic activities, volcanic eruption, forest fires, run off, and production of sea salt aerosols are among the various natural sources of heavy metal release into the environment (Nagajyoti et al. 2010). Among heavy metals, cadmium (Cd) is widely distributed in world soils both naturally as well as anthropogenically (Choppala et al. 2014; Du et al. 2014). Several studies have reported that Cd toxicity decreased the plant growth and biomass by generating reactive oxygen species at cellular and subcellular levels (Rizwan et al. 2016b, 2016c). The Cd toxicity also decreased the photosynthetic pigments and gas exchange characteristics in a variety of plant species (Li et al. 2015; Lysenko et al. 2015; Tauqeer et al. 2016). Cd mainly enters into humans via food crops grown in Cd-contaminated soil (Rizwan et al. 2016a, 2016b). The Cd toxicity in humans has caused a number of disorders such as bone fracture, kidney failure, mental abnormalities, hypertension, and various skin disorders (Song et al. 2015). Similarly, Cd is also toxic to animals, causing disturbances in their metabolic activities along with various lethal diseases, which occur by grazing or engulfing the Cd-contaminated fodder and other crops (Wang et al. 2016a).
Plants have developed a range of natural defense systems against metal stress comprising of both enzymatic and non-enzymatic antioxidants, production of osmolyte, and synthesis of chelating agents (Artiushenko et al. 2014; Adrees et al. 2015; Rizwan et al. 2015). Tolerance against metal stress varies depending upon plant species, variety, and the type of metal stress (Artiushenko et al. 2014). Maize (Zea mays L.) is a hyper accumulator plant and has the ability to accumulate and tolerate a certain Cd concentration without exhibiting toxicity symptoms (Yang et al. 2016). Maize has the potential to enhance its biomass production that act as supporting pillar for Cd tolerance in this crop (Rizwan et al. 2016b). Privileged biomass production accumulates the Cd in legislating volume and transports Cd towards the aerial parts (Broadhurst et al. 2015; Wang et al. 2016b). The genetic variations exist in Cd uptake by crop plants (Naeem et al. 2016; Rizwan et al. 2016c). For instance, Cd uptake and accumulation was higher in maize cultivar (31P41-Pioneer) sensitive to Cd stress compared to cultivar (3062-Pioneer) tolerant to Cd stress (Tanwir et al. 2015). Similarly, Cd stress decreased the growth and biomass of the Agatti-2002 maize cultivar compared to the EV-1098 cultivar and 50 μM Cd applied in the sand medium did not affect the growth parameters of the latter cultivar (Hussain et al. 2012). In another study, Cd was accumulated up to 45 mg kg−1 of maize dry weight without appearing with visible toxicity symptoms with a growth medium pH of greater than 6 (Broadhurst et al. 2015). Thus, selection of Cd-tolerant maize cultivars might be an effective strategy for the cultivation of Cd-contaminated soils (Rizwan et al. 2016b). However, varieties developed from traditional lines are not known for Cd accumulation potential, which may have variations in Cd uptake and root to shoot translocation compared to traditional parents (Naeem et al. 2016). This suggests that existing varieties/cultivars should be characterized for Cd uptake and translocation ability. The present study was conducted to evaluate the Cd accumulating ability of different maize hybrids and the interaction of Cd with micronutrient uptake and translocation.
