Abstract
Essential oils (EOs) from plants may be alternative sources of molecules toxic against mosquito vectors of public health relevance. Most of researches in this field focused on EOs as larvicides or ovicides, while limited efforts focused on the exploitation of EOs as oviposition deterrents. In the present study, the larvicidal and oviposition deterrent activity of Syzygium lanceolatum leaf EO was evaluated against six mosquito species, Anopheles stephensi, An. subpictus, Aedes aegypti, Ae. albopictus, Culex quinquefasciatus, and Cx. tritaeniorhynchus. The chemical composition of the S. lanceolatum EO was analyzed by GC-MS analysis, showing the presence of phenyl propanal, β-caryophyllene, α-humulene, and caryophyllene oxide as major constituents. S. lanceolatum EO showed high acute toxicity on An. stephensi (LC50 = 51.20 μg/ml), Ae. aegypti (LC50 = 55.11 μg/ml), Cx. quinquefasciatus (LC50 = 60.01 μg/ml), An. subpictus (LC50 = 61.34 μg/ml), Ae. albopictus (LC50 = 66.71 μg/ml), and Cx. tritaeniorhynchus (LC50 = 72.24 μg/ml) larvae. Furthermore, the EO was effective as oviposition deterrent against the six tested mosquito species, with OAI on An. stephensi, An. subpictus, Ae. aegypti, Ae. albopictus, Cx. quinquefasciatus, and Cx. tritaeniorhynchus reaching −0.83, −0.81, −0.84, −0.83, −0.84, and −0.86, respectively. The toxicity of S. lanceolatum EO against several biological control agents of mosquitoes, including water bugs (Anisops bouvieri and Diplonychus indicus) and fishes (Gambusia affinis and Poecilia reticulata), was extremely low, with LC50 ranging between 4148 and 15,762 μg/ml. Overall, our results pointed out the promising potential of the S. lanceolatum leaf EO as a source of environmental-friendly oviposition deterrents and larvicides effective against a wide number of mosquito species of importance for parasitology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquitoes constitute an important group of arthropods for public health. Anopheles, Aedes, and Culex vector a wide range of human diseases such as malaria, dengue, yellow fever, filariasis, Japanese encephalitis, St. Louis encephalitis, and Zika virus, causing millions of deaths worldwide each year (Mehlhorn 2015; Benelli et al. 2016a). Global patterns of climate change and urbanization have increased the threat of humans contracting arthropod-borne viral infections (Benelli 2016a; Benelli and Mehlhorn 2016). However, high levels of pesticide resistance have been developed through chemical control of arthropod vectors, threatening the effectiveness of current control programs (Hemingway and Ranson 2000; Naqqash et al. 2016). To overcome these problems, it is necessary to search for alternative, more environmentally benign mosquito control methods (Benelli 2015a, b, c; Benelli et al. 2015; Pavela and Benelli 2016a). In this framework, botanical-borne pesticides may provide a cheap and eco-friendly alternative to synthetic insecticides (e.g., Elango et al. 2010; Govindarajan and Benelli 2016a, b, c), due to their generally low toxicity to human health and the environment (Isman 2008; Benelli 2015b, 2016b,c; Pavela 2015).
Among botanical-based insecticides, plant essential oils (EOs) have a broad spectrum of bioactivity (Isman 2008; Govindarajan et al. 2016a, b, c, d, e) because of the presence of several active ingredients that exert toxicity through several mechanisms of action (Pavela and Benelli 2016b). Several plant species of the family Myrtaceae, which includes 4620 species and 140 genera distributed all over the world (Mabberly 1997), are being used in folk medicine due to their antidiarrheal, antimicrobial, antioxidant, antirheumatic, anti-inflammatory, and anti-cholesterol properties (Stasi and Hiruma-Lima 2002). Several species belonging to the genus Syzygium are employed to treat diabetes mellitus. The chemical composition of EOs from several Syzygium species has been previously reported, with special reference to S. aqueum, S. samarangense, S. malaccense, S. aromaticum, S. guineense, and S. caryophyllatum (Wong and Lai 1996; Raina et al. 2001; Lee et al. 2009; Noudogbessi et al. 2008; Nassar et al. 2007; Bhuiyan et al. 2010; Stalin and Swamy 2013; Ahmed et al. 2009). Sesquiterpenes (hydrocarbons and oxygenated derivatives) have been found the main class of volatile constituents responsible of the antibacterial, antifungal, anti-inflammatory, and cytotoxic activities. In addition, monoterpenes and phenylpropanoids have been also reported as important constituents of Syzygium EOs (Boulos 1983).
