Abstract
The aim of this study has been to measure the level of lead, cadmium, nitrates, and nitrites in the daily diets of children and adolescents from orphanages located in Krakow (Poland). Diets were collected over four seasons of 2009. The content of cadmium and lead was measured with flameless atomic absorption spectrometry. Nitrates and nitrites in diets were measured using the Griess colorimetric method. In all orphanages, the average intake of lead with daily diets, regardless of the season, ranged from 1.11 ± 0.15 to 22.59 ± 0.07 μg/kg bw/week. The average cadmium intake by children and adolescents ranged between 3.09 ± 0.21 and 20.36 ± 2.21 μg/kg bw/week and, for all orphanages, exceeded the tolerable weekly intake (TWI) level. Daily intake of nitrates and nitrites ranged respectively from 27 to 289 % and from 9 to 99 % of the acceptable daily intake (ADI). The youngest children, with lower body mass, were particularly sensitive to the excessive intakes of cadmium and nitrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of heavy metals in the environment results from human activity and the development of civilization (Järup & Åkesson 2009; Ociepa-Kubicka & Ociepa 2012; WHO 2010a; WHO 2010b; EFSA 2010; EFSA 2012). In this context, particular importance is attached to lead and cadmium, heavy metals which are absolutely harmful to the human body (Ociepa-Kubicka & Ociepa 2012; Engström et al. 2012; WHO 2010a; WHO 2010b; EFSA 2012).
Lead has been commonly used in industry, particularly in developing countries. This heavy metal exists in the environment in nonorganic form and is nonbiodegradable, which is the major reason for its high occurrence (EFSA 2010; Liu et al. 2010a; Liu et al. 2010b; Liu et al. 2012). It can be found in soil, water, various food products (cereal products, potatoes, leafy vegetables, tap water), as well as in buildings, especially old ones. It penetrates the human body through inhalation and ingestion (EFSA 2010; Faulk et al. 2014; Kapusta-Duch et al. 2010; Liu et al. 2010a; Liu et al. 2012; Järup & Åkesson 2009). Some data suggest that even the lowest level of exposure to this metal may cause health problems and may affect, in particular, the function of the central nervous system, the liver, and the kidneys. Lead is especially harmful for children. The US Centers for Disease Control and Prevention decreased the guidelines for children’s exposure to this metal, based on its concentration in blood, from 10 μg/dL to the level below 5 μg/dL. It has also been suggested that the concentration of lead in children’s blood should not exceed 2 μg/dL, in order to protect the central nervous system (Betts 2012; Gilbert & Weiss 2006; Faulk et al. 2014). The European Food Safety Authority (EFSA) CONTAM Panel concluded that the provisional tolerable weekly intake (PTWI) of 25 μg/kg bw established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) is not appropriate. For this reason, EFSA established the benchmark dose lower confidence limits (BMDL) in the case of neurotoxic doses in young children (12 μg/L in the blood), nephrotoxicity (15 μg/L in the blood), and cardiovascular effect (systolic blood pressure of 36 μg/L in the blood) in adults. The above mentioned values correspond to the dietary intake values of 0.50, 0.63, and 1.50 μg/kg bw/day, respectively (EFSA 2010). To the best knowledge of the authors, JECFA did not make any changes (WHO 2010b).
Cadmium occurs naturally in soil but is also used in industry. One of the major sources of cadmium for humans are foods of plant origin (cereals, leafy green vegetables, potatoes, root vegetables) providing even up to 80 % of the total amount of this metal (Engström et al. 2012; Martí-Cid et al. 2008; Kapusta-Duch et al. 2011; Wilk et al. 2013). The time-prolonged effect of even small amounts of Cd contributes to its accumulation, particularly in the liver, kidneys, and bones, influencing various disorders in the human body. These include neuronal, nephro, carcino, teratogenic, and immune disorders (Enli et al. 2010; Engström et al. 2012).
The EFSA CONTAM Panel established the tolerable weekly intake (TWI) of cadmium at the level of 2.5 μg/kg bw in 2009. Two years later, this value was also approved by the Joint Experts Committee on Food Additives (www.efsa.europa.eu; EFSA 2009; EFSA 2012). What is more, in 2010, the JECFA established the provisional tolerable monthly intake of cadmium at 25 μg/kg bw (WHO 2010a).
