Abstract
There are great concentrations of toxic metallic and metalloid elements such as lead, arsenic, mercury, cadmium or silver in many species of mushrooms comparative to other fruits and vegetables. In this study, contamination with heavy and toxic metallic and metalloid elements in the cultivated mushroom of (Pleurotus florida (Mont.) Singer) is investigated. P. florida was cultivated on different substrates; wheat straw (as blank), wheat straw + pine cone, wheat straw + soybean straw and wheat straw + urea and the effects of these substrates on contamination levels of Mn, Fe, Cu, Zn, As, Cd, and Pb were analyzed. The results showed that the concentrations of essential elements (Mn, Fe, Cu, and Zn) in the target mushroom are at the typical levels. The estimated daily intakes of studied metallic and metalloid elements were below their oral reference dosage mentioned by the international regulatory bodies. Health risk index (HRI) was calculated to evaluate the consumer’s health risk assessment from the metal intake that contaminated in the cultivated mushroom of P. florida on the different nutrient sources. In this study, the individual HRIs were less than 1, which indicates insignificant potential health risk associated with the consumption of target mushroom from the studied substrates. Based on the HRIs values among the toxic metallic and metalloid elements, As in the target mushroom in the substrate of the wheat straw + pine cone is the main sources of risk, and it may cause severe health problems. Thus, this study suggests that the concentrations of heavy and toxic elements should be periodically monitored in cultivated mushrooms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the major public health concern is the presence of heavy metals in soil, air, water, and living objects because of rapid industrialization and urbanization in the developing countries (Granero and Domingo 2002; Govil et al. 2008; Huang et al. 2007; Li et al. 2008). Although some heavy metals such as Fe, Co, Mn, Zn as bio-metals are common constituents of mushroom flesh and are required in trace amounts by living organisms, but some other metallic and metalloid elements such as cadmium (Cd), lead (Pb), mercury (Hg), silver (Ag), and arsenic (As), which can be well bio-concentrated from soil and other substrate by many mushrooms collected in the wild and from a farm (Jarzyńska et al. 2012; Saba et al. 2016a, b; Falandysz and Rizal 2016; Jarzyńska and Falandysz 2012) are considered to be harmful. Cadmium is toxic to cardiovascular, kidneys and bones. Lead and mercury decrease the intelligence quotients (IQ) in children due to the pathological changes of organs and damnification of the central nervous system. Arsenic can affect human health and there is a strong association between arsenic exposure and increased risks of both carcinogenic and systemic health effects. Arsenic exposure affects almost all organ systems including the cardiovascular, dermatologic, nervous, hepatobilliary, renal, gastro-intestinal, and respiratory systems (Tchounwou et al. 2003). The heavy metals are generally dangerous due to tendency to bioaccumulate, a process that increase the chemical element’s concentration in a biological organism occurs over time, compared to the concentration of a chemical element in the environment. Therefore, it is desirable to monitor and control amounts of the heavy metals in foods such as cultivated edible mushroom due to their detrimental effects on the human health.
Mushrooms have been used as food and food components in many countries for a long time (Prabu and Kumuthakalavalli 2014). They are healthy source for vegetarian people (Mallikarjuna et al. 2013; Nnorom et al. 2012; Vetter et al. 2005; Nnorom et al. 2013). They are popular in many developed and being accepted in many developing countries like India (Menaga et al. 2012). Mushrooms can use the agricultural wastes because of having the ability to biodegrade the substrate, so they have an important role in the ecosystem (Mleczek et al. 2016). The genus Pleurotus (Fr.) P. Kumm has various edible mushroom species. This genus has the ability to grow on the agro-industrial lignocellulosic wastes. Pleurotus Ostreatus is the second most cultivated edible mushroom globally after Agaricus bisporus (Sanchez 2010). In fact, P. ostreatus and P. florida, represent a single species (Gonzalez and Labarere 2000). The genus Agaricus especially A. bisporus with some A. brasilensis is the major genus with about 30% of the world’s cultivated mushrooms. Pleurotus, a close second, with 5 to 6 cultivated species, constitutes about 27% of produced cultivated mushrooms worldwide (Royse 2014). The highest number of studies on the mushrooms has been done in the European continent area. Also, there are at least 75,000 species on this continent, and more than 15,000 species of them are macrofungi (Falandysz and Borovička 2013). Pleurotus species are popular and widely cultivated throughout the world mostly in Asia, America and Europe because of their simple, low cost production technology and high biological efficiency (Hoa et al. 2015). One of the most important species of this genus is P. florida. It is commonly called as the white oyster mushroom.