Materials and methods
Plant culture and treatments
A hydroponic experiment was conducted in the warehouse of the Institute of Soil and Environmental Sciences, University of Agriculture Faisalabad (UAF) Pakistan. Seeds of seven maize hybrids, approved for general cultivation in Punjab, Pakistan, were collected from Monsanto (DKC 65-25, DKC 61-25, and DKC 919), Pioneer (32-T-16, 32-B-33, and 31-P-41), and Syngenta (Syn-hybrid) seed distributors in Faisalabad. Seeds were surface sterilized with 10% H2O2 and rinsed thoroughly with distilled water. The disinfected seeds were sown in polyethylene-lined iron trays containing moist sand, prewashed with a hydrochloric acid solution (10%) followed by washing with distilled water. Ten-day-old seedlings were transplanted into foam-plugged holes (one seedling per hole) of polystyrene sheets floating on plastic tubs with 25 L capacity. Each tube was filled with a modified nutrient solution containing the following: 16.0 mM KNO3, 2.0 mM NH4H2PO4, 6.0 mM KCl, 4.0 mM Ca(NO3)2.4H2O, 1.0 mM MgSO4.7H2O, 1.5 mM MgSO4.7H2O, 2.0 μM ZnSO4.7H2O, 0.5 μMn CuSO4.5H2O, 2.0 μM MnSO4. H2O, 0.5 μM H2MoO4, 25 μM H3BO4, and 50 μM Fe-EDTA (Johnson et al. 1957). Additional N produced by NH4H2PO4, and Ca(NO3)2.4H2O supplementation was subtracted from KNO3. After 2 weeks of growth in the nutrient solution, the plants were subjected to different Cd concentrations (0, 5, 10, and 15 μM as Cd(NO3)2) in each plastic tube. After every week of growth, the old nutrient solution was replaced with fresh one. To ensure oxygen supply to the roots, nutrient solution was kept aerated continuously using aeration pumps connected to each tub via plastic tubes. The pH of the nutrient solution was monitored daily and adjusted to 6.5 ± 0.05 with 0.1 N HCl or NaOH. The experiment was laid out in completely randomized factorial design with four replicates.
Measurements of gas exchange parameters and chlorophyll contents
Two weeks after Cd exposure, gas exchange parameters such as photosynthetic rate, transpiration rate, and stomatal conductance were measured from the third upper fully expanded leaf, using system LCA-4 ADC portable infrared gas analyzer (Analytical Development Company, Hoddesdon, England). Readings were taken by clamping the central part of the leaf in the chamber of the instrument between 11:00 am to 12:00 am to ensure unchanged photon flux density and temperature. Chlorophyll measurement, in terms of SPAD value, was performed on the third upper leaf with the help of chlorophyll SPAD meter (SPAD-502).
Plant harvesting and biomass
The plants were harvested after 3 weeks of Cd stress then washed with tap water followed by two washings with distilled water. Plants were blotted dry and separated into roots and shoots while their fresh biomasses were recorded. Root and shoot length was recorded using stainless steel scale prior to plant drying. Then, samples were stored in labeled paper bags and air-dried. Followed by air drying, samples were dried at 65 ± 5 °C in a forced air-driven oven till a constant weight and dry biomass of root and shoot was recorded.
Measurement of Cd and micronutrients
Plant samples (1.0 g each sample) were mixed in 5 ml of each concentrated HNO3 and HClO4 in a conical flask and kept for overnight. Then, 5 ml of concentrated HNO3 was added in the next day and digestion was performed using hot plate until the material became clear. Followed by digestion, samples were cooled and diluted to 50 ml with distill water (AOAC 1990). This diluted material was filtered with Whatman filter paper number 42 and stored in airtight plastic bottles.
Cadmium, zinc (Zn), copper (Cu), and manganese (Mn) concentrations were measured by using calibrated atomic absorption spectrometer (Model Thermo Electron S-Series). The regression relation between concentration and absorbance of the standard solutions were used to calculate the concentration of unknown samples. The enrichment factor (ability of plants to accumulate metal) was calculated as the ratio of shoot Cd concentration to Cd concentration in nutrient solutions (Chen et al. 2004). The translocation factor was calculated as the ratio of shoot Cd concentration to root Cd concentration (Baker and Whiting 2002). Both roots and shoots fresh biomass Cd tolerance index (FBTI) of maize hybrids was calculated by the following formula;
Statistical analysis
The data were statistically analyzed following the ANOVA technique (Steel et al. 1996). The least significant difference (LSD) test was applied to differentiate the treatment means differences using the Statistix 8.1 computer software (version 8.1 Software package).
