Abstract
Contaminant concentrations, sources, seasonal variation, and eco-toxicological risk of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in surface water around Beijing from summer to winter in 2015 and 2016 were investigated. The concentrations of ∑OCPs and ∑PCBs ranged from 9.81 to 32.1 ng L−1 (average 15.1 ± 7.78 ng L−1) and from 7.41 to 54.5 ng L−1 (average 21.3 ± 1.87 ng L−1), respectively. Hexachlorocyclohexane (HCHs) were the dominated contamination both in aqueous and particulate phase. For PCBs, lower chlorinated PCBs were the major contaminants. Compositions of HCHs, dichlorodiphenyltrichloroethane (DDTs), and PCBs indicated that the sources of OCPs and PCBs in water were due to historical usage in the study areas. For OCPs, there was an obvious variation among three seasons, while insignificant change was shown for PCBs. Water quality standards for China’s surface water were not exceeded in this study. Concentrations at Miyun Reservoir, the primary source of drinking water to Beijing, when compared to the USEPA’s criterion for cancer risk was below the level of risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) are ubiquitous in environment due to their extensive usage and POPs characteristics which pose great risk to human (Zhang et al. 2013; Mrema et al. 2013) and the ecological environment (Nozar et al. 2014). OCPs were widely employed around the world in the late twentieth century and were mainly applied to agriculture and disease controlling. PCBs, with extremely stable physicochemical property and excellent electric insulation and thermal stability, were mainly applied to electronic productions. Due to their bioaccumulation, degradation resistance and carcinogenesis, tetratogenesis, and mutagenesis to human, the chlorine substituted pollutions gained great concerns all over the world (Wang et al. 2016; Xu et al. 2015). Therefore, 15 OCPs, such as hexachlorocyclohexane (HCHs), dichlorodiphenyltrichloroethane (DDTs) and chlordane, as well as PCBs, were banned with the issue of the Stockholm Convention in 2004 (Sharma et al. 2014).
In China, OCPs and PCBs were widely produced and used in 1960s. For example, the total use of DDTs and HCHs during 1950s to 1980s was 4.0 × 105 and 4.9 × 106 t, respectively (Hua and Shan 1996), accounting for about 20 and 30 % of the world total use, respectively. According to previous investigations, the total use of industrial PCBs around the world was 1.0 × 106 t before 1980s, and 1.0 × 104 t was manufactured in China (China SEPA 2003). Although the total use of OCPs and PCBs was sharply decreased after the signing of the Stockholm Convention, some OCPs were still in use, such as DDT used as an intermediate in the production of dicofol. Furthermore, due to the extensive usage, POPs characteristics, and poor management, these substances entered into various environmental compartments in China (Zhang et al. 2013). As one important issue, OCPs and PCBs could be transferred and accumulated into the aquatic environment by many ways, such as surface runoff, sewage discharge, atmospheric deposition, etc. (Yang et al. 1997; Yang et al. 2015; Zhang et al. 2010). In the past, many studies had been performed to characterize the contents of OCPs and PCBs in soil, atmosphere, precipitation, and surface water in Beijing, China (Zhang et al. 2014a; Li et al. 2009; Yang et al. 2012a; Yang et al. 2012b; Zhang et al. 2004). As one of the important environmental media of OCPs and PCBs, water presented a decline temporal contamination trend in Beijing. For example, the mean concentrations of ∑HCH, ∑DDT, and ∑PCBs in Tonghui River in 2002 were 356.38, 91.81, and 105.5 ng L−1, respectively (Zhang et al. 2004). However, in 2005, their concentrations declined to 18.0, 6.22 and 20.8 ng L−1, respectively (Li et al. 2008). Furthermore, HCHs and DDTs were still the major OCPs in the Chao River in 2011 with low concentrations of 1.57 and 2.19 ng L−1, respectively (Yu et al. 2014). After the signing of the Stockholm Convention, OCPs and PCBs had been extensively studied and their pollution had decreased to extremely low with strict policy in China. However, studies of OCPs and PCBs in surface water of Beijing were limited and their latest contamination situations were not available. Therefore, for the continuous implementation of the Stockholm Convention, it is necessary to study the latest concentrations of OCPs and PCBs in surface water around Beijing.
In the present study, three water systems in downtown and one water system in suburbs of Beijing were studied. Contamination levels of 17 OCPs and 84 PCBs in these systems were investigated during summer, autumn, and winter to study their spatial distributions and temporal variations. Finally, the recent sources of OCPs and PCBs were estimated, and their eco-toxicological risks were also assessed.