Pleurotus species have unique flavor and aromatic properties nutritionally and can be used for their nutritive and medicinal properties (Agrahar-Murugkar and Subbulakshmi 2005). These mushrooms have low cholesterol and fat levels and can be a rich source for protein, some essential amino acids, fiber, potassium, vitamins (Gupta 2014). Alam et al. (2008) had a study on nutritional analysis of cultivated mushrooms; Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica in Bangladesh. They reported that these mushrooms have 20 ~ 25% proteins, 13 ~ 24% fibers and contained a lower amount of lipid about 4 to 5%. Khan (2010) showed that P. florida can be a rich source of protein, lipids, carbohydrates, vitamin and minerals content but low in calories and fat content. The content (g/100 g dried mushroom) was 17–42 g protein, 37–48 g carbohydrates, 24–31 g fiber, and 4–10 g minerals whereas lipid content was so low around 0.5–5 g. Fruit bodies in mushrooms are rich in vitamins, especially vitamin-B1, vitamin- B2, vitamin-C, and vitamin-D2 (Manzi et al. 2004). Wang and Ng (2000) and Mattila et al. (2006) demonstrated that the vitamins contents (mg/100 g of dried product) of P. ostreatus are respectively 30–65 mg/100 g niacin; 28–35 mg/100 g ascorbic acid, 1.9–2.0 mg/100 g thiamin, 1.8–5.1 mg/100 g riboflavin, and 0.3–0.7 mg/100 g folate. Cooking and other processing methods can be decreased or increased the main composition of mushrooms (Falandysz and Borovička 2013). Manzi et al. (2001) reported a study on the nutritional value of mushrooms widely consumed in Italy. They used fresh and proceed mushrooms (deep frozen, canned, and dried), raw and after cooking. The results showed that technological process can be affected significantly on the protein, carbohydrate ash, and fibers contents. Nutrient concentrations were increased significantly by cooking procedure. Also, the results of their study showed that the nutrient amounts loss during deep freezing might be because of structural damage of the vegetable cells. The most contents of protein, carbohydrate, fat, fiber, and ash belonged to the dried mushroom especially after cooking. In conclusion, their research showed that the cooking does not harm the nutritional quality of mushrooms. On the other hand, preservation and cooking methods can effect on some heavy metals. Falandysz and Drewnowska (2015) performed a cooking experiment to test the effect of short term blanching (cooking in boiling water for 10 min) of fresh fruiting bodies of Amanita fulva on the Hg content of the blanched mushrooms. The results showed that there was not more than 10% loss of Hg (per volume/weight of the product per unit of dry matter content). This is because, during the 10 min blanching time, there was a loss of a portion of Hg and a corresponding loss of a portion of the water soluble organic constituents of fruiting bodies and also of its original moisture. Pleurotus sp. is considered as medicinal mushrooms, exhibiting hematological, antiviral, antitumor, antibiotic, antibacterial, hypocholesterolic, and immunomodulation activities (Patil et al. 2010). The compounds such as lectins, polysaccharides, polysaccharide-peptides, polysaccharide-protein complex have been isolated from mushrooms and they have been found to have antioxidant, anticancer, antimicrobial, antidiabetic, antihypercholestrolemic, and immunomodulatory properties (Cohen et al. 2002; Bobek and Galbavy 2001). Patel et al. (2012) have been done a review study on medicinal properties of Pleurotus species (Oyster Mushroom). The information of their review showed that oyster mushroom has many therapeutic characteristics. Although, more high-tech approaches is required for deeper exploration. They reported the medicinal properties of Pleurotus including; antimicrobial, anti-Human Immunodeficiency Virus (HIV), antitumor, antimutagenic, antioxidant, antilipidemic, hyperglycemic, hypotensive, anti-Inflammatory, hepatoprotective, hypocholesterolemic and immunomodulatory.
There are different studies that they have focused considerable attention on the accumulation of heavy metals in several mushroom species (Busuioc et al. 2011; Chen et al. 2009; Cocchi et al. 2006; Demirbaş 2000; Zhang et al. 2010). The existence of high metal concentrations in mushrooms is considered important because of a potential toxicological hazard due to their relative position in the food chain (Garcia et al. 1998; Zhu et al. 2011). Therefore, investigating heavy metal contents of cultivated edible mushrooms has tremendous significance for consumers in the whole world.
In this study, the effects of different nutrient sources on concentrations of metallic and metalloid elements in the cultivated mushroom (P. florida) was investigated. The findings from this study can be used to estimate the contamination level and distribution of the heavy metallic and metalloid elements in a cultivated mushroom (P. florida) on the different substrates. But it should be mentioned that the species of mushrooms can differ in their ability of bioconcentration of the different elements in fruiting bodies. On the other hand, as the nutrient sources have weaker ability in absorbing of the heavy metals, the results obtained can be beneficial. Another objective of this study is to evaluate the potential health risks associated with the heavy metallic and metalloid elements via consumption of target cultivated mushroom from the different substrates using the estimated daily intake (EDI) and health risk index (HRI) from single toxic heavy metallic and metalloid elements.
Materials and methods
Cultivation of mushroom sample in different nutrient sources
The Pleurotus florida spawn was obtained from a local market in Birjand (South Khorasan, Iran). The wheat straw was collected from local agricultural filed. The experiment was conducted in randomized block design with three replications. Treatment of substrate namely were wheat straw alone (as a blank) and in combination with soybean, urea, and pine cone. The wheat straw was sun dried and chopped into small bits (3–5 cm long) then was soaked in the water for about 24 h and then boiled in water 60 °C for 15 min. After that it was cooled to the environment temperature and excess of water was drained out to moisture 70%. The above prepared substrates were used for spawning with mushroom. The autoclavable (121 °C for 30 min) polythene bags were used for above purpose. After complete sterilization, the wheat straw was laid to the height of 5–6 cm and the spawn of P. florida species and three different supplements were broadcasted on this straw layers. Likewise, 5–6 layers of spawn were spread in these bags. The bags were tied up and then perforated with a needle at the regular intervals. From the first day of cultivation up to 22 days after that, the temperature was kept at 25 °C and the humidity should be 85 to 90% using humidifier. There is no need for light because the light is not appropriate for oyster mushrooms in the growth phase. Approximately 10 days after inoculation the mycelium growth can be seen. After 15–18 days, the complete growth takes place. When the fruiting bodies developed the mushrooms were harvested. The cleaned mushrooms were sun dried and preserved in polythene bags as well.
Analytical method
For the analysis of the heavy metallic and metalloid elements in the target mushroom with inductively coupled plasma atomic emission spectroscopy (ICP-AES), the following digestion protocol was applied. Samples of P. florida were collected from each of the four different substrate sources used. The mean of fruiting bodies in a sample (each substrate) was 26. But for each substrate, a total of 5 samples were collected to examine in the next steps. Mushrooms were individually cleaned, cut and finally dried at 105 °C for 24 h. An agate homogenizer was used to homogenize dried samples then they were kept at +4 °C in pre-cleaned polyethylene bottles until analyzed. Deionized water from a Milli-Q system (Millipore, USA) was used to prepare all aqueous solutions. All chemicals such as mineral acids and oxidants (HNO3 and H2O2) were of the analytical grade (Merck, Darmstadt, Germany). All of the plastic equipment and glassware were cleaned by soaking overnight in a 10% nitric acid solution and subsequently washed several times with deionized water before using. For the elemental analysis, a Varian 735-ES ICP-AES was used. The working conditions of ICP-AES are shown in Table 1.