Results
Physiological traits
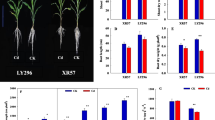
Effect of maize hybrids (V), Cd levels, and their interactions were significant (p ≤ 0.05) for studied plant physiological traits (photosynthesis rate, transpiration rate, chlorophyll content, and stomatal conductance) except for V × Cd interaction for leaf chlorophyll content (Table 1). By increasing the Cd levels in the growth medium, a decline in maize physiological traits was observed and the highest reduction in these parameters was attained at maximum Cd stress (15 μM Cd) compared to the control. Effects of Cd stress were more prominent on transpiration rate and stomatal conductance as compared to photosynthesis rate and chlorophyll content. Maize hybrids denoted variable susceptibility to Cd stress depending upon applied Cd concentrations and studied physiological traits. The maize hybrid DKC 61-25 reported significantly higher photosynthesis rate and stomatal conductance while the hybrid DKC 919 and Syn resulted in a maximum transpiration rate and chlorophyll content, respectively. Among hybrids, Cd-associated photosynthesis reduction was minimum in 32-B-33 and maximum in DKC 65-25 as compared to other hybrids. Least tolerance to applied Cd stress was observed for DKC 65-25 for all studied traits at all Cd levels.
Agronomic traits
Plant agronomic traits were significantly (p ≤ 0.05) affected under Cd stress except for root dry biomass at 10 and 15 μM Cd levels (Table 1). No significant interaction of V × Cd was documented for most of the studied traits except shoot dry biomass, which was significant (Table 1). By increasing Cd levels, a gradual decline in studied agronomic traits was observed for all hybrids.
Micronutrient uptake
The potential of maize hybrids for micronutrients (Cu, Zn, and Mn) accumulation in plant shoot and root was evaluated both under Cd-stressed and non-stressed conditions (Table 2). Both Cd concentration and maize hybrid background significantly (p ≤ 0.01) affected the plant shoot and root potential to uptake these elements. No significant interaction of V × Cd was evaluated for both root and shoot for Cu and Mn while Zn accumulation was unaffected by this combination (Table 2). Accumulation of micronutrients was higher at a minimum Cd stress level and gradually decreased with increasing Cd concentrations in the nutrient solution. All hybrids responded differently depending upon Cd levels and plant part for studied elements accumulation. The maize hybrid 32-B-33 accumulated the highest Cu concentrations while the DKC 65-25 hybrid accumulated the highest Zn concentrations in both shoot and roots compared to the other hybrids. 31-P41 and DKC 919 exhibited greater accumulation of Mn in shoot and roots, respectively. The difference in shoot and root Mn concentration was the least compared to Cu and Zn accumulation. The Cd stress produced the highest negative effect on Zn accumulation in both shoot and roots in all hybrids.
Cadmium tolerance and translocation
Plant Cd tolerance index (CTI) responded similarly as for micronutrient accumulation. Increasing Cd levels in the growth medium gradually decreased the CTI in all maize hybrids compared to the control (Fig. 1). Effects of Cd levels and hybrids were highly significant for all calculated CTI except for root length CTI that was non-significant. The hybrid 32-B-33 showed minimum CTI for shoot biomass accumulation (0.24) while DKC 919 exhibited least CTI (0.62) for shoot length. Root biomass accumulation documented minimum value for CTI for DKC-61-25 while CTI for root length was statistically non-significant among hybrids (Fig. 1). Except the 32-B-33 hybrid, all maize hybrids showed non-significant effect for root length CTI at all levels of Cd treatments.