Nitrates and nitrites can penetrate food products from the environment as contaminants and may also be added intentionally as preservatives: in meat production as the chief components of curing mixtures and in rennet cheese production. Major sources of nitrates in human daily diets are vegetables providing up to 80 % of the total amount of these compounds (Hord et al. 2009; Leszczyńska et al. 2009). The problem of the excessive accumulation of these compounds in plants occurs most frequently due to the application of high doses of nitrogen as fertilizer (WHO 2006). Nitrates belong to the group of chemical compounds of relatively low toxicity, but in the human body, they are converted to the nitrites responsible for methemoglobinemia in children. Moreover, they manifest their toxic effects in the human body by affecting reproduction, thyroid function, as well as the inhibition of body weight gain. Nitrites can also affect the human body through participation in the formation of N-nitroso compounds exhibiting carcinogenic, mutagenic, and embryotoxic effects (Volkmer et al. 2005; WHO 2006). On the other hand, several clinical trials are being carried out in order to determine the broad therapeutic potential of increasing nitrite bioavailability on human health and disease, including studies related to vascular aging. Inorganic nitrite, as well as dietary nitrate supplementation, represents a promising therapy for the treatment of arterial aging and prevention of age-associated cardiovascular diseases in humans (Hord et al. 2009; Kapil et al. 2010; Sindler et al. 2014). Adequate daily intake (ADI) for nitrates (sodium and potassium) and nitrites (sodium and potassium) is up to 5.0 and 0.2 mg/kg bw/day, respectively (WHO 2006).
Based on the abovementioned results of other authors, the following research hypothesis has been defined: meals for children and adolescents in orphanages, prepared based on commonly available food products, may pose a risk for the excessive intake of lead, cadmium, nitrates, and nitrites, especially in relation to the population of children with the lowest body mass.
The major objective of this study has been to increase knowledge concerning the intake of lead, cadmium, nitrate, and nitrite with daily diets by children and adolescents from orphanages. The results of these studies were used in the workshop prepared for persons involved in the planning as well as preparation of daily diets, in order to teach them how to reduce or eliminate food products constituting the main source of the abovementioned pollutants. Based on the data obtained, recommendations can be given for other orphanages in Poland.
Materials and methods
Children’s diets
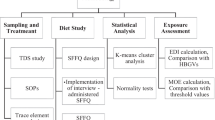
The description of diet preparation was previously reported (Pysz et al. 2016). Shortly, the study foresees receiving one daily diet from the each orphanage per day. Daily diets were collected from the seven orphanages located in Krakow, Poland, for 4 days of the week, according to the recommendations (Thomson and Byers 1994), including Sunday and Friday, when the meals consumed are different owing to the Polish traditions. Diets were collected during spring, summer, autumn, and winter of 2009. A total of 112 diets of children and adolescents have been analyzed. Average meals were collected during the time of consumption. Meals were put into certified, sealed containers, which were kept in temperatures of 4 °C, also during their transport to the laboratory. At the laboratory, the daily diets were weighed. The obtained weight of the daily diet was compared with the mass calculated based on the same day’s menu. The mass of each daily diet was corrected for leftovers i.e., 10 % of which are accepted principles in studies of this type (Kunachowicz et al. 2007; Gawęcki 2010). Daily diets were homogenized using a homogenizer (CAT type X 120, Paso Robles, CA, USA).
A part of each diet was used for the analysis of nitrates and nitrites as well as dry mass. The remaining diets were freeze dried (Christ Alpha 1-4, Gefriertrocknungsanlangen, Germany) and stored in temperatures of −20 °C until to the other analyses. In freeze-dry samples, the dry mass was determined.
Nitrates and nitrites analysis
Homogenized samples were used for the analysis of nitrate and nitrite contents using the Griess colorimetric method according to the Polish norm PN-92 /A-75112 (Polish Standard 1992). Analyses were performed in triplicate. Nitrate content was assessed using Griess I (sulfanilamide, Sigma-Aldrich, Saint Louis, USA) and Griess II (n-(1-Naphtyl)ethylene-diamine dihydrochloride, water solution, Sigma-Aldrich, Saint Louis, USA). In this method, the reaction of nitrite with n-(1-Naphtyl)ethylene-diamine dihydrochloride in acidic conditions results in a color complex and absorbance is measured at the wavelength of 538 nm. For the best knowledge of authors, daily diets with certified concentrations of nitrates and nitrites are not commonly available. This method was verified on the basis of the recovery ratio of analyzed compounds (analysis of spiked samples) which were previously used for recovery studies. To daily diets with a lower content of nitrates and nitrites, KNO2 or NaNO3 was added in three various known concentrations. For each sample, analyses were made in triplicate. Sample recovery ranged between 80 and 110 % which was correct according to the principles (Konieczka & Namieśnik (ed.) 2007).
The determination of lead and cadmium in daily diets
The concentration of lead and cadmium in freeze-dry diets was measured using flameless atomic absorption spectrometry. The determination of heavy metals content was carried out via a validated method employing electrothermal atomization with graphite cuvette (Varian AA240Z; Varian Palo Alto, CA, USA) according to the EN 14084: 2003 (EN 14084: 2003). Wet mineralization was conducted using the pressure microwave method (MarsXPres, CEM, Matthews, NC, USA) with nitric acid (cat no. 1.00441, Suprapur MERCK, Darmstadt, Germany) in the amount of 10 mL per 0.5 g of sample in the temp of 200 °C. For the assessment of cadmium content, the buffer solution was applied (cat. No. 1.14282, MERCK, Darmstadt, Germany) in the amount of 5 μL per 20 μL of the diet sample.