Materials and methods
Materials and reagents
Seventeen OCPs, including α-HCH, β-HCH, γ-HCH, δ-HCH, hexachlorobenzene, cis-chlordane, trans-chlordane, mirex, α-endosulfan, β-endosulfan, endosulfansulfat, p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, p,p′-DDD, and o,p′-DDD, were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Standard solution containing 84 PCBs and the internal standard solution (PCB 155 and TCmX) was obtained from Accu Standard Inc. (New Haven, CT, USA). All of the standard solutions were diluted by toluene into 10 mg L−1 respectively and stored at −18 °C prior to analysis.
All solvents (acetone, hexane, toluene, and dichloromethane (DCM)) were in HPLC grade and purchased from Fisher Scientific (Pittsburgh, PA, USA) or J. T Baker (Center Valley, PA USA.). Amberlite XAD-2 resin was obtained from Sigma-Aldrich (St. Louis, MO, USA). In addition, silica gel (100–200 mesh) was purchased from Merck (Kenilworth, NJ, USA). For silica gel treatment, it was activated at 300 °C for 10 h and then deactivated with Milli-Q water (3.5 % weight of silica). Sodium sulfate was baked at 650 °C for 10 h to decompose organics. The water was prepared from a Milli-Q system (18.2 MΩ cm−1, Billerica, MA, USA).
Sampling information
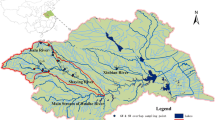
To understand the current concentration of OCPs and PCBs, nine surface water sampling sites located at Miyun reservoir (one site), Chaobai River (one site), Tonghui River (four sites), and Yuyuan Lake (three sites) were selected. The details of sampling sites were shown in Fig. 1. The Miyun reservoir is the biggest and the only drinking water source of Beijing whose water source originates from Hebei Province. As the most vital water resource, the quality of Miyun reservoir water is responsible to the health and the drinking water safety of 20 million citizens of Beijing. The downstream of Miyun reservoir is Chaobai River which is the biggest river of Beijing. The river traverses the most agriculture region of east Beijing, and it takes the responsibility of irrigation water function for the nearby farmland. Therefore, because of the geographical location and the agricultural function, the usage and the residual of pesticides are the main reason of the river’s pollution. Tonghui River starts from east second ring road and extends its way to the eastern suburb of Beijing, where the biggest sewage plant and some chemical plants located. At the same time, it is one of the landscape rivers in the downtown and the receiver of processed waste water from the chemical plants and the sewage plant. Therefore, its major pollution source is the waste water. The Yuyuantan Lake, originating mainly from Yongdinghe River and connecting the moat, is located in the west third ring road of Beijing. The contamination source came from the upstream, Yongdinghe River, which is severely polluted. The OCPs and PCBs contamination levels in these water systems can reflect their recent pollutions in downtown and suburb surface waters of Beijing. A total of 25 surface water samples were collected from July 2015 to January 2016. For every sampling site, 20-L water was collected and transferred to the lab in five 4-L PE bottles. Then the dissolve phase and particulate phase was separated via 0.45 μm GF/F fiber (Jiudinggaoke, China). The dissolved phase was transferred to brown glass container and kept in 4 °C, and the particulate phase was kept in −20 °C prior to analysis respectively. All the samples were extracted within 3 days.
Sample extraction and purification
For the dissolved phase, the target compounds were concentrated with a column which contained about 8.0 g XAD-2 resin. The water in the XAD-2 resin was removed using an oven setting at 60 °C. Before the extraction procedure, the internal standards were spiked into all of samples. The XAD-2 extraction was applied to accelerate solvent extraction (ASE) according to established procedures elsewhere, with little modification (Mokbel et al. 2016; Raeppel et al. 2014). The detail parameters were as follows: the extraction solvent was DCM, the extraction temperature was 100 °C, the static time was 10 min, and the extraction cycle was 3. After the extraction, DCM was concentrated and solvent-changed into hexane solution. Subsequently, the solution was purified with a silica gel solid phase extraction column (from top to bottom: 1 cm sodium sulfate, 8.0 g of 3.5 % deactivation silica gel, 1 cm sodium sulfate). After precondition with 20 mL hexane, the column was eluted with 80 mL hexane followed by 80 mL hexane and DCM (1:1, V/V) (Xu et al. 2005). After that, the eluent was further concentrated with a rotary evaporation and exchanged into toluene solution. Finally, the solution was concentrated to 0.2 mL under a gentle nitrogen stream and stored in −18 °C prior to GC-MS analysis.
The GF/F fiber contained particulate phase was triply ultrasonic extracted with 30 mL DCM for 30 min, and the extraction solvent was treated the same as the dissolved phase.