Shoot fresh biomass cadmium (Cd) tolerance index (SFBCTI), shoot dry biomass Cd tolerance index (SDBCTI), shoot length Cd tolerance index (SLCTI), root fresh biomass Cd tolerance index (RFBCTI), root dry biomass Cd tolerance index (RDBCTI), and root length Cd tolerance index (RLCTI) for different studied maize hybrids under exogenous applied Cd stress levels
Cadmium translocation index and Cd concentration in shoot and root were significantly higher in those pots where the highest Cd level was applied (Fig. 2, Table 3). The genotypic differences in Cd translocation and accumulation in studied hybrids demonstrated the highest Cd translocation index for the 23-T-16 hybrid. The Cd concentration increased in both shoots and roots of all maize hybrids in a dose-additive manner (Table 3). At all Cd stress, the Syn hybrid accumulated the highest Cd concentration in shoots while the 31-P-41 hybrid accumulated the highest Cd concentration in the roots.
Discussion
In the present study, six maize hybrids were tested in hydroponic medium to observe Cd-induced changes in growth and nutrient accumulation. Significant reduction in plant growth and gas exchange processes was recorded with increasing Cd stress in all maize hybrids with different extent. The highest negative effect was attained where the maximum level of Cd was applied. Cadmium-induced growth reduction as recorded in this study is in line with previous studies such as shown in Brassica juncea (Iqbal et al. 2005) and Pisum sativum (Metwally et al. 2005). There are evidences of Cd-associated stunted growth and physiological traits under hydroponic conditions (Rizwan et al. 2016a). The Cd-based growth reduction in maize is possibly due to a reduction in photosynthetic activity and disturbance in leaf photosystems (Rizwan et al. 2016b). Cadmium denatures protein by disrupting the H-S (hydrogen-sulfur) bond which is responsible for stunted growth and development (Lin et al. 2007). In the present study, chlorophyll contents and gas exchange parameters as well as plant dry biomass decreased with increasing Cd levels in the growth medium (Table 1). Similar results related to Cd-mediated reduction in plant growth and photosynthesis have been observed in many plant species (Lysenko et al. 2015; Arshad et al. 2016; Rizwan et al. 2016a, 2016b). The maize hybrid DKC 61-25 reported significantly higher photosynthesis rate and stomatal conductance that may be due to its higher tolerance and adaptability to Cd treatments. The Cd-induced photosynthetic limitation is might be due to stomatal opening and closing and metabolic processes impairment (Wu et al. 2004, 2006). Another possible reason of the photosynthesis reduction under Cd stress is attributed to limited chlorophyll contents and decreased activity of rubisco and due to lowered sub-stomatal CO2 concentration (Wu et al. 2004; Cui and Wang 2006). Cd-mediated variation in gas exchange parameters in maize hybrids might be due to genetic variation in maize hybrids (Anjum et al. 2015). It is well documented that plants potentially develop a wide range of defense systems to minimize the toxic effects of metal stress (Artiushenko et al. 2014; Rizwan et al. 2016b). This defensive system may comprise of enzymatic and non-enzymatic antioxidants, production of osmolyte and chelate synthesis or enhanced cell wall lignification, and suberin lamella formation (Lux et al. 2011; Adrees et al. 2015). Higher level of Cd accumulation may have affected the antioxidant enzymes (ascorbate peroxidase, superoxide dismutase, and glutathione reductase) that have resulted in inhibition of plant physiological activities. Plants could tolerate metal stress to a certain level by enhancing the activities of antioxidant enzymes, metallothioneins, and stress proteins; the response varies with plant species and metal applied (Xu et al. 2014; Anjum et al. 2015; Parrotta et al. 2015).