For digestion, a Milestone Ethos-Up microwave closed system was used. Samples (0. 5 g) were digested with 9 mL of HNO3 (65%) and 1 mL of H2O2 (30%) for 7 min, and finally diluted to 50 mL with deionized water. A blank digestion was performed similarly. For the digestion procedure, the heat was reached to 180 °C in 5 min and kept for 2 min constantly. This process was done two times (Sarikurkcu et al. 2012).
Method valuation
The limits of detection (LODs) and limits of quantitative (LOQs) were determined at a critical concentration where the signal-to-noise (S/N) ratios were 3 and 10, respectively. The corresponding results are given in Tables 2 and 3. In order to validate the method for accuracy and precision, a certified reference material (CRM), namely poplar leaves (GBW07605) was analyzed for the corresponding elements. As shown in Table 4, the result of the analysis of the CRM showed good agreement with the certified levels.
Health risk index (HRI)
Health risk index (HRI) can be calculated by investigating of the contamination of heavy metals for the human body. In this research, mushrooms grown on the different nutrient sources and the concentration of elements were used to calculate the HRI. The HRI value depends on the daily intake of metals (DIM) and oral reference dose (RfD). RfD is an evaluated per day contamination of metal to the human body that has no harmful effect in their life time (US-EPA IRIS 2006). The HRI was calculated for As, Cd and Pb. According to the data in the integrated risk information system (IRIS), oral reference doses for As, Cd and Pb are 0.0003, 0.001 and 0.004 mg kg−1 day−1, respectively (US-EPA IRIS 2006). The HRI for As, Cd and Pb was calculated using Eq. (1) (Jan et al. 2010):
where DIM indicates the used amount of metals per day and RfD shows the dosage of the reference oral. DIM was calculated according to the following equation:
In Eq. (2), CM, DFI and BAW demonstrate the heavy metal concentrations in mushrooms (mg/kg), daily intake of mushrooms and average body weight, respectively.
Results and discussion
Nutrient sources in cultivation of P. florida
There is a wide range of different cellulosic substrates are used to cultivate mushrooms (Amin et al. 2010). Actually, mushrooms can be cultivated on decayed organic matters rich in lignin, cellulose, and other complicated carbohydrates (Alananbeh et al., 2014). Carbon, nitrogen, and inorganic compounds as their nutritional sources are required for Pleurotus species to grow. In this study, the wheat straw has been used as main substrate source combined with three nutritional supplements; urea, soybean, and pine cone. Fungus absorbs nitrogen as organic nitrogen. Urea, amines, amino acids, and proteins have organic nitrogen. Urea is also converted to ammonium (NH4 +) and nitrate (NO3 −) and then taken up by the fungus hyphae. Natural organic sources of N for the cultivation of oyster mushrooms are always preferred. The oyster cultivation has been developed on mixtures of wheat and urea, so organic wastes of agriculture and food industry origin that have nitrogen as protein can be used (Nunes et al. 2012). One of the main sources of supplying protein and oil in the world is soybean (Moraditochaee 2012). It has 52% nitrogen and can be a good fertilizer for edible mushrooms cultivation. Soybean as agro-waste can be used in combination with cereal agro-waste to increase the yield of P. ostreatus (Patil et al. 2010). Pinus eldarica was one of the softwood species planted in many parts of Iran, and it has shown good adaptation to the environmental condition (Kiaei 2011). One of the most important non-wood forest products is pine nuts that they also have export value. They include of numerous health promoting phyto-chemicals, vitamins, antioxidants, and minerals. Annually, the large quantities of cones are produced mainly in pine farms throughout the world. They are collected, dried to facilitate seed release, and generally discarded. P. eldarica is widely planted in many regions of Birjand and there are plenty of pine cones as waste things, so they can be a useful substrate for cultivation of mushrooms. On the other hand, edible mushrooms especially oyster mushrooms are efficient lignin degrades which can grow on the wide variety of agricultural waste (Patil et al. 2010). Using pine cones as mushrooms cultivation substrate has economic efficiency because there are large amounts of pine cone per annum.
Factors that have a significant influence on mushroom productivity include the type and amount of substrate and complement foods and their interaction with mushroom strains (Jafarpour and Eghbalsaeed 2012). Substrate source, substrate quality, spawn, strain, compost and complement are the various numbers of parameters that effect on the growth and performance of oyster mushroom (Royse et al. 2004; Jafarpour et al. 2010). The high content of protein and nitrogen source has an effect on shortening growth period and increasing both yield and biological efficiency (Peksen and Yakupoglu 2009; Adebayo et al. 2009; Fanadzo et al. 2010; Jafarpour et al. 2010). One of the obstacles for mushroom culture is high nitrogen content of substrates that can cause the raising the media temperature and subsequently postponing the mycelium run (Jafarpour and Eghbalsaeed 2012). However, when the substrates were improved by supplements of the plant origin led to the slow release of organic materials which could be absorbed by mycelium structures (Royse et al. 1991). Jafarpour et al. (2010) demonstrated that enrichment of sugar beet pulp, palm fiber, and boll complemented with wheat bran, rice bran, carrot pulp, and soya cake powder improved characters of growth of P. ostreatus. Kazemi Jeznabadi et al. (2016) had the study on King oyster mushroom production using various sources of agricultural wastes in Iran. The results showed that supplement combinations and substrate type are beneficial for mushroom production because of enhancing the physical and enzymatical accessibility of the ingredients for fungus growth and development.