Instrumental analysis
An Agilent 7890 GC system coupled to a 5975C inert XL MSD with Triple-Axis Detector MS was employed for the sample analysis. The GC system was equipped with a DB-5MS (60 m × 0.25 mm i.d., 0.25 μm film thickness) capillary column for analyte separation. One microliter of the final extraction solution was injected in splitless model. The temperature of injector was 290 °C. The oven temperature program was as follows: from 90 °C (holding for 1 min) to 170 °C at 20 °C min−1, then to 280 °C (holding for 5 min) at 2 °C min−1. The temperatures of ion source and Quadrupole are 230 and 150 °C, respectively.
Quality assurance and quality control
In order to validate the analytical method, 6 samples were spiked with all of the selected congeners using the same analytical method. The recoveries of the target compounds in dissolved phase and particulate phase were in the range of 67.7–123 and 42.3–131 %, respectively. For every batch of nine samples, a procedural blank and a OCPs and PCBs spiked sample were carried out and blank correction was performed. The average recoveries of the internal standards were in the range of 74–118 %. The correlation coefficients of OCPs and PCBs for calibration curves were >0.995. The limit of detection (LOD) is defined to a signal to noise of 3. The method detection limits (MDLs) were characterized to be 0.10–0.38 ng L−1.
Results and discussion
OCPs in surface water in Beijing
The concentration range and average concentration of OCPs in surface waters of Beijing are described in Table 1. Seventeen OCPs were detected in 25 samples with different levels, indicating that the contamination of OCPs in Beijing was still very serious. The average concentrations of ∑17OCPs (dissolved phase and particular phase) in summer, autumn, and winter were 20.0 ± 14.8, 6.15 ± 3.55, and 19.3 ± 12.5 ng L−1. Generally, HCHs (α- + β- + γ- + δ-HCH) and DDTs were the dominated contaminants in 17 OCPs with high concentrations and high detection frequencies. The concentration of ∑HCHs in summer, autumn, and winter was in the range of 4.40–45.4 ng L−1 (average 15.3 ± 12.7 ng L−1), 1.21–13.4 ng L−1 (average 5.98 ± 3.49 ng L−1), and 9.19–45.3 ng L−1 (average 18.6 ± 12.2 ng L−1), respectively. At the same time, the average concentration of ∑DDTs in three seasons was 0.46 ± 0.73 ng L−1. High levels of HCH concentration were also detected in precipitation (33.0 ng L−1) (Yang et al. 2012b), soil (1.31 ng g−1) (Yu et al. 2014), and surface water (18.0 ng L−1) (Li et al. 2008) of Beijing, indicating the contamination of HCHs was extensive. On the other hand, other water sources in China, such as the Yangzi River, Honghu Lake, and Taihu Lake (Tang et al. 2013; Yuan et al. 2013; Wu et al. 2014), were also detected with high concentration of HCHs. Besides, HCB was another OCP which was detected in all samples, with an average concentration of 0.28 ± 0.20 ng L−1 in summer, 0.08 ± 0.03 ng L−1 in autumn, and 0.03 ± 0.03 ng L−1 in winter.
The average concentrations of OCPs in three seasons (15.1 ± 7.78 ng L−1) were compared with recent studies around Beijing district and other regions. There are few studies of OCPs in water around Beijing which mainly focusing on Haihe River of Tianjin, Tonghui River, Yongding-Guanting Reservoir districts, and rain water (Wang et al. 2007; Zhang et al. 2004; Li et al. 2008; Luo et al. 2009; Xue and Xu 2006; Yang et al. 2012b). From Fig. 2, the concentrations of OCPs around Beijing with different sampling period have an obvious decreasing tendency, indicating the efficient implement of Stockholm Convention in China. The concentrations of OCPs in Haihe River and Tonghui River during 2002 and 2003 were relatively higher with average of 709 and 529 ng L−1, respectively. In the meanwhile, higher concentration of OCPs in Guanting Reservoir during 2003 and 2004 was observed with an average of 202 ng L−1. However, in recent studies, the concentration of OCPs in water around Beijing was obviously decreased, such as Guanting Reservoir, Gaibeidian, and rain waters and was similar to concentration in the present study. When compared to other regions in China, the concentrations of OCPs were similar to the low reaches of Yangzi River, where the average concentration of ∑24OCPs was 14.2 ± 7.07 ng L−1 (Tang et al. 2013) and higher than that of Honghu Lake (the mean concentration of ∑HCH, ∑DDTs and ∑chlordans was 4.06 ng L−1) (Yuan et al. 2013). Furthermore, the contamination levels were relatively lower than that from heavily polluted agricultural region of Poyang Lake, where concentration of ∑19OCPs in 40 water samples was in the range of 19.1–112 ng L−1 (Zhi et al. 2015). Additionally, comparing to other countries, the contamination levels of OCPs in Beijing were similar to the same ∑10OCPs of rural-urban river basin in Tanzania during 2009 and 2011(7.05 ng L−1) (Hellar-Kihampa et al. 2013) and higher than that the same ∑10OCPs in Sarno River in southern Italy in 2008 (2.11 ng L−1) (Montuori et al. 2014), but lower than that of Owan river in Nigeria during 2012 and 2013 (1.26 μg L−1) (Ogbeide et al. 2015). Therefore, it can be concluded that present OCPs concentration levels observed in the surface water of Beijing were relatively low.