Our results reveal that on average, the concentration of Zn, Cu, and Mn in roots and shoots of the seven maize genotypes significantly decreased by the application of 15 μM Cd in the growth medium. The reduction in shoot and root Mn concentration due to Cd toxicity was minimum as compared to Cu and Zn (Table 2). Cd stress induced the highest negative effect on Zn accumulation in both shoot and root (Rizwan et al. 2016a). Cadmium reduces the uptake of these micronutrients by modifying the permeability of plasma membranes and contending for the same membrane transporters. This phenomenon alters the nutrient concentration and composition in plants (Sarwar et al. 2010). Resultantly, Cd causes nutrient deficiency and imbalance in plants (Rizwan et al. 2012). Furthermore, the alteration in micronutrient concentrations and uptake in plants could also be due to a Cd-induced reduction in enzyme activity (i.e., catalase, peroxidase, polyphenol oxidase, superoxide dismutase) and inhibition of root emergence and growth (Chen et al. 2003). In the present study, there existed a significant variation among maize genotypes for root and shoot micronutrient concentration, as affected by Cd levels. The maize hybrid DKC 65-25 showed higher potential for accumulation of Zn, Cu, and Mn, even with incremental Cd toxicity, as compared to other genotypes. It has been shown that there were genotypic differences in the five wheat genotypes for Fe, Zn, and Cu uptake and translocation by adding of 1 mg Cd L−1 to the nutrient solution (Zhang et al. 2002). Their conclusion was that the effect Cd in solution on micronutrient content varies among elements, plant organ, and genotype. A study by Wang et al. (2007) revealed the variation in Cd, Fe, Cu, Mn, and Zn accumulation in two maize genotypes; one had a higher capacity to uptake Cd from solution and could be a potential hyperaccumulator for Cd toxicity.
Cadmium uptake by roots and shoots varied greatly among the hybrids and increased linearly with increasing concentration of applied Cd (Table 3). The Cd uptake and translocation varied among the wheat cultivars (Naeem et al. 2016) and maize cultivars (Hussain et al. 2012; Broadhurst et al. 2015; Tanwir et al. 2015). The variation in Cd uptake among maize hybrids might be due to the difference in the genetic makeup of the plants. The roots accumulated the higher amount of Cd compared to shoots (Table 3). The first strategy of plants exposed to Cd stress is an accumulation or deposition of Cd in the cell wall (Fernandez et al. 2014; Rizwan et al. 2016a). The higher accumulation of Cd in roots compared to shoots is quite similar to the phenomenon that the maize plants increase their root biomass to cope with the heavy metal stress like Cd (Rizwan et al. 2016b). Root played a vital role in rhizosphere modification by transforming the architecture, solubility, mobility, and uptake of nutrients (Adrees et al. 2015; Keller et al. 2015). Uptake of Cd in roots mainly depends on root structure and root activities (Stritsis et al. 2014). The plant cell wall serves as a first barrier blocking heavy metal entry into cells and is considered as a pivotal site for storage and deposition of Cd that resulted as a crucial mechanism for plant heavy metal tolerance. Moreover, owing to being negatively charged, the plant cell wall of maize has significant potential for heavy metal binding and retention (Polle and Schützendübel 2004). Overall, the higher accumulation of Cd in roots may enhance the plant tolerance to Cd stress.
Conclusion
Cadmium effects on growth, biomass, chlorophyll contents, and gas exchange parameters varied greatly among the maize hybrids and decreased with increasing Cd concentrations in the growth medium. The uptake of micronutrients varied greatly among maize hybrids and decreased with increasing the Cd levels. Among the studied hybrids, 31-P-41 and Syn hybrid accumulated the highest Cd concentrations in roots and shoots, respectively. The higher Cd accumulating maize hybrids may be used for both phytoremediation of Cd-contaminated soils and in the selection and breeding for Cd-tolerant genotypes. However, the molecular basis of such differential accumulation needs to be further investigated.