Syzygium lanceolatum (synonyms: Eugenia lanceolata Lam.; Syzygium wightianum Wall. ex Wt. and Arn.) belongs to the family Myrtaceae, which has 10 genera and 154 species in the Indian subcontinent. Of these, Syzygium Gaertn. is the largest genus, with 11 species that are endemic to Western Ghats of Tamil Nadu, India (Gamble and Fischer 1923). Plants of this family are known to be rich in volatile EOs, which are reported for their uses in Indian traditional medicine (Mahmoud et al. 2001; Reynertson et al. 2005). However, to the best of our knowledge, no information is available on the chemical composition and insecticidal activity of S. lanceolatum EO.
Notably, most of the researches in the field of mosquito control with plant-borne pesticides focused on plant EOs as larvicides or ovicides (see Benelli (2015b) and Pavela (2015) for recent reviews), while only limited efforts focused on the exploitation of EOs as oviposition deterrents (Prajapati et al. 2005; Autran et al. 2009; Khandagle et al. 2011; Rajaganesh et al. 2016. Therefore, in the present study, the larvicidal and oviposition deterrent activity of the S. lanceolatum leaf EO was evaluated against six mosquito species, the malaria vectors Anopheles stephensi and An. subpictus, the dengue and Zika virus vectors Aedes aegypti and Ae. albopictus, the filariasis and St. Louis encephalitis and West Nile vector Culex quinquefasciatus, and the Japanese encephalitis vector Cx. tritaeniorhynchus. Furthermore, the chemical composition of the S. lanceolatum EO was analyzed by gas chromatography-mass spectroscopy (GC–MS) analysis. Lastly, the non-target toxicity of the S. lanceolatum EO was evaluated against several biological control agents of mosquitoes, including water bugs (Anisops bouvieri and Diplonychus indicus) and fishes (Gambusia affinis and Poecilia reticulata).
Materials and methods
Extraction and GC–MS analysis of the S. lanceolatum essential oil
Fresh leaves of S. lanceolatum were collected during May 2016 in the Munnar mountains (India 10° 05′ 21″ N, 77°03′35″ E, 1700 m a.s.l.). S. lanceolatum samples were identified, and authenticated voucher specimens were deposited at the Herbarium of the Faculty of Science, Annamalai University (India). 400 grams of S. lanceolatum fresh leaves were hydrodistilled using a modified Clevenger-type apparatus for 3 h; then, S. lanceolatum EO was dried over anhydrous Na2SO4 and stored into amber-colored vials at 5 °C until the testing phase (Govindarajan and Benelli 2016a, b). S. lanceolatum EO was stored in an airtight container prior to analysis by gas chromatography (GC) and GC–MS.
GC and GC–MS analyses
Analytical gas chromatography was carried out using an HP gas chromatograph. The separation was achieved by use of a HP1 (fused silica) capillary column (30 m × 0.25 mm; film thickness 0.25 μm), split ratio, 1:25, and using a flame ionization detector. GC settings were programmed as reported by Govindarajan and Benelli (2016c). He was employed as carrier gas; the flow rate was 1 ml/min. GC–MS was performed on Agilent Technology 5973 mass selective detector connected with a HP 6890 gas chromatograph. Separation was achieved relying to the HP1 MS capillary column described above, with split ratio 1:25, equipped with a flame ionization detector (FID). MS was operated at 70 eV ionization energy. Quantitative data on S. lanceolatum EO composition were obtained from the electronic integration of FID peak areas.
The identification of S. lanceolatum EO components was based on retention indices, which were calculated by using retention times of n-alkanes injected after the S. lanceolatum EO at the same chromatographic conditions. The components of the S. lanceolatum EO were identified by comparison of their mass spectra and retention indices (Table 1) with the ones from Wiley library and Adams (2007).
Larvicidal and oviposition deterrence assays
Pathogen- and parasite-free strains of An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus were reared as recently described by Govindarajan and Benelli (2016c), at 27 °C, 12:12 L/D photoperiod, 80 ± 10 % R.H. Early third instar larvae and adult females (5–7 days old) were used for larvicidal and oviposition deterrence experiments, respectively (Govindarajan et al. 2016d).
The larvicidal activity of the S. lanceolatum EO was evaluated following the method by WHO (2005) slightly modified by Govindarajan and Benelli (2016c). Various doses of the S. lanceolatum EO were dissolved in 1 ml DMSO, and then diluted in 249 ml of filtered tap water to obtain the tested concentrations (Table 2). Control was 1 ml of DMSO diluted in 249 ml of water. Within each replicate, 20 early third instar larvae were tested. No food was given to the larvae (WHO 2005). For each concentration, five replicates were performed. Mortality was recorded after 24 h of exposure.
In oviposition deterrent assays, the S. lanceolatum EO was evaluated at various concentrations (40–250 μg/ml) prepared in DMSO. As reported above for larvicidal experiments, DMSO diluted in water served as a control. The experiments were carried out as described by Xue et al. (2001) slightly modified by Benelli and Govindarajan (2016). As oviposition support, we used a filter paper strip placed on the internal surface of treated and control bowls of 500-ml capacity filled with 100 ml distilled water, and the filter paper was half submerged in water. Twenty gravid females (5–7 days old) of An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, or Cx. tritaeniorhynchus were released in the bioassay cage (60 × 60 × 45 cm). After 30 min, the treated and control bowls were placed in diagonal position inside bioassay cage. After 24 h, the number of eggs laid in treated and control bowls were counted under a stereomicroscope (Olympus, Japan).