The average concentrations of OCPs compared with other studies around Beijing. OCPs = HCHs + DDTs + HCB + endosulfans. The sampling time for Haihe River, Tonghui River, Guanting Reservior-1, Guanting Reservior-2, Gaobeidian, and rain water were 2004, 2002, 2003–2004, 2006, 2006 and 2009–2011, respectively
PCBs in surface water in Beijing
The concentration range and average concentration of PCBs in surface waters of Beijing are also described in Table 1. The wide range, high detection frequency, and high concentration of PCBs in 25 samples indicated that the PCB contamination in surface water was widely spread around Beijing. The total concentration of ∑84PCBs in water samples in summer was in the range of 5.43–58.0 ng L−1 (dissolved phase + particulate phase), with an average value of 23.4 ± 16.5 ng L−1. In autumn and winter, the concentrations ranged from 7.48 to 51.1 ng L−1 (average 20.9 ± 16.9 ng L−1) and 3.35 to 68.2 ng L−1 (average 19.7 ± 23.3 ng L−1), respectively. The major components of PCB compounds were CB-180 (1.79 ± 1.29 ng L−1), CB-153 (1.09 ± 0.44 ng L−1), and CB-31&28 (0.87 ± 0.69 ng L−1). The sum of lower chlorine PCB homologues (Di-CBs, Tri-CBs, Tetra-CBs, and Penta-CBs accounted for 62.3 %) was the main PCBs contaminations in surface water of Beijing which was similar to the PCBs products in China (China SEPA 2003). In the meanwhile, higher chlorinated PCB homologues were abundant in particulate phase due to their low hydrophilicity. For example, the sum of Hexa-CBs, Hepta-CBs, and Octa-CBs and Nona-CBs in particulate phase of three seasons accounted for 71.1, 54.9, and 35.8 %, respectively, while in dissolved phase, the values were 24.8, 20.6, and 8.14 %, respectively.
The average concentration of ∑84PCBs was compared with other studies around Beijing and other districts in China. Similar to the concentration of OCPs in water around Beijing, the concentration of PCBs has an obvious decrease tendency either. From Fig. 3, we can see that ∑6PCBs in Gaobeidian in 2006 was similar to the concentration of ∑84PCBs in the same sampling site in the present study (Li et al. 2008). In the meanwhile, the concentration in Tonghui River in 2002 was 6.3 times higher than that of in the present study (Zhang et al. 2003). On the other hand, the concentration of PCBs in rain water of Beijing with an average concentration of 40.5 ng L−1 was a little higher than that in surface water (Yang et al. 2012a). When compared to other districts in China, the concentration of PCBs in Beijing was lower than that in Baiyangdian Lake (45.35 ng L−1) (Dai et al. 2011), similar to that detected in surface water of the Three Gorges Dam region (17.5 ng L−1) (Ge et al. 2014b), but much higher than that from the surface waters of the middle reaches of the Yangzi River (6.1 pg L−1) (Zhang et al. 2014b) and the East Lake (2.62 ± 1.91 ng L−1) (Ge et al. 2014a). When compared to other countries, the PCB concentration in present study was much higher than that in Sarno River in Southern Italy in 2008 (12.1 ng L−1) (Montuori et al. 2014), Chenab River in Pakistan in 2013 (5.72 ng L−1) (Mahmood et al. 2014), and Great Lake in America in 2011 (2.0–55 pg L−1) (Khairy et al. 2015), but much lower than Umgeni River in South Africa in 2013 (1089 ng L−1) (Gakuba et al. 2015). Therefore, it can be concluded that more efforts should be offered to further reduce present PCBs pollution in the surface water of Beijing.