For the validation of the method, the reference material (i.e., cabbage) was used (NCS ZC 73012 GSB-5) approved by the China National Analysis Center for Iron and Steel (Beijing China). The concentration of Cd was 35 ± 6 μg/kg and for Pb 190 ± 30 μg/kg. For Cd precision repeatability was 3.79 % and for the recovery of the sample, 105.02 %. For Pb precision repeatability was 9.52 % and for the recovery of the sample, 108.7 %.
Interpretation of results
The content of contaminants in daily diets has been calculated per real intake, on the basis of the weight of diets as well as the body mass of children from each orphanage. The average body mass of children from each orphanage was calculated (Table 1). Lead intake was compared to the provisional tolerable weekly intake (PTWI) i.e., 25 μg/kg bw (WHO 2010b). Cadmium intake was compared to the tolerable weekly intake (TWI) which is 2.5 μg/kg bw (www.efsa.europa.eu; EFSA 2012). Intake of nitrates and nitrites was compared to the acceptable daily intake value (ADI). In accordance with the data from JECFA, the acceptable daily intake (ADI) of nitrates and nitrites from diets was set to 5.0 mg NaNO3/kg bw/day and 0.2 mg KNO2/kg bw/day, respectively (WHO 2006).
Statistical analysis
In order to establish the significance of differences between the content of contaminants in the diets of children from orphanages depending on the season, the one-way analysis of variance (ANOVA) has been applied. The evaluation of significant differences was performed using Duncan’s test at the significance level of p ≤ 0.05.
Results and discussion
Lead
In all the orphanages, the average intake of lead with daily diets, regardless of the season, ranged from 1.11 ± 0.15 to 22.59 ± 0.07 μg/kg bw/week, which was from 4.76 to 90.4 % of the PTWI value (Table 2). The highest amount of lead intake per kilogram body mass was measured in the youngest children from orphanage I (46.3 % of PTWI). The smallest amounts (about 12 % of the PTWI) were determined in the diets from orphanages III and VI. The largest content of this metal has been found in the diets collected in winter season, while the lowest level in the diets collected during the summer season. In orphanage I, significantly higher concentration of lead was measured in winter as compared to the diets collected in spring (p = 0.015) and summer (p = 0.003). Daily diets from the orphanages II, III, VI, and VII provided significantly highest level of lead in winter season as compared to the other seasons (p = 0.004, 0.002, 0.004; p = 0.005, 0.006, 0.004; p = 0.001, 0.000, 0.001; and p = 0.007, 0.005, 0.006, respectively). In the orphanage IV, the highest level of lead was measured in winter as compared to diets collected in spring (p = 0.012) and autumn (p = 0.016). In orphanage V, significantly higher content of lead was measured in diets from winter as compared to the diets collected in summer (p = 0.023) as well as in autumn (p = 0.031).
The highest concentration of lead in daily diets collected in the winter season may be explained by higher consumption of cereal products and vegetables, especially carrot, red beets, cabbages as well as juice which may contain carrots. During the summer season, probably the consumption of juice containing carrot was lower. Additionally, vegetables used for meals preparation were shortly cultivated (early vegetables) and it could cause lower concentration of lead in the daily diets of children collected in summer season. Based on the results obtained in our study, the average intake of lead may be referred as safe for children and adolescent. Albeit, the children with lower body mass may be more sensitive than adolescents or adults.
For the best knowledge of authors, in the available literature lacks data on lead and cadmium contaminants in daily diets of children from orphanages. Therefore, the results obtained in this study were compared to the results for other population groups.