Toxicity on biological control agents
The effect of S. lanceolatum EO on the four non-target organisms was assessed following the method by Sivagnaname and Kalyanasundaram (2004) with minor modifications by Govindarajan et al. (2016a, b). The toxicity of the S. lanceolatum EO was tested against adults of the water bugs A. bouvieri and D. indicus and the larvivorous fishes G. affinis and P. reticulata. The non-target species were reared as described by Govindarajan and Benelli (2016c), maintaining them in cement tanks (diam.: 85 cm; depth: 30 cm) filled with tap water, 27 ± 3 °C, and external R.H. 85 %. S. lanceolatum EO was evaluated at doses 50 × LC50 calculated for the tested mosquito larvae. Ten replicates were performed for each dose plus 4 control replicates (where no EO was added to the water). Mortality of each non-target species was assessed after 48 h of exposure (Govindarajan and Benelli 2016b).
Data analysis
All mortality data were analyzed by probit analysis. LC50 and LC90 were estimated following the method by Finney (1971). The oviposition activity index (OAI) was calculated as follows (Kramer and Mulla 1979):
Effective repellency (ER %) evoked by S. lanceolatum EO was calculated as indicated by Xue et al. (2001):
NT was the total number of eggs in the treated solution. NC was the total number of eggs in the control solution.
Concerning non-target organisms, the suitability index (SI) was calculated for each species as follows (Deo et al. 1988):
All data were analyzed using the SPSS Statistical Software Package version 16.0. P < 0.05 was used to assess the significance of differences among values.
Results
Yield and chemical composition
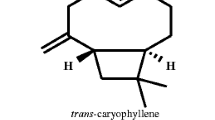
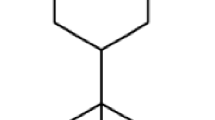
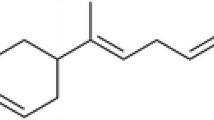
Yield of S. lanceolatum leaf EO was 6.3 ml/kg of leaf fresh weight. Table 1 shows the constituents of S. lanceolatum EO, their percentage composition, and the Kovats Index (KI) values listed in order of elution. 18 compounds representing 96.3 % of the S. lanceolatum EO composition were identified. Major constituents of this oil were phenyl propanal (18.3 %), β-caryophyllene (12.8 %), α-humulene (14.5), and caryophyllene oxide (10.7 %). The chemical structures of the four major compounds were shown in Fig. 1. The percentage compositions of remaining 14 compounds ranged from 1.2 to 6.5 %.
Larvicidal activity and oviposition deterrent activity
The acute toxicity of the S. lanceolatum EO on larvae of An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus is presented in Table 2. The EO extracted from the leaves of S. lanceolatum exhibited effective larvicidal activity, with the LC50 values of 51.20, 55.11, 60.01, 61.34, 66.71, and 72.24 μg/ml, respectively. No mortality was recorded in controls.
Results obtained from oviposition deterrent assays testing the S. lanceolatum EO on An. stephensi, A. aegypti, C. quinquefasciatus, A. subpictus, A. albopictus, and C. tritaeniorhynchus are reported in Table 3. The mean number of eggs laid in sites treated with the EO tested at the highest concentration (i.e., 250–300 μg/ml) was 46.2, 44.6, and 37.5 eggs per bowl for A. stephensi, A. aegypti, and C. quinquefasciatus and 52.3, 47.1, and 39.7 eggs per bowl for An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus. These data showed significant oviposition deterrent activity if compared to the relative controls (P < 0.05) (Table 3). Overall, S. lanceolatum EO was effective as oviposition deterrent against the six tested mosquito species, with OAI on An. stephensi, An. subpictus, Ae. aegypti, Ae. albopictus, Cx. quinquefasciatus, and Cx. tritaeniorhynchus reaching −0.83, −0.81, −0.84, −0.83, −0.84, and −0.86, respectively.
Toxicity on biological control agents
The acute toxicity of S. lanceolatum EO tested on four non-target mosquito natural enemies A. bouvieri, D. indicus, P. reticulata, and G. affinis is given in Table 4. LC50 values were 8133, 6189, 14,528, and 15,762 μg/ml, respectively. SI/PSF indicated that S. lanceolatum EO showed low toxicity on A. bouvieri, D. indicus, P. reticulata, and G. affinis, if compared to the targeted mosquito species (Table 5). As a final remark, our focal observations outlined that the survival and swimming activity of the non-target species were not altered during the exposure to LC50 and LC90 doses of the S. lanceolatum EO calculated on mosquito larvae.