Potential sources of OCPs and PCBs in surface waters of Beijing
HCH was the dominated contaminant in the present study. Previous studies have showed that the composition of HCH isomers can be used as the evidence of its sources. In China, the technical HCHs and lindane were widely used before the implementation of the ban. The composition of technical HCHs is about 60–70 % α-HCH, 5–12 % β-HCH, 10–15 % γ-HCH, and 6–10 % δ-HCH, and the ratio of α-HCH/γ-HCH is about 4–7. Compared with technical HCHs, lindane contains about 99 % γ-HCH (Willett et al. 1998). In the present study, the ratio of α-HCH/γ-HCH was in the range of 0.61–2.66, with an average 1.09 ± 0.65 which was much lower than that of technical HCHs. The lower ratio indicated the recent usage of lindane around Beijing district. The concentration of ∑DDTs ranged from 0.07 to 4.65 ng L−1 in summer, from below detection (n.d.) to 0.25 ng L−1 in autumn, and from 0.01 to 0.07 ng L−1 in winter, with an average value of 0.36 ± 0.52 ng L−1. The highest level of ∑DDTs in summer was observed in S1 with a concentration of 4.65 ng L−1, followed by S9 (1.38 ng L−1) and S3 (0.50 ng L−1). In autumn, the highest concentration was presented in S4 (0.25 ng L−1). However, in winter, the concentration of ∑DDTs was decreased to extremely low with the highest concentration of 0.07 ng L−1 detected in S3. In general, the ∑DDTs in summer was much higher than that in autumn and winter. DDE and DDD were not used as the insecticides and mainly resulted from the usage of technical DDT or the degradation of DDT. Many studies mentioned that DDT can degrade to DDE and DDD through biological effects (Yuan et al. 2013; Zhi et al. 2015; Hellar-Kihampa et al. 2013). Therefore, the ratio of DDT/(DDE + DDD) could be applied to identify whether there was a new DDT input or not (Xu et al. 2005). In the present study, the ratio of DDT/(DDE + DDD) ranged from 0.15 to 1.79, and the highest ratio of DDT/(DDE + DDD) was found in S6 and S9 (>1), indicating new discharge of DDTs to the two sampling sites. For the other sampling sites, the result indicated that DDT was degraded to DDD or DDE by microorganism biodegradation. Moreover, the higher ratio of o,p′-DDT/p,p′-DDT (0.27–103) in water samples indicated that the potential sources of DDTs were the combination of historical usage of technical DDT and dicofol in Beijing.
The difference of PCBs composition in nine sampling sites to that of six technical Aroclor was shown in Fig. 4. The dominated PCB homologues were Tetra-CB, Hexa-CB, and Hepta-CB in all sampling sites except S5 (Penta-CB, Hexa-CB, and Hepta-CB accounted for 66.7 %) and S8 (Tri-CB and Tetra-CB accounted for 74.3 %). While in Aroclor 1262 and Aroclor1260, the dominated PCB homologues were Hexa-CB and Hepta-CB, and that of Aroclor1248 was Tetra-CB. The results indicated the historical usage of Aroclor 1262, Aroclor1260, and Aroclor1248 in Beijing. While the composition in S5 (Tetra-CB, Penta-CB and Hexa-CB accounted for 94.4 %) was similar to that of Aroclor 1254, indicating numerous usage of Aroclor 1254 in this area. In the meanwhile, the component of PCB component in S8 was similar to Aroclor 1242 which meant that the historical usage of Aroclor 1242. Similar historical usage of Aroclor mixtures was also observed in East Lake (Ge et al. 2014a) and water source in South China (Yang et al. 2015).
The distribution and seasonal variation of OCPs and PCBs
Figure 5 shows the distribution variation of OCPs and PCBs in different sampling sites. For OCPs, the highest concentration was detected in S1, located far away from the downtown of Beijing, where a large quantity of OCPs was applied for agricultural usage. Another high concentration was located at the entrance of Yuyuan Lake with the concentration of 21.3 ng L−1 whose contamination source was the seriously polluted Yongding River. With the water flowing from S7 to S3, it turned out that insignificant OCPs concentration fluctuation was observed. Higher residual of PCBs was observed in S1 and S2. The Miyun Reservoir was built in 1960s. At the same time, some old devices contained PCBs oil may leak out into the environment. Therefore, the concentration of PCBs in S1 and S2 was higher than other sampling sites. Additionally, similar to the distribution of OCPs from S9 to S3, insignificant variation of PCBs contaminations was observed. It should be noted that the largest sewage treatment plant of Beijing and some large chemical plants located near S5. The disposed industrial wastewater and sewage runoff were directly discharged to Tonghui River. Therefore, higher level of PCB in S3 to S5 was observed compared to urban sampling sites.