Results obtained in our study are similar to data published by other Polish and foreign authors. Kłos et al. (2009) determined the level of lead in average daily diets obtained from preschool gardens from various parts of Poland. The content of lead was 125.5 ± 31.4 μg (46.8 % of PTWI). Analytically assessed lead concentration in daily diets of children and adolescent (age 10–19 years, average body mass (58.9 ± 15.9 kg) from educational centers located in Lublin region (eastern part of Poland) was in the range 227–364 μg/week (20.7–21.5 % PTWI) (Marzec & Łukasiewicz 2010). The average intake of analytically assessed lead by persons from rural area of Świętokrzyskie region (Poland) was 6.26 μg kg bw/week (25 % of PTWI) (Leszczyńska & Gambuś 2001). What is more Marzec & Schlegel-Zawadzka (2004), also reported that intake of lead (assessed by chemical analysis) by Polish population living in the Eastern part of Poland was in the range 66.5–106.0 μg/person/day (31–49 % of PTWI). The major sources of lead in diets were vegetables, cereals, meat and its products, and fruits. Liu et al. (2010b) showed that the mean intake of lead with daily diets among Chinese 30 children (Jinhu, southeastern part of China) in age 1.9–7.0 was 15.66 μg kg bw/week. The dietary intake of lead by adult with body mass 70 kg from Catalonia region (Spain) was 45.13 μg/day (Martí-Cid et al. 2008). Muñoz et al. (2005) reported that the intake of lead with food products by population of Santiago (Chile, average body mass 68 kg) was 206 μg/day (85 % of PTWI) and was lower than PTWI established by JECFA. Leblanc et al. (2000) reported that the estimated intake of lead calculated based on chemical analysis of meals obtained from French mass catering establishments was 216 μg/day/person. Based on our data as well as results from other researches, it can be suggested that intake of lead with food product by various groups of populations is lower than PTWI value.
Cadmium
The average cadmium intake with diets by children and adolescents ranged between 3.09 ± 0.21 and 20.36 ± 2.21 μg/kg bw/week. In all orphanages, the TWI value was exceeded, which is an important finding of our studies (Table 3). As in the case of lead, the largest cadmium intake per kilogram body mass was found in orphanage I, in each season. In this orphanage are the children aged 4–6. High level of cadmium was measured in diets form orphanage II, IV as well as V. The significantly higher content of cadmium was found in diets collected from winter as compared to the diets collected in spring in orphanage IV. A reason for higher intake of cadmium with consumed food was similar like in the case of lead. Persons who plan menu and prepare diets should pay more attention in selecting food products. They can decrease the amount of cereal flakes and groats in diets, which are major sources of this heavy metal. Washing and peeling of vegetables may reduce the concentration of pollutants (cadmium, lead) in final product.
Intake with daily diets too large amounts of heavy metals hinders the use of many nutrients by blocking the active sites of the enzymes; they can also react with nucleic acids, increase urinary excretion of microelements (as the effect of renal tubular damage), and reduce hematological indices (Chowdbury & Chandra 1987; Goyer 1997).
Results obtained in this study are similar to data published by EFSA (EFSA 2012). Albeit in some cases, in our study, the content of cadmium in diets of assessed subpopulation was higher than in EFSA report (EFSA 2012). When we have compared the exposure of children to cadmium to the previous value of provisional tolerable weekly intake 0.007 mg/kg bw/week, the exposure to this metal was still too high. Similar results were published by Watanabe et al. (2013), who reported that the intake of cadmium with daily diets, by Japanese children aged of 3–6, was 4.20 μg/kg bw/week. Our results differ from those reported by the majority of other authors, who determined cadmium content in diets of children, adolescents, and adults at the lower level compared to the PTWI value. Marzec & Łukasiewicz (2010) reported lower content of cadmium in daily diets of children from educational centers (Lubelskie region, Poland). The cadmium content was in the range 121–214 μg/week (34.2–45.2 % PTWI). Also, Liu et al. (2010b) reported that the daily diets of Chinese children contained cadmium in amount 1.49 μg/kg bw/week, which was lower than PTWI value. Concentration of cadmium was analytically assessed. In previously published papers by other Polish researchers, usually the concentration of cadmium in diets of various groups of populations has not exceeded PTWI value. Leszczyńska & Gambuś (2001) reported lower content of cadmium in average daily diets from households of rural area of Świetokrzyskie region (Poland). The level of cadmium was in the range 2.03–6.63 μg/kg bw/week. Marzec & Schlegel-Zawadzka (2004) measured the content of this heavy metal in household diets from Eastern Poland, on the level 16.4–34.5 μg/person/day (27–58 % PTWI). They have found that major source of this heavy metal in diets were cereals, vegetables, and meat products. Kłos et al. (2008) reported that concentration of cadmium in selected diets, obtained from military health resorts located in various parts of Poland, was in the range 27.0 ± 17.5–76.0 ± 22.2 μg/day (20.13–30.39 % PTWI, PTWI = 0.007 mg/kg bw). Additionally, published data from other countries usually show lower cadmium content in daily diets than PTWI value. Sand & Becker (2012) reported mean dietary cadmium exposure for adults from Belgium on the level 2.3 μg/kg bw/week. The highest concentration of this heavy metal was in potatoes, wheat flour, and fish and seafood. Exposure to cadmium with average British daily diets was 12 μg/day (Ysart et al. 2000). Muñoz et al. (2005) showed that for adult person from Santiago (Chile), the estimated cadmium intake with food products was 20 μg/day. The major sources of cadmium were fish and shellfish, spices, cereals, potatoes, meat, milk and its products, and vegetables. Also in Belgium, chemically assessed mean intake of cadmium was 23.1 ± 6.6 μg/day (Van Cauwenbergh et al. 2000). Martí-Cid et al. (2008) reported that the intake of cadmium with food products by average Spanish person from Catalonia region was 9.80 μg/day. The highest concentration of cadmium was determined in fish and shellfish, potato, milk and dairy products, vegetables, fruits, and meat and meat products. According to the data published by Leblanc and co-workers (2000), the dietary exposure of French consumer to cadmium intoxication was 60 μg/day/person. Rubio et al. (2006) showed higher level of cadmium in food products frequently consumed by Canary Island (Spain) population, i.e., 72.8 μg/day (for person weighted 70 kg), which was 29.12 % of PTWI. Additionally, the highest mean concentration of cadmium was in fish, legumes, red meat, and vegetables. This value also did not exceed PTWI. Based on literature review, Van Cauwenbergh et al. (2000) reported that daily intake cadmium by populations of various countries around the world was in the range 7.1–56.3 μg/day.