Discussion
Chemical composition of the S. lanceolatum essential oil
Our GC and GC–MS results showed that at least 18 compounds were present in the S. lanceolatum EO, with phenyl propanal (18.3 %), β-caryophyllene (12.8 %), α-humulene (14.5), and caryophyllene oxide (10.7 %) as main constituents. This is a quite different chemical composition, if compared to the EOs extracted from other Syzygium species, such as S. aromaticum (Gurib-Fakim 2006), S. zeylanicum (Govindarajan and Benelli 2016b), and S. cumini (Ayyanar and Subash-Babu 2012). Indeed, in EO of close related species S. zeylanicum, the main components were α-humulene (37.8 %) and β-elemene (10.7 %), while only low amounts of phenyl propanal (4.2 %), β-caryophyllene (2.3 %), and caryophyllene oxide (4.9 %) were detected. Moreover, in S. cumini, a completely different composition of the EO was found, with high percentages of α-terpineol (16.67 %) and α-pinene (17.53 %) (Ayyanar and Subash-Babu 2012), which could be responsible of the high antioxidant activity of this EO (see Kim et al. 2004). Indeed, as a general trend, Syzygium plant parts, with special reference to seeds, are well documented as sources of natural antioxidants in traditional Thai medicine (Maisuthisakul et al. 2007), while information on their toxic activity against insect pests is extremely scarce (Govindarajan and Benelli 2016b).
Larvicidal and oviposition deterrent activity of the S. lanceolatum essential oil
In latest years, a wide array of plant EOs have been tested against arthropods pests, including mosquitoes, ticks, and other important vectors of medical and veterinary relevance, with promising results (see Benelli 2015b; Benelli et al. 2016b; Pavela et al. 2016 for reviews). EOs from plants may represent a valuable source of mosquitocidal products. Indeed, many studies focused on the effectiveness of EOs and related constituents against mosquito young instars, with special reference to eggs (Benelli 2015b) and larvae (Pavela 2015).
Our results showed that the S. lanceolatum EO was effective as larvicide against An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus, leading to LC50 values of 51.20, 55.11, 60.01, 61.34, 66.71, and 72.24 μg/ml, respectively. In a review of all the plant EOs tested as mosquito larvicides, Pavela (2015) recently pointed out that only 77 EOs showed LC50 values lower than 50 ppm, while only 7 of them showed LC50 lower than 10 ppm. On the other hand, Komalamisra et al. (2005) considered products showing LC50 ≤ 50 mg/l as active, 50 mg/l < LC50 ≤ 100 mg/l as moderately active, 100 mg/l < LC50 ≤ 750 mg/l as effective, and L50 > 750 mg/l as inactive. In addition, Ravi Kiran et al. (2006) considered compounds with LC50 < 100 mg/l as significant mosquito larvicides. However, it should be stressed that these criteria must be directly correlated with the time of exposure and the origin of larvae, which are variables that can alter the LC50 values. In this framework, our results are promising, at variance with a wide number of EOs which led to LC50 values higher than 100 ppm. Good examples are Achillea millefolium EO (LC50 = 211.3 μg/ml), Helichrysum italicum EO (LC50 = 178.1 μg/ml), and Foeniculum vulgare EO (LC50 = 142.9 μg/ml) tested on Ae. albopictus (Conti et al. 2010), as well as the A. conyzoides EO evaluated against fourth instar larvae of Ae. aegypti (LC50 = 148 μg/ml) (Mendonca et al. 2005).
To our mind, the larvicidal action of S. lanceolatum EO on the six mosquito species tested in our research can be mainly due to the presence of α-humulene, β-caryophyllene, caryophyllene oxide, and phenyl propanal as the main compounds. Indeed, α-humulene, which is also one of the main components of S. zeylanicum EO, has been reported as highly toxic against An. subpictus (LC50 = 6.19 μg/ml), Ae. albopictus (LC50 = 6.86 μg/ml), and Cx. tritaeniorhynchus (LC50 = 7.39 μg/ml), while caryophyllene oxide exhibited larvicidal activity on Ae. albopictus larvae with a LC50 of 65.6 μg/ml (Cheng et al. 2009).
Furthermore, we recently showed that β-caryophyllene was toxic against third instar larvae of An. subpictus (LC50 = 41.66 μg/ml), Ae. albopictus (LC50 = 44.77 μg/ml), and Cx. tritaeniorhynchus (LC50 = 48.17 μg/ml) (Govindarajan et al. 2016a). β-caryophyllene has been also reported as a good larvicide against Ae. aegypti larvae with a 48-h LC50 value of 34 μg/ml (Cheng et al. 2004). Chaubey (2012) also noted that β-caryophyllene from the EO of Zingiber officinale (Zingiberaceae) and Piper cubeba (Piperaceae) was toxic to adults and larvae of Tribolium castaneum (Coleoptera: Tenebrionidae) and adults of Sitophilus oryzae (Coleoptera: Curculionidae). After 24 h of exposure, β-caryophyllene showed LD50 of 0.173 μl/cm−2 on T. castaneum adults, 0.17 μl/cm−2 on T. castaneum larvae, and 0.159 μL/cm−2 on S. oryzae adults. Benelli et al. (2012) also highlighted that β-caryophyllene from the EO of Hyptis suaveolens (Lamiaceae) showed 65 % of repellence activity against Sitophilus granarius (Coleoptera: Curculionidae).