Figure 6 shows the concentration range of OCPs and PCBs in the three seasons. From Fig. 6a, the concentrations of OCPs in summer and winter have a much wider range than that in autumn. At the same time, the average concentration in autumn is lower than that in summer and winter. The most important input of OCPs in surface water is surface runoff. At the same time, 80 % of the annual precipitation of Beijing is in summer. Therefore, the high volume precipitation and surface runoff in summer caused the higher concentration of OCPs in surface water of Beijing. However, the wet deposition, as well as surface runoff, decreased a lot in autumn. Therefore, with the water flowing, the concentration of OCPs contamination decreased. The total suspended particulate (TSP) deposition which contained OCPs and the “concentration effect” of dry season led to the phenomenon of higher concentration of OCPs in winter. The seasonal variability of PCBs was shown in Fig. 6b. Different from OCPs in three seasons, there were little variations of the average concentration of PCBs in Beijing district. The most important input of PCBs in surface water is the TSP deposition, the surface runoff, and air-water-sediment exchange (Duan et al. 2013). Therefore, the PCBs in soil were transferred to the river water through the surface runoff which caused the higher concentration of PCBs in summer. While in autumn, though the surface runoff was decreased, the water/sediment distribution and the “concentration effect” became the main factors, which caused the insignificant variations of PCBs contamination in autumn. In addition, the TSP deposition combined with the concentration effect became the main factor in winter. Therefore, particulates which adsorbed higher concentration of PCBs were transferred to the “concentrated” surface water causing insignificant variations of PCBs in winter.
Potential eco-toxicological risks
In order to estimate the OCPs and PCBs contamination levels and potential eco-toxicological risks in surface water of Beijing, the environmental quality standards for surface water of China (MEP P.R.C 2006) were compared to the present study. The limits of HCHs, HCB, DDTs, and PCBs in China were 5000, 1000, 1000, and 500 ng L−1, respectively. According to the present study, the contamination levels of HCHs, HCB, DDTs, and PCBs were all far less than the maximum level of the standard values of China. Therefore, the OCP and PCB pollution in these sites was in the safe range, though some residuals were still in water.
Furthermore, as the biggest drinking water source of Beijing, the cancer risk caused by OCPs and PCBs in S1 was taken into account. According to EPA health risk assessment model and combining the OCPs and PCBs concentration in present study(Wu et al. 2014; Yang et al. 2015), the cancer risks of PCBs, α-HCH, β-HCH, γ-HCH, HCB, p,p′-DDT, p,p′-DDE, and p,p′-DDD for adults was 0.12 × 10−6, 0.96 × 10−6, 0.62 × 10−6, 0.13 × 10−6, 1.79 × 10−8, 0.98 × 10−9, 0.98 × 10−9, and 0.92 × 10−9, respectively. The acceptable level of OCPs and PCBs recommended by EPA was 1.00 × 10−6. Therefore, the concentration of OCPs and PCBs in water of S1 was in an acceptable level and posed no cancer risk to the people of Beijing.
Conclusions
The contaminated levels, distribution, seasonal variation, and potential eco-toxicological risk of ∑17OCPs and ∑84PCBs in the surface water around Beijing were investigated in the present study. Through this study, we can know the current concentration level of two important categories of POPs in aqueous environment around Beijing. At the same time, we can also know the implementing efficiency of Stockholm Convention in China. The results showed that HCHs were the dominated contamination both in water phase (accounted for 85.4 %) and particulate phase (accounted for 93.8 %). Moreover, lower chlorinated PCB homologues, such as Tri-CBs and Tetra-CBs, were the major contaminants in water phase, whereas, in particulate phase, higher chlorinated PCB were the dominated compounds. According to the composition of OCPs and PCBs, the main sources of OCPs and PCBs in water were the historical usage. In the meanwhile, OCPs exhibited obvious seasonal variation, whereas insignificant variation was shown in PCBs. At the same time, none of the 17 OCPs and 84 PCBs was higher than the environmental quality standards for surface water of China and no cancer risk was posed to the public according to the criterion set by the EPA.