Data on minerals
The concentrations of K, Mg, Al, Mn, Fe, Cu, and Zn were presented in Table 2. K content ranged from 180 to 400 mg kg−1. The highest K content reported on combination wheat straw and pine cone (400 mg kg−1). The K content reported on the combination of wheat straw and urea (180 mg kg−1) was least. As K is the co-factor of several enzymatic reactions so it is one of the minerals that are necessary for mushrooms. K can be found in plenty in mushrooms. Usually, K in the form of phosphate is available for the fungus to provide two essential minerals for metabolism (Patil et al. 2010). Magnesium content ranged from 9.3 to 21 mg kg−1. Maximum magnesium content of 21 mg kg−1 was recorded with the combination of wheat straw and pine cone while least was related to the combination of wheat straw and urea (9.3 mg kg−1). The results showed, the more and less abundant of K and Mg were recorded from the same nutrient sources. Al content was the lower compared to other minerals in P. florida. The quantity of Al was recorded from 0.72 to 6.4 mg kg−1 and the highest value was related when mushrooms were cultivated on the combination of wheat straw and soybean straw and the least was due to the mixture of wheat straw and urea. Fe, Zn, Mn, and Cu are among the bio-elements that were frequently studied for the growth and composition of many mushroom species (Sales-Campos et al. 2009; Brzezicha-Cirocka et al. 2016). The highest Mn content was recorded on the combination of wheat straw and pine cone (5.6 mg kg−1) and the minimum was obtained on combination wheat straw and urea (2.3 mg kg−1). Fe and Zn were found in highest content in the same treatment. The highest Fe content was 93 mg kg−1 and the highest Zn content was 31.5 mg kg−1 that were recorded on the combination of wheat straw and pine cone while the lowest concentrations for both of Fe (9.0 mg kg−1) and Zn (6.40 mg kg−1) were obtained from the mixture of wheat straw and urea. The highest Cu content was reported when P. florida was cultivated on wheat straw lonely as a blank sample (11 mg kg−1) and the least was due to the combination of wheat straw and urea (2.0 mg kg−1). In this study, K was the highest in its concentration compared to other minerals, followed by Fe, Mg, and Zn. As previously mentioned, substrates have an effect on mushroom properties (Alananbeh et al. 2014). Oyetayo and Ariyo (2013) have done the approximate and mineral analysis for P. ostreatus that cultivated on different agro-wastes. They found that phosphorus had the highest value of among the other minerals analyzed. After phosphorus, K had high amount than the other minerals. Patil et al. (2010) and Alananbeh et al. (2014) showed based on their results that K had the highest concentration compared to the other nutrients. This could be related the high content of K in the agro-wastes that used to cultivate mushrooms.
The fruiting bodies of Pleurotus mushrooms are characterized by high level of mineral constituents same as all of living organisms. The whole mineral level depends on different things such as the species and age of the mushrooms, the diameter of the pilei and the substrate (Deepalakshmi and Mirunalini 2014). The type and age of mushrooms, pilei/substratum diameter and the substrates in which they grow, might be affected on the variations of their mineral content. Accumulation of greater amounts of elements in pileus than in the stipe has been observed with several mushroom species (Demirbas, 2001; Falandysz et al., 2001; Mattila et al., 2001). Bernas et al. (2006) reported a study on edible mushrooms as a source of valuable nutritive constituents. They demonstrated in their study that the protein content in the mushrooms depends on the composition of the substrate, the size of pileus, harvest time, and species of mushrooms. They illustrated the distribution of mineral content is usually grater in pileus than in stipe. Falandysz et al. (2016) in a research on mercury in forest mushrooms and topsoil from the Yunnan highlands and the subalpine region of the Minya Konka summit in the Eastern Tibetan Plateau showed the concentration of Hg in the cap is more than the stipe in all the mushroom species and, these concentrations are varied in different species of mushrooms. As earlier mentioned, the accumulation of heavy metals in the mushrooms depends on the environmental and fungal related factors. Organic matter content, pH, and metal concentration in the substrate can be considered as the environmental factors, in contrast, the kind of species, morphological part of the fruit body, developmental stages, the age of the mycelium, intervals between fructifications and biochemical composition is the fungal factors. They contribute significantly to the amount of heavy metals in the mushroom samples (Radulescu et al. 2010; Gebrelibanos et al., 2016). Vetter (1994) and Watanable et al. (1994) showed that the pilei of P. ostreatus has greater content of Cu, Fe, K, Mg, P, and Zn while the stipe has a greater content of Na. Furthermore, minerals are important in the diet because of having metabolic reactions, healthy bone formation, transmission of nerve impulses, regulation of water and salt balance (Patil et al. 2010). The minerals are needed for the fruiting of mushrooms same as plants. Some of them like P, K, Mg, and S are essential nutrients for the fungal growth (Wang et al. 2001). In this study, the content of some mineral elements of cultivated mushroom (P. florida) is harvested varied with different nutrient sources.
Toxic metallic and metalloid elements analysis
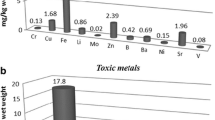
There are several methods to remove the heavy metals from wastes. Mushrooms uptake the heavy metals from a substrate by their mycelium (Das 2005). In this study, the content of three toxic metallic and metalloid elements, Cd, Pb, and As were measured and the results have been showed in Table 3. The highest absorption of As was reported on the wheat straw + pine cone (0.32 mg kg−1), while the least amount was related to the combination of wheat straw + urea (0.08 mg kg−1). Cd content varied from 0.3 to 0.9 mg kg−1 when cultivated on different substrates. The mixture of wheat straw and pine cone showed maximum content (0.9 mg kg−1); whereas minimum absorption of Cd was found in the combination of wheat straw and urea (0.3 mg kg−1). Finally, Pb content ranges from 0.4 to 1.8 mg kg−1. The maximum content was related to the combination of wheat straw and pine cone and the minimum was found on the mixture of wheat straw and urea. Regarding to the results of this study, among three toxic metallic and metalloid elements the maximum absorption for all of the nutrient sources was reported for Pb.