It can be argued that the use of pure compounds, such as in the case of α-humulene (Govindarajan and Benelli 2016b), should be preferred over the employ of whole EO. However, to our mind, the employ of the EOs is also an important alternative, for two main reasons. First, the EOs from local aromatic plants are easy to obtain, cheap, and still effective for a number of marginalized rural populations worldwide. Second, the substances contained in an EO, such as the one from S. lanceolatum, represent a rich blend with good larvicidal potential, which is exerted through multiple mechanism(s) of action, including inhibition of cytochrome P450 (CYPs), damage of GABA receptors, inhibition of cholinergic system, and modulation of octopaminergic system (Pavela and Benelli 2016b). This strongly reduced the chances of development of EO resistance in mosquito populations (Benelli 2015a).
As a further confirmation of the importance of screening close-related botanical as mosquitocidal products, we would like to point out the higher efficacy of the S. lanceolatum EO tested in this study, if compared to the EO extracted from S. zeylanicum, which achieved LC50 values of 83.11, 90.45, and 97.96 μg/ml on third instar larvae of An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus, respectively (Govindarajan and Benelli 2016b).
Notably, most of the researches in mosquito control focused on plant EOs as larvicides or ovicides, while limited efforts focused on their exploitation as oviposition deterrents (Prajapati et al. 2005; Khandagle et al. 2011). Our findings showed that S. lanceolatum EO was effective as oviposition deterrent against the six tested mosquito species, with OAI reaching −0.83, −0.81, −0.84, −0.83, −0.84, and −0.86 on An. stephensi, An. subpictus, Ae. aegypti, Ae. albopictus, Cx. quinquefasciatus, and Cx. tritaeniorhynchus, respectively. In this framework, we hypothesize that EO component phenyl propanal may be one of the main molecules responsible of the larvicidal and ovideterrent action exerted by the S. zeylanicum EO on mosquitoes, since it has been reported that phenyl propyl compounds showed larvicidal activity in recent literature. (Nascimento et al. 2013; Silva et al. 2016). In particular, Bezerra-Silva et al. (2016) reported that the aldehyde dodecanal showed deterrent effect against Ae. egypti mosquitoes, allowing us to formulate that close-related aldehyde compounds, such as phenyl propanal, may evoke comparable deterrent effects. Further research on the activity of these compounds is ongoing.
More generally, in agreement with our oviposition deterrence assays, Autran et al. (2009) tested EOs from stems, leaves, and inflorescences of Piper marginatum showing that in presence of 50 and 100 ppm of the EOs, the A. aegypti females laid 40 % fewer eggs if compared to the controls. Oviposition deterrence was also observed testing the EO from inflorescences of Alpinia purpurata at a minimum concentration of 100 ppm, which lead to a reduction of at least 50 % in the number of eggs laid in test vessels in comparison with controls (Santos et al. 2012). Also, the Commiphora leptophloeos leaf EO at concentrations of 25, 50, and 100 ppm exerted a strong effect on the oviposition of Ae. aegypti females, resulting in a reduction ranging from 59 to 63 % in the number of eggs laid (Prajapati et al. 2005). Lastly, we noted that the oviposition deterrent action of S. lanceolatum EO can be partially due to the presence of α-humulene and β-caryophyllene, which have been recently reported as an effective oviposition deterrent against Ae. aegypti (da Silva et al. 2015).
Biotoxicity on biological control agents
In our experiments, acute toxicity of S. lanceolatum EO and its major compounds against mosquito biocontrol agent A. bouvieri, D. indicus, G. affinis, and P. reticulata was extremely low, with LC50 values higher than 4148.34 μg/ml. Recently, plant EOs are gaining increasing attention important sources of biopesticides for control of agricultural and urban arthropod pests. This is mostly due to the fact that they do not induce resistance and have limited toxic effects on non-target organisms (Benelli 2015a; Pavela 2015). In agreement with our work, recent research showed little acute toxicity of S. zeylanicum EO on G. affinis, with a LC50 value of 20,374.26 μg/ml (Govindarajan and Benelli 2016b). Similarly, the biotoxicity of Heracleum sprengelianum EO and its two major compounds lavandulyl acetate and bicyclogermacrene on A. bouvieri, D.indicus, and G. affinis was also moderate, with LC50 values ranging from 1840 to 4219 μg/ml (Govindarajan and Benelli 2016a). Taken together, all these findings highlight the eco-friendly nature of plant-borne molecules extracted from Asian plant species, supporting their potential employ as mosquito larvicides in aquatic breeding sites (Benelli 2015a).