Nitrates
Nitrate intake calculated per NaNO3 ranged from 70 to 339 mg/person/day and in some cases exceeded the ADI value (Table 4). The largest exceeding of the ADI value for nitrates was found in the orphanage I, in which there were the youngest children. Intake of these compounds was in the range 74–197 mg/person/day (2–189 % of ADI). Nitrates do not pose a threat to human health, but they can be converted to nitrite, which are toxic. The lowest intake of nitrates with the daily diets, compared to the ADI, was found in orphanages III and VI. Generally, the highest content of nitrates was measured in diets collected in winter season. During this period, ADI for nitrates was exceeded from 3 to 189 %, in the five orphanages.
The significantly higher content of nitrates was measured in diets collected in winter season from orphanages I and VII as compared to the diets collected in other seasons (p = 0.003, 0.004, 0.003 and p = 0.035, 0.034, 0.037, respectively). The major sources of nitrates in daily diets, especially coming from the winter season, were vegetables (carrot, red beets, cabbages). Intake of these substances from daily diets can be reduced by proper washing of vegetables. A good option may be also purchasing these products from the controlled organic farms.
Our results are similar to data published by Wawrzyniak et al. (2008a). They reported that estimated intake of nitrates with daily diets by children from selected preschools located in Warsaw, Poland, was high and significantly exceeded the ADI value (range 121.9–172.3 % of ADI). Assessment of nitrate intake was performed based on menu from 10 days of the autumn, winter, and spring season. The major sources of these nitrates in diets were vegetables. Additionally, they concluded that the amount of nitrates contained in the consumed food, when calculated per 1 kg of children body weight, was much higher than identically calculated for adults. Also, Bawa et al. (2008) reported that estimated intake of nitrates by children aged 2–6 was 59.2 ± 36.8 mg/person/day (106.6 % ADI). These authors also found that in case of other subgroups (children aged 7–10, adolescents, pregnant women), ADI was not exceeded. Opposite to our results, it was reported by other authors that daily nitrate intake with diets by children and adults was lower, compared to the ADI. Leszczyńska (2000) reported that analytically assessed concentration of nitrates, in daily diets of households collected in autumn and spring from rural area of Świętokrzyskie region (Poland), was 140.9 mg/person/day (44 % ADI) and 167.3 mg/person/day (57 % ADI), respectively. Also, Wawrzyniak et al. (2008b) estimated that daily intake of nitrates in Polish households was lower than in our studies (132–190 mg NaNO3 person/day 56.8 % ADI). Additionally calculated, average intake of nitrates with daily diets by students from Poland did not exceed ADI value (77.3 mg NaNO3/person/day i.e., 25.1 % ADI). Vaessen & Schothorst (1999) reported that median intake of nitrates with diets in Nederland was 80 mg/person/day. Tamme et al. (2006) reported that intake of nitrates by Estonian population was 58 mg/day.
Nitrites
Despite the fact that in same days of the week, the content of nitrites in food diets of children from orphanages (calculated per KNO2) exceeded the tolerable daily intakes; averages established for all the research days in different seasons did not exceed these values (Table 5). The highest nitrite intake from diets was in the spring season. The ADI coverage ranged from 8.7 % (autumn) to 98.5 % (spring). Intake of nitrite with some diets, by children from orphanage I, in three research seasons was even up to 200 % of ADI value. An average daily intake ranged between 54 % of the ADI value in winter to 99 % in spring. In this institution are the youngest children, for whom such a high supply of nitrite may be particularly harmful, since these contaminants may inhibit weight gain as well as cause methemoglobinemia. Nitrite (and nitrate too) causes degradation of vitamins A, C, and B groups and tryptophan in the human body. They also cause worse metabolism of proteins and iodine (Majchrzak 1985; Dudka et al. 1997; Myshkin et al. 1997).