According to the results, most uptakes of heavy metallic and metalloid elements from the substrates were related to the pine cones powder and wheat straw as one of the treatment. The lowest amount of minerals was related to the urea as the supplement. Also, the lowest amount for toxic metallic and metalloid elements belonged to the mushrooms cultivated on wheat straw with urea. Which shows urea can be a suitable substrate for oyster mushrooms cultivation. Urea has 46% nitrogen and is one of the important fertilizers in the world. Urea can be a good supplement to increase the yield and nutritional quality of edible mushroom (Demirer et al. 2005). The maximum amounts of most measured minerals and all toxic metallic and metalloid elements were related to the pine cone treatment. Although pine cones can be used as a good substrate with economic efficiency for mushrooms cultivation, this substrate showed a high biosorption capability for toxic metallic and metalloid elements that is not good from health point of the consumer’s view.
Health risk assessment
Provisional tolerable daily intakes (PTDI)
Among the metallic and metalloid elements measured in this study, Cd, Pb, and As present a potential health hazardous for humans. The Joint FAO/WHO Expert Committee on Food Additives (JEFCA) recommended that provisional tolerable intakes of Cd, Pb, and As are 7, 25 and 15 μg per kg body weight weekly, respectively (Fang et al. 2014; Yang et al., 2010). Therefore, for a person with 60 kg weight, the weekly acceptable usage amount of Cd, Pb, and As would be 0.42, 1.50 and 0.9 mg, respectively. For this calculation, a body with 60 kg weight and an intake amount of fresh mushrooms per day of 300 g (containing 30 g of dry matter) can be assumed (Kalač and Svoboda 2000; Svoboda et al. 2000). Based on these data and assuming the lack of decrease in minerals content because of a culinary treatment of fruit bodies, estimated daily intake of the above metallic and metalloid elements can be calculated and the results have been shown in Table 5.
The mean intakes of Cd, Pb, and As for target cultivated mushroom of P. florida in all of the studied nutrient sources were far below RfD values recommended by the international regulatory bodies (US-EPA IRIS 2006). These metallic and metalloid elements do not pose a risk to human health since the EDIs calculated for these elements were far below the RfD values. However, there is the need for a continuous monitoring of contamination level of these elements especially As and Cd since they can accumulate to toxic levels. This will help to detect any change in their accumulation pattern that could become a hazard to human safety.
Health risk index (HRI)
Using HRI, the health risk of consumers because of intake of metal contaminated target mushroom was evaluated. There is no obvious risk to the exposed population if the HRI is less than 1; whereas a risk can be present if the HRI is above 1. The HRI was also calculated according to this assumption (a body weight of 60 kg and the used amount of dried mushrooms per day of 30 g). Result for the HRI of the Cd, Pb, and As has been presented in Table 5. The HRI of Cd, Pb, and As for P. florida in all of the nutrient sources were less than 1, indicating that above elements in target cultivated mushroom do not pose a risk to human health. In conclusion, As and Cd in wheat straw + pine cone as one of the substrate had the highest health risk among the toxic metallic and metalloid elements examined. Based on results in Table 5, As has a relatively higher potential health risk, while Pb has the lowest one.
Conclusions
Heavy metal pollution in cultivated edible mushrooms from the different nutrition source can be a serious problem. In this study, contamination with heavy and toxic elements in the cultivated mushroom of P. florida on different substrates; wheat straw (as blank), wheat straw + pine cone, wheat straw + soybean straw and wheat straw + urea was investigated. Besides of because of economic reasons, the mentioned substrates can be beneficial in cultivation of P. florida. Since yield was good and the levels of toxic elements (Cd, Pb, As) in edible product were within hygienic limits, and the bio-elements (K, Mg, Mn, Fe, Cu, Zn) were at satisfactory levels. A health risk assessment for consumers based on their intake of metal-contaminated target mushrooms was assessed using health risk index (HRI). According the results, the HRI of Cd, Pb, and As for P. florida in all of the nutrient sources were less than 1, indicating that above toxic elements in target cultivated mushroom do not pose a risk to human health. Therefore, cultivation of edible mushrooms from the health point of the consumer’s view needs more attention.