Conclusions
Overall, our study highlights the promising larvicidal and oviposition deterrent activity of the S. lanceolatum leaf EO on six mosquito species of public health importance. Notably, the EO was effective as oviposition deterrent with maximum OAI ranging from −0.81 to −0.86. GC and GC–MS studies showed that the toxic action of the tested EO can be due to the presence of phenyl propanal, β-caryophyllene, α-humulene, and caryophyllene oxide as major constituents. In particular, the latter three molecules have been recently reported as effective larvicides against anopheline and culicine species. In addition, α-humulene and β-caryophyllene are able to effectively deter Ae. aegypti females from egg laying. Notably, the toxicity of S. lanceolatum EO against several biological control agents of mosquitoes, including water bugs (A. bouvieri and D. indicus) and fishes (G. affinis and P. reticulata) was extremely low. On this basis, we pointed out the potential of the S. lanceolatum leaf EO as a source of environmental-friendly oviposition deterrents and larvicides effective against a wide number of mosquito species of importance for parasitology.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectroscopy, 4th edn. Allured Publishing Corporation, Carol Stream
Ahmed F, Chandra JNNS, Timmaiah NV (2009) An in vitro study on the inhibitory activities of Eugenia jambolana seeds against carbohydrate hydrolyzing enzymes. J Young Pharm 1(4):327–331
Autran ES, Neves IA, Silva CSB, Santos GKN, Câmara CAG, Navarro DMAF (2009) Chemical composition, oviposition deterrent and larvicidal activity against Aedes aegypti of essential oils from Piper marginatum Jacq. (Piperaceae). Bioresour Technol 100:2284–2288
Ayyanar M, Subash-Babu P (2012) Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed 2(3):240–246
Benelli G (2015a) Research in mosquito control: current challenges for a brighter future. Parasitol Res 114:2801–2805
Benelli G (2015b) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114:3201–3212
Benelli G (2015c) The best time to have sex: mating behavior and effect of daylight time on male sexual competitiveness in the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae). Parasitol Res 114:887–894
Benelli G (2016a) Spread of Zika virus: the key role of mosquito vector control. Asian Pac J Trop Biomed 6:468–471
Benelli G (2016b) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res 115:23–34
Benelli G (2016c) Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer – a brief review. Enzym Microb Technol. doi:10.1016/j.enzmictec.2016.08.022
Benelli G, Govindarajan M (2016) Green-synthesized mosquito oviposition attractants and ovicides: towards a nanoparticle-based "lure and kill" approach? J Clust Sci. doi:10.1007/s10876-016-1088-6
Benelli G, Mehlhorn H (2016) Declining malaria, rising dengue and Zika virus: insights for mosquito vector control. Parasitol Res 115:1747–1754
Benelli G, Flamini G, Canale A, Molffeta I, Cioni PL, Conti B (2012) Repellence of Hyptis suaveolens whole essential oil and, major constituents against adults of the granary weevil Sitophilus granarius. Bull Insect 65:177–183
Benelli G, Murugan K, Panneerselvam C, Madhiyazhagan P, Conti B, Nicoletti M (2015) Old ingredients for a new recipe? Neem cake, a low-cost botanical by-product in the fight against mosquito-borne diseases. Parasitol Res 114:391–397
Benelli G, Lo Iacono A, Canale A, Mehlhorn H (2016a) Mosquito vectors and the spread of cancer: an overlooked connection? Parasitol Res 115:2131–2137
Benelli G, Pavela R, Canale A, Mehlhorn H (2016b) Tick repellents and acaricides of botanical origin: a green roadmap to control tick-borne diseases? Parasitol Res 115:2545–2560
Bezerra-Silva PC et al (2016) Evaluation of the activity of the essential oil from an ornamental flower against Aedes aegypti: electrophysiology, molecular dynamics and behavioral assays. PLoS One 11:e0150008
Bhuiyan NI, Begum J, Nandi NC, Akter F (2010) Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum (L.) Alston). African J Plant Sci 4(11):451–454
Boulos L (1983) Medicinal plants of North Africa. Reference publications, Algonac
Chaubey MK (2012) Responses of Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae) against essential oils and pure compounds. Herba Pol 58:33–45
Cheng SS, Liu JY, Tsai KH, Chen WJ, Chang ST (2004) Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J Agric Food Chem 52:4395–4400
Cheng SS, Liu JY, Huang CG, Hsui YR, Chen WJ, Chang ST (2009) Insecticidal activities of leaf essential oils from Cinnamomum osmophloeum against three mosquito species. Bioresour Technol 100:457–464