In the orphanage II, the amounts of nitrites in daily diets were higher in the spring than in summer (p = 0.039) and autumn (p = 0.045).In orphanage IV, the content of nitrites was significantly higher in the diets from the summer than from autumn (p = 0.019). In addition, in the case of diets from the institution VI, statistically significant differences were found between winter and autumn (p = 0.043), spring and summer (p = 0.005) as well as spring and autumn (p = 0.003). Additionally, diets from the winter and spring season were characterized by higher contents of these compounds. The good way to reduce the consumption of nitrite may be decreased in the diet of cured meat and rennet cheese.
Results obtained in this study are similar to data published by Wawrzyniak et al. (2008b). They reported that daily diets of children from preschools located in Warsaw, Poland, contained 0.52–0.72 mg NaNo2 (34.5 ± 18.6–48.2 % ± 17.6 ADI). In other studies, daily intakes of nitrites with diets of students and in households from Poland did not exceed the ADI value (Leszczyńska 2000; Wawrzyniak et al. 2008a; Wawrzyniak et al. 2010). Similar trends were also observed by other researchers in Nederland (Vaessen & Schothorst 1999), Japan (Ishiwata et al. 2000) as well as in Estonia (Tamme et al. 2006).
Conclusion
Results obtained in this study demonstrate that, in any case, the intake of lead and nitrites by children and adolescents from orphanages did not exceed the permitted values. Intake of cadmium exceeded the TWI value in every season in all orphanages. Intake of nitrates exceeded the ADI value in winter season almost in all orphanages. On the other hand, the youngest children due to their low body mass and consequently, low values of the acceptable daily intake (ADI) as well as the provisional tolerable weekly intake (TWI), were particularly exposed to the excessive intakes of cadmium (667 % of TWI per year) and nitrates (156 % of ADI per year). The consumption of vegetables as well as cereal-based dishes, including breakfast flakes and groats, must be lowered to reduce intake of cadmium and lead with daily diets. In winter season, the major source of nitrates and nitrites were root vegetables (carrot, redroots) as well as cruciferous vegetables, especially cabbage, additionally cured meat and rennet cheese in case of nitrite. Consumption of these products should be also reduced. Proper preparation, including washing of vegetables, may reduce the content of lead, cadmium, nitrates, and nitrites. A good way may be purchasing mentioned group of products from the controlled organic farms, as well.
Based on our results, it can be suggested that the intake of lead, cadmium, nitrates, and nitrites with daily diets should be regularly monitored, especially in the case of children.
Abbreviations
- ADI:
-
Acceptable daily intake
- PTWI:
-
Provisional tolerable weekly intake
- TWI:
-
Tolerable weekly intake
- BMDL:
-
Brenchmark dose lower confidence limits
- EFSA:
-
European Food Safety Authority
- JECFA:
-
The Joint FAO/WHO Expert Committee on Food Additives
References
Bawa S, Rutkowska A, Starbała A (2008) Assessment of the intake of nitrates and nitrites in the selected population groups. Bromat Chem Toksykol 3:519–524 in Polish
Betts KS (2012) CDC updates guidelines for children’s lead exposure. Environ Health Perspect 120:a268. doi:10.1289/ehp.120-a268
Chowdbury BA, Chandra RK (1987) Biological and health implications of toxic heavy metal and essential trace element interactions. Prog Food Nutr Sc 11:55–59
Dudka J, Szczepaniak S, Mazur M (1997) Assessment of chronic effect of lead and sodium nitrite on selected biochemial parameters in the blond of rat’. Part I. The effect on hemoglobin, sulphur groups and tryptophan. Roczn PZH 48:23–29 in Polish
EN-14084:2003 Food products. Determination of trace elements. Determination of Pb, Cd, Zn, Cu, and Fe content by the use of atomic absorption spectroscopy (AAS) after microwave mineralization (in Polish)
Engström AK, Vahter M, Julin B, Wolk A, Åkesson A (2012) Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 6(50):1372–1378
Enli Y, Turgut S, Oztekin S, Demir S, Enli H, Turgut G (2010) Cadmium intoxication of pregnant rats and fetuses: interactions of copper supplementation. Arch Med Res 41(1):7–13
European Food Safety Authority (2009) Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on cadmium in food. The EFSA Journal 980:1–139