References
China SEPA (2003) GEF Project Brief (GF/CPR/02/010)
Dai GH, Liu XH, Liang G, Han X, Shi L, Cheng DM, Gong WW (2011) Distribution of organochlorine pesticides (OCPs) and poly chlorinated biphenyls (PCBs) in surface water and sediments from Baiyangdian Lake in North China. J Environ Sci 23:1640–1649
Duan XY, Li YX, Li XG, Li MF, Zhang DH (2013) Distributions and sources of polychlorinated biphenyls in the coastal East China Sea sediments. Sci Total Environ 463-464:894–903
Gakuba E, Moodley B, Ndungu P, Birungi G (2015) Occurrence and significance of polychlorinated biphenyls in water, sediment pore water and surface sediments of Umgeni River, KwaZulu-Natal, South Africa. Environ Monit Assess 187:568–581
Ge J, Liu MX, Yun XY, Yang YY, Zhang MM, Li QX, Wang J (2014a) Occurrence, distribution and seasonal variations of polychlorinated biphenyls and polybrominated diphenyl ethers in surface waters of the East Lake, China. Chemosphere 103:256–262
Ge J, Yun XY, Liu MX, Yang YY, Zhang MM, Wang J (2014b) Distribution, potential source and ecotoxicological risk of polychlorinated biphenyls and polybrominated diphenyl ethers in the surface water of the three Gorges Dam region of the Yangtze River, China. Ecotoxicology 23:978–987
Hellar-Kihampa H, Wael KD, Lugwisha E, Malarvannan G, Covaci A, Grieken RV (2013) Spatial monitoring of organohalogen compounds in surface water and sediments of a rural–urban river basin in Tanzania. Sci Total Environ 447:186–197
Hua XM, Shan ZJ (1996) The production and application of pesticides and factor analysis of their pollution in environment in China. Adv Environ Sci 4:33–45 in Chinese
Khairy M, Muir D, Teixeira C, Lohmann R (2015) Spatial distribution, air–water fugacity ratios and source apportionment of polychlorinated biphenyls in the lower Great Lakes Basin. Environ. Sci. Technol. 49:13787–13797
Li XM, Zhang QH, Dai JY, Gan YP, Zhou J, Yang XP, Cao H, Jiang GB, Xu MQ (2008) Pesticide contamination profiles of water, sediment and aquatic organisms in the effluent of Gaobeidian wastewater treatment plant. Chemosphere 72:1145–1151
Li YM, Zhang QH, Ji DS, Wang T, Wang YW, Wang P, Ding L, Jiang GB (2009) Levels and vertical distributions of PCBs, PBDEs, and OCPs in the atmospheric boundary layer: observation from the Beijing 325-m meteorological tower. Environ. Sci. Technol. 43:1030–1035
Luo JP, Ma M, Liu C, Zha JM, Wang ZJ (2009) Impacts of particulate organic carbon and dissolved organic carbon on removal of polycyclic aromatic hydrocarbons, organochlorine pesticides, and nonylphenols in a wetland. J Soils Sediments 9:180–187
Mahmood A, Malik RN, Li J, Zhang G (2014) Levels, distribution profile, and risk assessment of polychlorinated biphenyls (PCBs) in water and sediment from two tributaries of the river Chenab. Pakistan Environ Sci Pollut Res 21:7847–7855
MEP, P.R.C. (Ministry of Environmental Protection of the People’s Republic of China) (2006) Environmental quality standards for drinking water (GB5749–2006), Beijing (in Chinese)
Mokbel H, Dine EJA, Elmoll A, Liaud C, Millet M (2016) Simultaneous analysis of organochlorine pesticides simultaneous analysis of organochlorine pesticides simultaneous analysis of organochlorine pesticides (SPME) coupled to gas chromatography dual electron (SPME) coupled to gas chromatography dual electron. Environ Sci Pollut Res 23:8053–8063
Montuori P, Cirillo T, Fasano E, Nardone A, Esposito F, Triassi M (2014) Spatial distribution and partitioning of polychlorinated biphenyl and organochlorine pesticide in water and sediment from Sarno River and Estuary, Southern Italy. Environ Sci Pollut Res 21:5023–5035
Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C (2013) Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 307:74–88
Nozar SLM, Ismail WR, Zakaria MP (2014) Distribution, sources identification, and ecological risk of PAHs and PCBs in coastal surface sediments from the Northern Persian Gulf. Hum Ecol Risk Assess 20:1507–1520
Ogbeide O, Tongo I, Ezemonye L (2015) Risk assessment of agricultural pesticides in water, sediment, and fish from Owan River, Edo State, Nigeria. Environ Monit Assess 187:654
Raeppel C, Fabritius M, Nief M, Appenzeller BMR, Millet M (2014) Coupling ASE, sylilation and SPME–GC/MS for the analysis of current-used pesticides in atmosphere. Talant 121:24–29
Sharma BM, Bharat GK, Tayal S, Nizzetto L, Cupr P, Larssen T (2014) Environment and human exposure to persistent organic pollutants (POPs) in India: a systematic review of recent and historical data. Environ Int 66:48–64