Conti B, Canale A, Bertoli A, Gozzini F, Pistelli L (2010) Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol Res 107:1455–1461
Da Silva RCS, Milet-Pinheiro P, Bezerra da Silva PC, da Silva AG, da Silva MV, Navarro DMAF, Silva NH (2015) (E)-Caryophyllene and α-humulene: Aedes aegypti oviposition deterrents elucidated by gas chromatography-electrophysiological assay of Commiphora leptophloeos leaf oil. PLoS One 9:1–14
Deo PG, Hasan SB, Majumdar SK (1988) Toxicity and suitability of some insecticides for household use. Int Pest Control 30:118–129
Elango G, Rahuman AA, Kamaraj C, Zahir AA, Bagavan A (2010) Studies on effects of indigenous plant extracts on filarial vector Culex tritaeniorhynchus Giles. Parasitol Res 107:167–176
Finney DJ (1971) Probit analysis. Cambridge University Press, London, pp. 68–72
Gamble JS, Fischer CEC (1923) Flora of the presidency of madras, vol I–III. Adlard and Son, London, pp. 1915–1935
Govindarajan M, Benelli G (2016a) Eco-friendly larvicides from Indian plants: effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotox Environ Saf 133:395–402
Govindarajan M, Benelli G (2016b) α-humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol Res 115:2771–2778
Govindarajan M, Benelli G (2016c) Artemisia absinthium-borne compounds as novel larvicides: effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol Res. doi:10.1007/s00436-016-5257-1
Govindarajan M, Rajeswary M, Benelli G (2016a) Chemical composition, toxicity and effects on non-target organisms of Pinus kesiya essential oil: an eco-friendly larvicide against mosquito vectors. Ecotox Environ Safe 129:85–90
Govindarajan M, Rajeswary M, Hoti SL, Bhattacharyya A, Benelli G (2016b) Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol Res 115:807–815
Govindarajan M, Rajeswary M, Benelli G (2016c) δ-Cadinene, Calarene and δ-4-Carene from Kadsura heteroclita essential oil as novel larvicides against malaria, dengue and filariasis mosquitoes. Comb Chem High Throughput Screen 19(7):565–571
Govindarajan M, Rajeswary M, Arivoli S, Samuel T, Benelli G (2016d) Larvicidal and repellent potential of Zingiber nimmonii (J. Graham) Dalzell (Zingiberaceae) essential oil: an eco-friendly tool against malaria, dengue and lymphatic filariasis mosquito vectors? Parasitol Res 115(5):1807–1816
Govindarajan M, Shine K, Naiyf S, Alharbi NS, Benelli G (2016e) Acute toxicity and repellent activity of the Origanum scabrum Boiss. & Heldr. (Lamiaceae) essential oil against four mosquito vectors of public health importance and its biosafety on non-target aquatic organisms. Environ Sci Pollut Res. doi:10.1007/s11356-016-7568-2
Gurib-Fakim A (2006) Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Asp Med 27:1–93
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391
Isman MB (2008) Botanical insecticides: for richer, for poorer. Pest Manag Sci 64:8–11
Khandagle AJ, Tare VS, Raut KD, Morey RA (2011) Bioactivity of essential oils of Zingiber officinalis and Achyranthes aspera against mosquitoes. Parasitol Res 109:339–343
Kim H, Chen F, Wu C, Wang X, Chung H, Jin Z (2004) Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J Agric Food Chem 52:2849–2854
Komalamisra Y, Trongtokit Y, Rongsriyam Y, Apiwathnasorn C (2005) Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian J Trop Med 36(6):1412–1422
Kramer WL, Mulla MS (1979) Oviposition attractants and repellents of mosquitoes: oviposition responses of Culex mosquitoes to organic infusions. Environ Entomol 8:1111–1114
Lee S, Najiah M, Wendy W, Nadirah M (2009) Chemical composition and antimicrobial activity of the essential oil of Syzygium aromaticum flower bud (clove) against fish systemic bacteria isolated from aquaculture sites. Front Agric China 3(3):332–336
Mabberly DJ (1997) The plant book. A portable dictionary of the vascular plants, 2nd edn. Cambridge University Press, Cambridge
Mahmoud II, Marzouk MS, Moharram FA, El-Gindi MR, Hassan AM (2001) Acylated flavonal glycoside from Eugenia jambolana leaves. Phytochemistry 58(8):1239–1244
Maisuthisakul P, Suttajit M, Pongsawatmanit R (2007) Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem 100:1409–1418
Mehlhorn H (ed) (2015) Encyclopedia of parasitology, 4th edn. Springer, New York 893
Mendonca FAC, Silva KFS, Santos KK, Ribeiro JKAL, Sant'Ana AEG (2005) Activities of some Brazilian plants against larvae of the mosquito Aedes aegypti. Fitoterapia 76:629–636
Naqqash MN, Gökçe A, Bakhsh A, Salim M (2016) Insecticide resistance and its molecular basis in urban insect pests. Parasitol Res 115:1363–1373