European Food Safety Authority (2010) EFSA panel on contaminants in the food chain (CONTAM); scientific opinion on lead in food. EFSA J 8(4):1570
European Food Safety Authority (2012) Cadmium dietary exposure in the European population. Scientific report of EFSA. EFSA J 10(1):2551
Faulk C, Barks A, Sánchez BN, Anderson OS, Peterson KE, Dolinoy DC (2014) Perinatal lead (Pb) exposure results in sex-specific effect on food intake, fat, weight, and insulin response across the murine life course. PLoS One 9(8):e104273. doi:10.1371/journal.pone.0104273
Gawęcki J (2010) Human nutrition basic of science nutrition. Warszawa PWN: 525 (In Polish)
Gilbert SG, Weiss B (2006) A rationale for lowering the blood lead action level from 10 to 2 μg/dL. Neurotoxicology 27(5):693–701
Goyer RA (1997) Toxic and essential metal interactions. Ann Rev Nutr 17:37–50
Hord NG, Tang Y, Bryan NS (2009) Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 90:1–10
Ishiwata H, Sugita T, Kawasaki Y, Takeda Y, Yamada T, Nishijima M, Fukasawa Y (2000) Estimation of inorganic food additive (nitrite, nitrate, and sulfur dioxide) concentration in foods and their daily intake based on official inspection results in Japan in fiscal year 1996. J Food Hyg Soc Jpn 41(1):79–85
Järup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208. doi:10.1016/j.taap.2009.04.020
Kapil V, Webb AJ, Ahluwalia A (2010) Inorganic nitrate and the cardiovascular system. Heart 96:1703–1709
Kapusta-Duch J, Leszczyńska T, Filipiak-Florkiewicz A (2010) Comparison of nitrates and nitrites contents in the cruciferous vegetables cultivated in the T. Sendzimir Steelworks protection zone, on organic farms and commercially available on local markets. In the monograph: Rembiałkowska E. (red.). The impact of organic production methods on the vegetable product quality. Polish-Norwegian Research Fund, Norway Grants, Warszawa, 131–143
Kapusta-Duch J, Leszczyńska T, Forkiewicz A, Filipiak-Florkiewicz A (2011) Comparison of lead and cadmium contents in cruciferous vegetables grown under diversified ecological conditions: Cracow Region of Poland. Ecol. Food Nutr 50(2):137–154
Kłos A, Długaszek M, Bertrandt J (2008) Cadmium content in selected hospital diets. Bromat Chem Toksykol 2:99–104
Kłos A, Bertrandt J, Stężycka E, Długaszek M (2009) Lead and cadmium content in the daily food rations used in soldiers’ alimentation within the space of years. Bromat Chem Toksykol 3:766–770 in Polish
Konieczka P, Namieśnik J (ed) (2007) Assessment and control of quality results of analytical measurements. Wyd Naukowo-Techniczne, Warszawa (jn Polish)
Kunachowicz H, Czarnowska-Misztal E, Turlejska H (2007) Principles in human nutrition. Wyd. Szkolne i Pedagogiczne Sp. A Warszawa, p. 180 (in Polish)
Leblanc JC, Lawal JA, Ogunkeye AA, Orejimi BM (2000) Estimation of dietary intake of pesticide residues, lead, cadmium, arsenic and radionuclides in France. Food Addit Contam 17(11):925–932
Leszczyńska T (2000) An assessment of intake of nitrates and nitrites with daily food rations by selected rural inhabitants. Pol J Food Nutr Sci 9/50(1):67–70
Leszczyńska T, Gambuś F (2001) Assessment of lead and cadmium intake with food rations by inhabitants of selected rural households. Pol J Food Nutr Sci 10/51(1):45–48
Leszczyńska T, Filipiak-Florkiewicz A, Cieślik E, Sikora E, Pisulewski PM (2009) Effects of some processing methods on nitrate and nitrite changes in cruciferous vegetables. J Food Comp Anal 22(4):315–321
Liu CM, Ma JQ, Sun Y-Z (2010a) Quercetin protects the rat Sidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environ Toxicol Pharmacol 30:264–271
Liu P, Wang C-N, Song X-Y, Wu Y-N (2010b) Dietary intake of lead and cadmium by children and adults—result calculated from dietary recall and available lead/cadmium level in food in comparison to result from food duplicate diet method. Intl J Hygiene Environ Health Ens 213(6):450–457
Liu CM, Ma JQ, Sun Y-Z (2012) Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Exp Toxicol Pathol 64:575–582
Majchrzak D (1985) The effect of nitrates and nitrites on human and animals organism. Żyw Człow Metab 12:298–304 in Polish
Martí-Cid R, Llobet J, Domingo JL (2008) Dietary intake of arsenic, cadmium, mercury, and lead by the population of Catalonia, Spain. Biol Trace Elem Res 2(125):120–132
Marzec Z, Łukasiewicz M (2010) Cadmium, lead and nickel in food rations of the canteens of welfare institutions. Bromat Chem Toksykol 3:281–286
Marzec Z, Schlegel-Zawadzka M (2004) Exposure to cadmium, lead and mercury in the adult population from Eastern Poland, 1990–2002. Food Addit Contam 10:963–970