References
Adebayo GJ, Omolara BN, Toyin AE (2009) Evaluation of yield of oyster mushroom (Pleurotus pulmonarius) grown on cotton waste and cassava peel. Afr J Biotechnol 8:215–218
Agrahar-Murugkar D, Subbulakshmi G (2005) Nutritional value of edible mushrooms collected from the Khasi hills of Meghalaya. Food Chem 89:599–603. doi:10.1016/S0308-8146(04)00257-2
Alam N, Amin R, Khan A, Ara I, Shim MJ, Lee MW, Lee TS (2008) Nutritional analysis of cultivated mushrooms in Bangladesh - Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica. Mycobiology 36:228–232. doi:10.4489/MYCO.2008.36.4.228
Alananbeh KM, Bouqellah NA, Al Kaff NS (2014) Cultivation of oyster mushroom Pleurotus ostreatus on date-palm leaves mixed with other agro-wastes in Saudi Arabia. Saudi J Biol Sci 21:616–625. doi:10.1016/j.sjbs.2014.08.001
Amin R, Khair A, Alam N, Lee TS (2010) Effect of different substrates and casing materials on the growth and yield of Calocybe indica. Microbiology 38:97–101. doi:10.4489/MYCO.2010.38.2.097
Bernas E, Jaworska G, Lisiewska Z (2006) Edible mushrooms as a source of valuable nutritive constituents. Acta Sci Pol Technol Aliment 5:5–20
Bobek P, Galbavy S (2001) Effect of pleuran (beta-glucan from Pleurotus ostreatus) on the antioxidant status of the organism and on dimethylhydrazine-induced precancerous lesions in rat colon. Br J Biomed Sci 58:164–168
Brzezicha-Cirocka J, Mędyk M, Falandysz J, Szefer P (2016) Bio- and toxic elements in edible wild mushrooms from two regions of potentially different environmental conditions in eastern Poland. Environ Sci Pollut Res 23:21517–21522. doi:10.1007/s11356-016-7371-0
Busuioc G, Elekes CC, Stihi C, Iordache S, Ciulei SC (2011) The bioaccumulation and translocation of Fe, Zn, and Cu in species of mushrooms from Russula genus. Environ Sci Pollut R 18:890–896. doi:10.1007/s11356-011-0446-z
Chen XH, Zhou HB, Qiu GZ (2009) Analysis of several heavy metals in wild edible mushrooms from regions of China. Bull Environ Contam Toxicol 83:280–285. doi:10.1007/s00128-009-9767-8
Cocchi L, Vescovi L, Petrini LE, Petrini O (2006) Heavy metals in edible mushrooms in Italy. Food Chem 98:277–284. doi:10.1016/j.foodchem.2005.05.068
Cohen R, Persky L, Hadar Y (2002) Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl Microbiol Biotechnol 58:582–594. doi:10.1007/s00253-002-0930-y
Das N (2005) Heavy metals biosorption by mushrooms. Nat prod radiance 4:454–459
Deepalakshmi K, Mirunalini S (2014) Pleurotus ostreatus: an oyster mushroom with nutritional and medicinal properties. J Biochem Tech 5:718–726
Demirbaş A (2000) Accumulation of heavy metals in some edible mushrooms from Turkey. Food Chem 68:415–419. doi:10.1007/s10661-010-1728-5
Demirbas A (2001) Concentrations of 21 metals in 18 species of mushrooms growing in the east Black Sea region. Food Chem 75:453–457. doi:10.1016/S0308-8146(01)00236-9
Demirer T, Ock-Okuyucu BR, Ozer I (2005) Effect of different types and doses of nitrogen fertilizers on yield and quality characteristics of mushrooms (Agaricus bisporus (Lange) sing) cultivated on wheat straw compost. J Agr Rural Dev Trop 106:71–77
Falandysz J, Borovička J (2013) Macro and trace mineral constituents and radionuclides in mushrooms: health benefits and risks. Appl Microbiol Biotechnol 97:477–501. doi:10.1007/s00253-012-4552-8
Falandysz J, Drewnowska M (2015) Distribution ofmercuryin Amanita fulva (Schaeff.) Secr. Mushrooms: accumulation, loss in cooking and dietary intake. Ecotoxicol Environ Saf 115:49–54. doi:10.1016/j.ecoenv.2015.02.004
Falandysz J, Rizal LM (2016) Arsenic and its compounds in mushrooms: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. doi:10.1080/10590501.2016.1235935
Falandysz J, Szymczyk K, Ichihashi H, Bielawski L, Gucia M, Frankowska A, Yamasaki S (2001) ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Addit Contamin 18:503–513. doi:10.1080/02652030119625
Falandysz J, Saba M, Liu HG, Li T, Wang JP, Wiejak A, Zhang J, Wang YZ, Zhang D (2016) Mercury in forest mushrooms and topsoil from the Yunnan highlands and the subalpine region of the Minya Konka summit in the eastern Tibetan plateau. Environ Sci Pollut Res. doi:10.1007/s11356-016-7580-6
Fanadzo M, Zireva DT, Dube E, Mashingaidze AB (2010) Evaluation of various substrates and complements for biological efficiency of Pleurotus sajor-caju and Pleurotus ostreatus. Afr J Biotechnol 9:2756–2761. doi:10.5897/AJB09.1259
Fang Y, Sun X, Yang W, Ma N, Xin Z, Fu J, Hu Q (2014) Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem 147:147–151. doi:10.1016/j.foodchem.2013.09.116
Garcia MA, Alonso J, Fernández MI, Melgar MJ (1998) Lead content in edible wild mushrooms in Northwest Spain as indicator of environmental contamination. Arch Environ Contam Toxicol 34:330–335. doi:10.1007/s002449900326
Gebrelibanos M, Megersa N, Taddesse AM (2016) Levels of essential and non-essential metals in edible mushrooms cultivated in Haramaya, Ethiopia. Int J Food Contamin 3:1–12. doi:10.1186/s40550-016-0025-7
Gonzalez P, Labarere J (2000) Phylogenetic relationships of Pleurotus species according to the sequence and secondary structure of the mitochondrial small-subunit rRNA V4, V6 and V9 domains. Microbiology 146:209–221