References
Adrees M, Ali S, Rizwan M, Rehman MZ, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
Anjum NA, Sofo A, Scopa A, Roychoudhury A, Gill SS, Iqbal M, Lukatkin AS, Pereira E, Duarte AC, Ahmad I (2015) Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res 22:4099–4121
AOAC (1990) Official methods of analysis. Association of Official Analytical Chemists, Inc, Virginia
Arshad M, Ali S, Noman A, Ali Q, Rizwan M, Farid M, Irshad MK (2016) Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch Agron Soil Sci 62:533–546
Artiushenko T, Syshchykov D, Gryshko V, Čiamporová M, Fiala R, Repka V, Martinka M, Pavlovkin J (2014) Metal uptake, antioxidant status and membrane potential in maize roots exposed to cadmium and nickel. Biologia 69:1142–1147
Baker AJ, Whiting SN (2002) In search of the Holy Grail—a further step in understanding metal hyperaccumulation? New Phytol 155:1–4
Broadhurst CL, Chaney RL, Davis AP, Cox A, Kumar K, Reeves RD, Green CE (2015) Growth and cadmium phytoextraction by Swiss chard, maize, rice, Noccaea caerulescens, and Alyssum murale in pH adjusted biosolids amended soils. Int J Phytorem 17:25–39
Chen YX, He YF, Luo YM, YL Y, Lin Q, Wong MH (2003) Physiological mechanism of plant roots exposed to cadmium. Chemosphere 50:789–793
Chen Y, Wang C, Wang Z, Huang S (2004) Assessment of the contamination and genotoxicity of soil irrigated with wastewater. Plant Soil 261:189–196
Choppala G, Saifullah Bolan N, Bibi S, Iqbal M, Rengel Z, Kunhikrishnan A, Ashwath N, Ok YS (2014) Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit Rev Plant Sci 33:374–391
Cui Y, Wang Q (2006) Physiological responses of maize to elemental sulphur and cadmium stress. Plant Soil Environ 52:523–529
Du YL, He MM, Xu M, Yan ZG, Zhou YY, Guo GL, Nie J, Wang LQ, Hou H, Li FS (2014) Interactive effects between earthworms and maize plants on the accumulation and toxicity of soil cadmium. Soil Biol Biochem 72:193–202
Fernandez R, Fernandez-Fuego D, Bertrand A, Gonzalez A (2014) Strategies for Cd accumulation in Dittrichia viscose (L.) greuter: role of the cell wall, non-protein thiols and organic acids. Plant Physiol Biochem 78:63–70
Hussain I, Iqbal M, Qurat-ul-Ain S, Rasheed R, Mahmood S, Perveen A, Wahid A (2012) Cadmium dose and exposure-time dependent alterations in growth and physiology of maize (Zea mays). Int J Agri Biol 14:959–964
Iqbal N, Masood A, Nazar R, Syeed S, Khan NA (2005) Photosynthesis, growth and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in cadmium tolerance. Agri Sci China 9:519–527
Johnson CM, Strout R, Broyer TC, Carlton AB (1957) Comparative chlorine requirements of different plant species. Plant Soil 8:327–353
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241:847–860
Li Y, Chen Z, Xu S, Zhang L, Hou W, Yu N (2015) Effect of combined pollution of Cd and B [a] P on photosynthesis and chlorophyll fluorescence characteristics of wheat. Pol J Environ Stud 24:157–163
Lin AJ, Zhang XH, Chen M, Cao Q (2007) Oxidative stress and DNA damages induced by cadmium accumulation. J Environ Sci 19:596–602
Lux A, Martinka M, Vaculik M, White PJ (2011) Root response to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Lysenko EA, Klaus AA, Pshybytko NL, Kusnetsov VV (2015) Cadmium accumulation in chloroplasts and its impact on chloroplastic processes in barley and maize. Photosynth Res 125:291–303
Metwally A, Safronova VI, Bellimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J Exp Bot 56:167–178
Naeem A, Saifullah, Rehman MZ, Akhtar T, Ok YS, Rengel Z (2016) Genetic variation in cadmium accumulation and tolerance among wheat cultivars at the seedling stage. Commun Soil Sci Plant Anal 47:554–562
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman JF (2015) Target or barrier? The cell wall of early and later diverging plants vs cadmium toxicity: differences in the response mechanisms. Front Plant Sci 6:1–16