Muñoz O, Bastias JM, Araya M, Morales A, Orellana C, Rebolledo R, Velez D (2005) Estimation of the dietary intake of cadmium, lead, mercury, and arsenic by the population of Santiago (Chile) using a total diet study. Food Chem Toxicol 43(11):1647–1655
Myshkin AE, Konyaeva VS, Gumargalieva KZ, Moiseev YV (1997) Oxidation of ascorbic acid in the presence of nitrites. J Agric Food Chem 44(10):2948–2950
Ociepa-Kubicka A, Ociepa E (2012) Toxic effects of heavy metals on plants, animals and humans. Eng Prot Environ 2:169–180 in Polish
Polish Standard 1992 PN-92/A-75112. Polish Committee for Standardization. Food products—determination of nitrites and nitrates (in Polish)
Pysz K, Leszczyńska T, Kopeć A (2016) Intake of vitamin C, β-carotene and polyphenolic compounds by children and adolescents from orphanages. J Am Coll Nutr 35(1):75–85. doi:10.1080/07315724.2014.987405
Rubio C, Hardisson A, Reguera JI, Revert C, Lafuente MA, Gonzalez-Iglesias T (2006) Cadmium dietary intake in the Canary Islands, Spain. Environ Research 100:123–129
Sand S, Becker W (2012) Assessment of dietary cadmium exposure in Sweden and population health concern including scenario analysis. Food Chem Toxicol 50:536–544
Sindler AL, Devan AE, Fleenor BS, Seals DR (2014) Inorganic nitrite supplementation for healthy arterial aging. J Appl Physiol 116(5):463–477
Tamme T, Reinik M, Roasto M, Juhkam K, Tenno T, Kiis A (2006) Nitrates and nitrites in vegetables and vegetable-based products and their intakes by the Estonian population. Food Addit Contam 23(4):355–361
Thomson FE, Byers T (1994) Dietary assessment resource manual. J Nutr 124:2245S–2317S
Vaessen HAMG, Schothorst RC (1999) The oral nitrate and nitrite intake in the Netherlands: evaluation of the results obtained by HPLC analysis of duplicate 24-hour diet samples collected in 1994. Food Addit Contam 16(5):181–188
Van Cauwenbergh R, Bosscher D, Robberecht H, Deelstra H (2000) Daily dietary cadmium intake in Belgium using duplicate portion sampling. Eur Food Res Technol 212:13–16
Volkmer BG, Ernst B, Simon J, Kuefer R, Bartsch G Jr, Bach D, Gschwend JE (2005) Influence of nitrate levels in drinking water on urological malignancies: a community-based cohort study. BJU Int 95(7):972–976
Watanabe T, Nakatsuka H, Shimbo S, Yaginuma-Sakurai K, Ikeda M (2013) High cadmium and low lead exposure of children in Japan. Int Arch Occup Environ Health 86:865–873
Wawrzyniak A, Szczepańska M, Hamułka J, Szymczyk K (2008a) Assessment of nitrates and nitrites contents in preschool food rations. Roczniki PZH 3:273–281 in Polish
Wawrzyniak A, Hamułka J, Pająk M (2008b) Evaluation of nitrates and nitrites food intake in Polish households in years 1996-2005. Roczniki PZH 1:9–18 in Polish
Wawrzyniak A, Hamułka J, Pankowska I (2010) Evaluation of nitrites and nitrates food intake in the students’ group. Ann Nat Inst Hyg 4:367–372 in Polish
WHO (2010b) Preventing disease through heath environments. Exposure to lead a major public health concern. World Heath Organization, Geneva http://www.who.int/ipcs/features/lead.pdf
Wilk A, Kalisińska E, Różański J, Łanocha N (2013) Cadmium, lead and mercury in human kidneys. J Environ Med 16(1):75–81
World Health Organization (2006) International Program on Chemical Safety, Environmental Health Criteria 5: nitrates, nitrites, and N-nitroso compounds. http://www.inchem.org/documents/pims/chemical/pimg016.htm version 29.01.2015
World Health Organization (2010a) Preventing disease through healthy environments exposure to cadmium: a major public health concern. Geneva, Swidzerland
Ysart G, Miller P, Croasdale M, Crews H, Robb P, Baxter M, L’Agry C, Harrison N (2000) UK total diet study—dietary exposures to aluminum, arsenic, cadmium, chromium, cooper, lead, mercury, nickel, selenium, tin and zinc. Food Addit Contam 17(9):775–786
Acknowledgments
The study was financed by Ministry of Science and Higher Education of Republic of Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Responsible editor: Vítor Pais Vilar
Rights and permissions
About this article
Cite this article
Pysz, K., Leszczyńska, T., Bieżanowska-Kopeć, R. et al. Chemical assessment of lead, cadmium, nitrate, and nitrite intakes with daily diets of children and adolescents from orphanages in Krakow, Poland. Environ Sci Pollut Res 23, 25200–25209 (2016). https://doi.org/10.1007/s11356-016-7550-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7550-z