Tang ZW, Huang QF, Yang YF, Zhu XH, Fu HH (2013) Organochlorine pesticides in the lower reaches of Yangtze River: occurrence, ecological risk and temporal trends. Ecotoxicol Environ Saf 87:89–97
Wang WT, Wang YH, Zhang RJ, Wang SP, Wei CS, Chaemfa C, Li J, Zhang G, Yu KF (2016) Seasonal characteristics and current sources of OCPs and PCBs and enantiomeric signatures of chiral OCPs in the atmosphere of Vietnam. Sci Total Environ 542:777–786
Wang T, Zhang ZL, Huang J, Hu HY, Yu G, Li FS (2007) Occurrence of dissolved polychlorinated biphenyls and organic chlorinated pesticides in the surface water of Haihe River and Bohai Bay. China Environment Science 28(4):730–735 In Chinese
Willett KL, Ulrich EM, Hites RA (1998) Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environ Sci Technol 32:2197–2207
Wu CF, Luo YM, Gui T, Huang YJ (2014) Concentrations and potential health hazards of organochlorine pesticides in shallow groundwater of Taihu Lake region. China. Sci. Total Environ. 470-471:1047–1055
Xu DD, Dan M, Song Y, Chai ZF, Zhuang GS (2005) Concentration characteristics of extractable organohalogens in PM2.5 and PM10 in Beijing. China. Atmos. Environ. 39:4119–4128
Xu PW, Lou XM, Ding GQ, Shen HT, Wu LZ, Chen ZJ, Han JL, Wang XF (2015) Effects of PCBs and PBDEs on thyroid hormone, lymphocyte proliferation, hematology and kidney injury markers in residents of an e-waste dismantling area in Zhejiang. China Sci Total Environ 536:215–222
Xue ND, Xu XB (2006) Composition, distribution, and characterization of suspected endocrine-disrupting pesticides in Beijing GuanTing reservoir (GTR). Arch Environ Contam Toxicol 50:463–473
Yang GS, Ma LL, Xu DD, Liu LY, Jia HL, Chen Y, Zhang YB, Chai ZF (2012a) Temporal trends of polychlorinated biphenyls in precipitation in Beijing. China. Atmos. Environ. 56:222–227
Yang GS, Ma LL, Xu DD, Liu LY, Jia HL, Chen Y, Zhang YB, Chai ZF (2012b) Temporal trends of organochlorine pesticides in precipitation in Beijing. China Atmos Environ 61:614–619
Yang YH, Sheng GY, Fu JM, Min YS (1997) Organochlorinated compounds in waters of the pearl river delta region. Environ Monit Assess 44:569–575
Yang YY, Xie QL, Liu XY, Wang J (2015) Occurrence, distribution and risk assessment of polychlorinated biphenyls and polybrominated diphenyl ethers in nine water sources. Ecotoxicol Environ Saf 115:55–61
Yu Y, Li YX, Shen ZY, Yang ZF, Mo L, Kong YH, Lou I (2014) Occurrence and possible sources of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) along the Chao River, China. Chemosphere 114:136–143
Yuan LX, Qi SH, Wu XG, Wu CX, Xing XL, Gong XY (2013) Spatial and temporal variations of organochlorine pesticides (OCPs) in water and sediments from Honghu Lake. China J Geochem Explor 132:181–187
Zhang Y, Fu S, Dong Y, Nie H, Li Z, Liu X (2014a) Distribution of polychlorinated biphenyls in soil around three typical industrial sites in Beijing. China. Bull. Environ. Contam. Toxicol. 92:466–471
Zhang Q, Gao LR, Zheng MH, Liu LD, Li C (2014b) Polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) in water samples from the middle reaches of the Yangtze River. China Bull Environ Contam Toxicol 92:585–589
Zhang ZL, Huang J, Yu G, Hong HS (2004) Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing. China Environ Pollut 130:249–261
Zhang K, Wei YL, Zeng EY (2013) A review of environmental and human exposure to persistent organic pollutants in the Pearl River Delta. South China Sci Total Environ 463-464:1093–1110
Zhang W, Ye YB, Hu D, Ou LB, Wang XJ (2010) Characteristics and transport of organochlorine pesticides in urban environment: air, dust, rain, canopy throughfall, and runoff. J Environ Monit 12:2153–2160
Zhi H, Zhao ZH, Zhang L (2015) The fate of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in water from Poyang Lake, the largest freshwater lake in China. Chemosphere 119:1134–1140
Acknowledgments
We are grateful to the co-workers in our laboratory for sample collection. We thank the financial supports from National Natural Science Foundation of China (Grant Nos. 11275216, 21407149, 11435002, 11575210, and 11375212), and Innovation Program of Institute of High Energy Physics, Chinese Academy of Sciences (Y451555), and the 2014 Beijing Natural Science Foundation-Beijing Institute of Science and Technology jointly funded projects (L140006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester heath
Rights and permissions
About this article
Cite this article
Shao, Y., Han, S., Ouyang, J. et al. Organochlorine pesticides and polychlorinated biphenyls in surface water around Beijing. Environ Sci Pollut Res 23, 24824–24833 (2016). https://doi.org/10.1007/s11356-016-7663-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7663-4