Govil PK, Sorlie JE, Murthy NN, Sujatha D, Reddy GL, Rudolph-Lund K, Krishna AK, Rama Mohan K (2008) Soil contamination of heavy metals in the Katedan industrial development area, Hyderabad, India. Environ Monit Assess 140:313–323. doi:10.1007/s10661-007-9869-x
Granero S, Domingo JL (2002) Levels of metals in soils of Alcalá de Henares, Spain: human health risks. Environ Int 28:159–164. doi:10.1016/S0160-4120(02)00024-7
Gupta N (2014) Preliminary phytochemical screening of different extracts of Pleurotus florida. Columbia. J Pharm Sci 1:23–26
Hoa HT, Wang CL, Wang CH (2015) The effects of different substrates on the growth, yield, and nutritional composition of two Oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 43:423–434. doi:10.5941/MYCO.2015.43.4.423
Huang SS, Liao QL, Hua M, XM W, Bi KS, Yan CY, Chen B, Zhang XY (2007) Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere 67:2148–2155
Jafarpour M, Eghbalsaeed S (2012) High protein complementation with high fiber substrates for oyster mushroom cultures. Afr J Biotechnol 11:3284–3289. doi:10.5897/AJB11.1473
Jafarpour M, Jalali Zand A, Dehdashtizadeh B, Eghbalsaied SH (2010) Evaluation of agricultural wastes and food complements usage on growth characteristics of Pleurotus ostreatus. Afr J Agric Res 5:3291–3296. doi:10.5897/AJAR10.623
Jan FA, Ishaq M, Khan S, Ihsanullah I, Ahmad I, Shakirullah M (2010) A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). J Hazard Mater 179:612–621. doi:10.1016/j.jhazmat.2010.03.047
Jarzyńska G, Falandysz J (2012) Trace elements profile of slate bolete (Leccinum durisculum) mushroom and associated upper soil horizon. J Geochem Explor 121:69–75. doi:10.1016/j.gexplo.2012.07.001
Jarzyńska G, Chojnacka A, Dryżałowska A, Nnorom IC, Falandysz J (2012) Concentrations and Bioconcentration factors of minerals in yellow-cracking bolete (Xerocomus Subtomentosus) mushroom collected in Noteć Forest, Poland. J Food Sci 77:202–206. doi:10.1111/j.1750-3841.2012.02876.x
Kalač P, Svoboda L (2000) A review of trace element concentrations in edible mushrooms. Food Chem 69:273–281. doi:10.1016/S0308-8146(99)00264-2
Kazemi Jeznabadi E, Jafarpour M, Eghbalsaied S (2016) King oyster mushroom production using various sources of agricultural wastes in Iran. Int J Recycl Org Waste Agricult 5:17–24. doi:10.1007/s40093-015-0113-3
Khan MA (2010) Nutritional composition and hypocholesterolemic effect of mushroom: Pleurotus sajor-caju and Pleurotus florida. LAP Lambert Academic publishing Gmbh & co. KG, Saarbrucken, Germany, pp. 1–11
Kiaei M (2011) Anatomical, physical, and mechanical properties of eldar pine (Pinus eldaricamedw.) grown in the Kelardasht region. Turk J Agric For 35:31–42. doi:10.3906/tar-1001-552
Li Y, Gou X, Wang G, Zhang Q, Su Q, Xiao G (2008) Heavy metal contamination and source in arid agricultural soil in Central Gansu Province. China. J Environ Sci 20:607–612. doi:10.1016/S1001-0742(08)62101-4
Mallikarjuna SE, Ranjini A, Haware DJ, Vijayalakshmi MR, Shashirekha MN, Rajarathenam S (2013) Mineral composition of four edible mushrooms. Hindawi J Chem 2013:1–5. doi:10.1155/2013/805284
Manzi P, Aguzzi A, Pizzoferrato L (2001) Nutritional value of mushrooms widely consumed in Italy. Food Chem 73:321–325. doi:10.1016/S0308-8146(00)00304-6
Manzi P, Marconi S, Aguzzi A, Pizzoferrato L (2004) Commercial mushrooms: nutritional quality and effect of cooking. Food Chem 84:201–206. doi:10.1016/S0308-8146(03)00202-4
Mattila P, Konko K, Eurola M, Pihlava JM, Astola J et al (2001) Contents of vitamins, mineral elements and some phenolic compounds in cultivated mushrooms. J Agric Food Chem 49:2343–2348. doi:10.1021/jf001525d
Mattila P, Suonpa K, Pilronen V (2006) Functional properties of edible mushroom. Nutr J 16:694–696. doi:10.1016/S0899-9007(00)00341-5
Menaga D, Mahalingam P, Rajakumar S, Ayyasamy P (2012) Evaluation of phytochemical characteristics and antimicrobial activity of Pleurotus florida mushroom. Asian J Pharm Clin Res 5:102–106
Mleczek M, Niedzielski P, Siwulski M, Rzymski P, Gąsecka M, Goliński P, Kozak L, Kozubik T (2016) Importance of low substrate arsenic content in mushroom cultivation and safety of final food product. Eur Food Res Technol 242:355–362. doi:10.1007/s00217-015-2545-4
Moraditochaee M (2012) Evolution energy indices of soybean production in north of Iran. ARPN J Agric Biol Sci 7:554–557
Nnorom IC, Jarzyńska G, Falandysz J, Drewnowska M, Okoye I, Oji-Nnorom Ch G (2012) Occurrence and accumulation of mercury in two species of wild grown Pleurotus mushrooms from southeastern Nigeria. Ecotoxicol Environ Saf 84:78–83. doi:10.1016/j.ecoenv.2012.06.024
Nnorom IC, Jarzyńska G, Drewnowska M, Dryżałowska A, Kojta A, Pankavec S, Falandysz J (2013) Major and trace elements in sclerotium of Pleurotus tuber-regium (Ósū) mushroom—dietary intake and risk in southeastern Nigeria. J Food Comp Anal 29:73–81. doi:10.1016/j.jfca.2012.10.001
Nunes MD, da Luz JMR, Paes SA, Ribeiro JJO, da Silva MCS, Kasuya MCM (2012) Nitrogen supplementation on the productivity and the chemical composition of oyster mushroom. J Food Res 1:113–119. doi:10.5539/jfr.v1n2p113
Oyetayo VO, Ariyo OO (2013) Micro and macronutrient properties of Pleurotus ostreatus (Jacq: fries) cultivated on different wood substrates. Jordan J Biol Sci 6:223–226. doi:10.12816/0001537
Patel Y, Naraian R, Singh VK (2012) Medicinal properties of Pleurotus species (oyster mushroom): a review. World J Fun Plant Bio 3:1–12. doi:10.5829/idosi.wjfpb.2012.3.1.303