Polle A, Schützendübel A (2004) Heavy metal signaling in plants: linking cellular and organismic responses. In Plant responses to abiotic stress. Springer, Berlin Heidelberg, pp 187–215
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rizwan M, Meunier JD, Hélène M, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209-210:326–334
Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Rehman MZ, Qayyum MF, Abbas F (2015) Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Pollut Res 22:15416-15431
Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, Keller C (2016a) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23:1414–1427
Rizwan M, Ali S, Qayyum MF, Ok YS, Rehman MZ, Abbas Z, Hannan F (2016b) Use of maize (Zea mays L.) for phytomanagement of Cd contaminated soils: a critical review. Environ Geochem Health. doi:10.1007/s10653-016-9826-0
Rizwan M, Ali S, Rizvi H, Rinklebe J, Tsang DCW, Meers E, Ok YS, Ishaque W (2016c) Phytomanagement of heavy metals in contaminated soils using sunflower—a review. Crit Rev Environ Sci Technol. doi:10.1080/10643389.2016.1248199
Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, Farida G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agr 90:925–937
Song WE, Chen SB, Liu JF, Li CH, Song NN, Ning LI, Bin LI (2015) Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J Int Agri 14:1845–1854
Steel RGD, Torrie JH, Dickey DA (1996) Principles and procedures of statistics: a biometrical approach, 3rd edn. McGraw Hill, New York
Stritsis C, Steingrobe B, Claassen N (2014) Cadmium fractions in an acid sandy soil and Cd in soil solution as affected by plant growth. J Plant Nutr Soil Sci 177:431–437
Tanwir K, Akram MS, Masood S, Chaudhary HJ, Lindberg S, Javed MT (2015) Cadmium-induced rhizospheric pH dynamics modulated nutrient acquisition and physiological attributes of maize (Zea mays L.). Environ Sci Pollut Res 22:9193–9203
Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Iftikhar U, Ahmad R, Farid M, Abbasi GH (2016) Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotoxicol Environ Saf 126:138–146
Wang M, Duan ZJ, Jiang X, Liu DW (2007) Cadmium accumulation and its effects on metal uptake in maize (Zea mays L.). Biores Technol 98:82–88
Wang HY, Wen SL, Chen P, Zhang L, Cen K, Sun GX (2016a) Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environ Sci Pollut Res 23:3781–3788
Wang A, Wang M, Liao Q, He X (2016b) Characterization of Cd translocation and accumulation in 19 maize cultivars grown on Cd-contaminated soil: implication of maize cultivar selection for minimal risk to human health and for phytoremediation. Environ Sci Pollut Res 23:5410–5419
Wu FB, Chen F, Wei K, Zhang GP (2004) Effect of cadmium on free amino acid, glutathione and ascorbic acid concentrations in two barley genotypes (Hordeum vulgare L.) differing in cadmium tolerance. Chemosphere 57:447–454
Wu F, Dong J, Jia G, Zheng S, Zhang G (2006) Genotypic difference in the responses of seedling growth and Cd toxicity in rice (Oryza sativa L.). Agri Sci China 5:68–76
Xu X, Liu C, Zhao X, Li R, Deng W (2014) Involve- ment of an antioxidant defense system in the adaptive response to cadmium in maize seedlings (Zea mays L.). Bull Environ Contam Toxicol 93:618–624
Yang Y, Xiong J, Chen R, Fu G, Chen T, Tao L (2016) Excessive nitrate enhances cadmium (Cd) uptake by up-regulating the expression of OsIRT1 in rice (Oryza sativa). Environ Exp Bot 122:141–149
Zhang G, Fukami M, Sekimoto H (2002) Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage. Field Crop Res 77:93-98
Acknowledgments
The authors greatly acknowledge the financial support from the University of Agriculture Faisalabad (UAF), Pakistan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Akhtar, T., Zia-ur-Rehman, M., Naeem, A. et al. Photosynthesis and growth response of maize (Zea mays L.) hybrids exposed to cadmium stress. Environ Sci Pollut Res 24, 5521–5529 (2017). https://doi.org/10.1007/s11356-016-8246-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8246-0