Nascimento JC et al (2013) Larvicidal activities and chemical composition of essential oils from Piper klotzschianum (Kunth) C. DC. (Piperaceae). Pest Manag Sci 69:1267–1271
Nassar MI, Gaara AH, El-Ghorab AH, Farrag A-RH, Hui Shen H, Huq E, Mabry TJ (2007) Chemical constituents of clove (Syzygium aromaticum, fam. Myrtaceae) and their antioxidant activity. Rev Latinoam Quim 35(3):47–57
Noudogbessi JP, Yedomonhan P, Sohounhloue DCK, Chalchat JC, Figueredo G (2008) Chemical composition of essential oil of Syzygium guineense (Willd.) DC. Var. Guineense (Myrtaceae) from Benin. Rec Nat Prod 2(2):33–38
Pavela R (2015) Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crop Prod 76:174–187
Pavela R, Benelli G (2016a) Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors - a review. Exp Parasitol 167:103–108
Pavela R, Benelli G (2016b) Essential oils as eco-friendly biopesticides? Challenges and constraints. Tr Plant Sci. doi:10.1016/j.tplants.2016.10.005
Pavela R, Canale A, Mehlhorn H, Benelli G (2016) Application of ethnobotanical repellents and acaricides in prevention, control and management of livestock ticks: a review. Res Vet Sci 109:1–9
Prajapati V, Tripathi AK, Aggarwal KK, Khanuja SPS (2005) Insecticidal, repellent and oviposition-deterrent activity of selected essential oil against Anopheles stephensis, Aedes aegypti and Culex quinquefasciatus. Bioresour Technol 96:1749–1757
Rajaganesh R, Murugan K, Panneerselvam C, Jayashanthini S, Aziz AT, Roni M, Suresh U, Trivedi S, Rehman H, Higuchi A, Nicoletti M, Benelli G (2016) Fern-synthesized silver nanocrystals: towards a new class of mosquito oviposition deterrents? Res Vet Sci 109:40–51
Raina VK, Srivastava SK, Aggarwal KK, Syamasundar KV, Kumar S (2001) Essential oil composition of Syzygium aromaticum leaf from little Andaman India. Flavour Frag J 16(5):334–336
Ravi Kiran S, Bhavani K, Sita Devi P, Rajeswara Rao BR, Janardhan Reddy K (2006) Composition and larvicidal activity of leaves and stem essential oils of Chloroxylon swietenia DC against Aedes aegypti and Anopheles stephensi. Bioresour Technol 97(18):2481–2484
Reynertson KA, Basile MJ, Kennelly EJ (2005) Antioxidant potential of seven myrtaceous fruits. Ethnobot Res Appl 3:25–35
Silva MF et al (2016) Composition and biological activities of the essential oil of Piper corcovadensis (Miq.) C. DC (Piperaceae). Exp Parasitol 165:64–70
Santos GKN, Dutra KA, Barros RA, Câmara CAG, Lira DD, Gusmão NB, Navarro DM (2012) Essential oils from Alpinia purpurata (Zingiberaceae): chemical composition, oviposition deterrence, larvicidal and antibacterial activity. Ind Crop Prod 40:254–260
Sivagnaname N, Kalyanasundaram M (2004) Laboratory evaluation of methanolic extract of Atlantia monophylla (family: Rutaceae) against immature stages of mosquitoes and non-target organisms. Mem Inst Oswaldo Cruz 99:115–118
Stalin N, Swamy PS (2013) Leaf essential oil composition and biochemical activity of an endangered medicinal tree Syzygium caryophyllatum (L.) Alston, (wild black plum). J Essent Oil Bear Pl 17(3):371–379
Stasi LCD, Hiruma-Lima CA (2002) Plantas Medicinais na Amazonia e na Mata Atlantica, 2nd edn. Editora Unesp, Sao Paulo
Wong KC, Lai FY (1996) Volatile constituents from the fruits of four Syzygium species. Flavour Frag J 11(1):61–66
World Health Organization (2005) Guidelines for laboratory and field testing of mosquito larvicides. Communicable disease control, prevention and eradication, WHO pesticide evaluation scheme. WHO, Geneva, WHO/CDS/WHOPES/GCDPP/1.3
Xue RD, Barnard DR, Ali A (2001) Laboratory and field evaluation of insect repellents as oviposition deterrents against the mosquito Aedes albopictus. Med Vet Entomol 15:126–131
Acknowledgements
The authors are grateful to the Professor and Head of the Department of Zoology, Annamalai University for the laboratory provisions granted.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Funding
G. Benelli is sponsored by PROAPI (PRAF 2015) and University of Pisa, Department of Agriculture, Food and Environment (Grant ID: COFIN2015_22). Funders had no role in the study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Benelli, G., Rajeswary, M. & Govindarajan, M. Towards green oviposition deterrents? Effectiveness of Syzygium lanceolatum (Myrtaceae) essential oil against six mosquito vectors and impact on four aquatic biological control agents. Environ Sci Pollut Res 25, 10218–10227 (2018). https://doi.org/10.1007/s11356-016-8146-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8146-3