Patil SS, Ahmed SA, Telang SM, Baig MM (2010) The nutritional value of Pleurotus ostreatus (Jacq.: Fr) Kumm cultivated on different lignocellulosic agro-wastes. Innov rom. Food Biotechnol 7:66–76
Peksen A, Yakupoglu G (2009) Tea waste as a complement for the cultivation of Ganoderma lucidum. World J Microbiol Biotechnol 25:611–618
Prabu M, Kumuthakalavalli R (2014) Nutritional and phytochemical studies on Pleurotus florida (Mont.) singer and Calocybe indica P&C. World J Pharm Res 3:4907–4913
Radulescu C, Stihi C, Busuioc G, Popescu IV, Gheboianu AI, Cimpoca VG (2010) Evaluation of essential elements and heavy metal levels in fruiting bodies of wild mushrooms and their substrate by EDXRF spectrometry and FAA spectrometry. Rom. Biotech Lett 15:5444–5456
Royse DJ (2014) A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. In: Singh M (ed) Proceedings of the 8th international conference on mushroom biology and mushroom products, New Delhi, India, pp 1–6
Royse DJ, Fales SL, Karunanandaa K (1991) Influence of formaldehyde-treated soybean and commercial nutrient complementation on mushroom (Pleurotus sajor-caju) yield and invitro dry matter digestibility of spent substrate. Appl Microbiol Biotechnol 36:425–429. doi:10.1007/BF00208169
Royse DJ, Rhodes TW, Ohga S, Sanchez JE (2004) Yield, mushroom size and time to production of Pleurotus cornucopiae (oyster mushroom) grown on switch grass substrate spawned and complemented at various rates. Bioresour Technol 91:85–91. doi:10.1016/S0960-8524(03)00151-2
Saba M, Falandysz J, Nnorom IC (2016a) Accumulation and distribution of mercury in fruiting bodies by fungus Suillus luteus foraged in Poland, Belarus and Sweden. Environ Sci Pollut Res 23:2749–2757. doi:10.1007/s11356-015-5513-4
Saba M, Falandysz J, Nnorom IC (2016b) Mercury bioaccumulation by Suillus bovinus mushroom and probable dietary intake with the mushroom meal. Environ Sci Pollut Res 23:14549–14559. doi:10.1007/s11356-016-6558-8
Sales-Campos C, Fereira DA, Eira A, Teixeira DE, Almeida M, Noguieira DE, Andrade MC (2009) Mineral composition of raw material, substrate and fruiting bodies of Pleurotus ostreatus in culture. Interciencia 34:432–436
Sanchez C (2010) Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol 85:1321–1337. doi:10.1007/s00253-009-2343-7
Sarikurkcu C, Tepe B, Solak MH, Cetinkaya S (2012) Metal concentrations of wild edible mushrooms from Turkey. Ecol Food Nutr 51:346–363. doi:10.1080/03670244.2012.674448
Svoboda L, Zimmermannova K, Kalač P (2000) Concentrations of mercury, cadmium, lead and copper in fruiting bodies of edible mushrooms in an emission area of copper smelter and a mercury smelter. Sci Total Environ 246:61–67. doi:10.1016/S0048-9697(99)00411-8
Tchounwou PB, Patlolla AK, Centeno JA (2003) Carcinogenic and systemic health effects associated with arsenic exposure-a critical review. Toxicol Pathol 31:575–588. doi:10.1080/714044691
US-EPA IRIS (2006) United States, Environmental Protection Agency, Integrated Risk Information System. <http://www.epa.gov/iris/substS>.
Vetter J (1994) Mineral elements in the important cultivated mushrooms Agaricus bisporus and Pleurotus ostreatus. Food Chem 50:277–279. doi:10.1016/0308-8146(94)90132-5
Vetter J, Hajdú J, Györfi J, Maszlavér P (2005) Mineral composition of the cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula eodes. Acta Alim 34:441–451. doi:10.1556/AAlim.34.2005.4.11
Wang H, Ng TB (2000) Isolation of a novel ubiquitin-like protein from Pleurotus ostreatus mushroom with anti-human immune deficiency virus, translation-inhibitory and ribonuclease activities. Biochem Biophys Res Commun 276:587–593. doi:10.1006/bbrc.2000.3540
Wang D, Sakoda A, Suzuki M (2001) Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beet grain. Bioresour Technol 78:293–300. doi:10.1016/S0960-8524(01)00002-5
Watanable T, Tsuchinasi N, Takai Y, Tanaka K, Suzuki A (1994) Effect of ozone exposure during cultivation of oyster mushroom Pleurotus ostreatus on chemical components of the fruit bodies. J Jpn Soc. Food Sci Technol 41:705–708. doi:10.3136/nskkk1962.41.705
Yang X, Zhao HT, Wang J, Meng Q, Zhang H, Yao L, Zhang YC, Dong AJ, Ma Y, Wang ZY, DC X, Ding Y (2010) Chemical composition and antioxidant activity of essential oil of pine cones of Pinus armandii from the southwest region of China. J Med Plants Res 4:1668–1672. doi:10.5897/JMPR10.217
Zhang D, Frankowska A, Jarzyńska G, Kojta AK, Drewnowska M, Wydmańska D, Bielwaski L, Wang J, Falandysz J (2010) Metals of king bolete (boletus edulis) bull.: Fr. Collected at the same site over two years. Afr J Agric Res 5:3050–3055
Zhu F, Qu L, Fan W, Qiao M, Hao H, Wang X (2011) Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environ Monit Assess 179:191–199. doi:10.1007/s10661-010-1728-5
Acknowledgements
We are grateful to the research council of the University of Birjand for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Khani, R., Moudi, M. & Khojeh, V. Contamination level, distribution and health risk assessment of heavy and toxic metallic and metalloid elements in a cultivated mushroom Pleurotus florida (Mont.) singer. Environ Sci Pollut Res 24, 4699–4708 (2017). https://doi.org/10.1007/s11356-016-8222-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8222-8