Abstract
Indoor dust samples were collected from 40 homes in Kocaeli, Turkey and were analyzed simultaneously for 14 polybrominated diphenyl ethers (PBDEs) and 16 poly aromatic hydrocarbons (PAHs) isomers. The total concentrations of PBDEs (Σ14PBDEs) ranged from 29.32 to 4790 ng g−1, with a median of 316.1 ng g−1, while the total indoor dust concentrations of 16 PAHs (Σ16PAHs) extending over three to four orders of magnitude ranged from 85.91 to 40,359 ng g−1 with a median value of 2489 ng g−1. Although deca-PBDE products (BDE-209) were the principal source of PBDEs contamination in the homes (median, 138.3 ng g−1), the correlation in the homes was indicative of similar sources for both the commercial penta and deca-PBDE formulas. The PAHs diagnostic ratios indicated that the main sources of PAHs measured in the indoor samples could be coal/biomass combustion, smoking, and cooking emissions. For children and adults, the contributions to ∑14PBDEs exposure were approximately 93 and 25 % for the ingestion of indoor dust, and 7 and 75 % for dermal contact. Exposure to ∑16PAHs through dermal contact was the dominant route for both children (90.6 %) and adults (99.7 %). For both groups, exposure by way of inhalation of indoor dust contaminated with PBDEs and PAHs was negligible. The hazard index (HI) values for BDE-47, BDE-99, BDE-153, and BDE-209 were lower than the safe limit of 1, and this result suggested that none of the population groups would be likely to experience potential health risk due to exposure to PBDEs from indoor dust in the study area. Considering only ingestion + dermal contact, the carcinogenic risk levels of both B2 PAHs and BDE-209 for adults were 6.2 × 10−5 in the US EPA safe limit range while those for children were 5.6 × 10−4 and slightly higher than the US EPA safe limit range (1 × 10−6 and 1 × 10−4). Certain precautions should be considered for children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid development of new building and insulation materials, furnishings, and consumer products has resulted in a corresponding increase in both the quantity and number of new chemicals in the built environment (Rudel and Perovich 2009; Weschler 2009). In this regard, the numbers of chemicals and exposure to these chemicals have been increasing dramatically every passing day. While the exposure levels to these chemicals are largely undocumented, they can be expected to increase as a wider variety of chemicals come into use, people spend more time indoors, and finally, air exchange rates decrease to improve energy efficiency (Zhang et al. 2011; Rudel and Perovich 2009; Weschler 2009).
Persistent organic pollutants (POPs) have seen wide use in commercial and industrial applications around the world, and as a result of their high chemical stability, resistance to degradation, persistence, lipophilic properties, and the tendency to bioaccumulate and be biomagnified in the food chain, they can be counted among the most ubiquitous pollutants in the environment (Vafeiadi et al. 2014). Among them, polybrominated diphenyl ethers (PBDEs) are a class of persistent organic compounds that have been used as flame retardants in many consumer products, including textiles, polyurethane foam in furniture, mattresses, carpet pads and automobile seats, as well as in plastic components of electrical household appliances, since the mid-1970s (Hassan and Shoeib 2015; Lim et al. 2014). PBDEs can act as endocrine disrupters, affecting the thyroid hormone-mediated pathways (Legler and Brouwer 2003), and evidence of cancer in animals has led BDE-209 to be classified as a possible human carcinogen by the US EPA (US EPA IRIS 2016a). Due to the growing environmental and human health concerns, Penta-BDE and Octa-BDE were banned in the European Union (summer 2004) and have been voluntarily phased out in the USA since 2004 (Betts 2008; La Guardia et al. 2009; Kemmlein et al. 2009). The phase out of two formulations led to an increase in the production of Deca-BDE mixture in the EU (Söderström et al. 2004). At last, all the technical mixtures of PBDEs were banned in the EU (Directive 76/769/CEE; Directive Penta- and Octa-PBDE formulations (2003/11/EC); Court Proceeding 2008/c116/02; Kemmlein et al. 2009). In 2009, PBDEs were added to the list of POPs under the Stockholm convention (UNEP/POPS/COP.4/17 2009). Turkey signed the Stockholm Convention on 23 May 2001 and it was adopted by the Council of Ministers (Law No. 5871) on 14 April 2009 (Oficial Gazette 30-07-2009, No. 27304). The Convention entered into force for Turkey officially on 12 January 2010 (NAP 2014). Despite the banning or restrictions imposed on the use of all the technical mixtures of PBDEs today, different groups of chemical classes of PBDEs are in common use or still exist in building materials, furnishings, and consumer products. Hence, the general population is still exposed to PBDEs due to the widely used and unregulated deca-BDE (La Guardia et al. 2009).
Poly aromatic hydrocarbons (PAHs), produced as a result of the incomplete combustion of organic materials for heating and cooking purposes, incense burning, and tobacco smoke in indoor environments, also have long histories of contribution to human exposure (Weschler and Nazaroff 2008; Maertens et al. 2008). Exposure to PAHs has been associated with several adverse human health effects, such as hematological changes/anemia, genotoxicity, hepatotoxicity, lung cancer, and neurological dysfunction (Kamal et al. 2015). A total of seven PAHs, including benzo(a)anthracene, benzo(a) pyrene, benzo(b)fluoranthene, benzo(k)fluoranthene, chrysene, dibenz(a,h)anthracene, and indeno(1, 2, 3-c, d)pyrene, have been ranked by the US EPA’s Integrated Risk System as probable human carcinogens (B2; US EPA 2016b).
The large contributions of PBDEs in both new and older residential use products and PAHs emitted during combustion suggest that the indoor environment may be a significant source of human exposure to these pollutants (Zhang et al. 2011). Consumption of contaminated foods, inhalation of contaminated air, and ingestion of dust, in particular house dust, are the main routes of human exposure to PBDEs and PAHs (Yu et al. 2012; Harrad et al. 2016). Given the large percentage of time people spend inside (approximately 90 % of the total, most of this being at home), there is potential for significant human exposure to the PBDEs and PAHs found in indoor air and dust, both through direct inhalation and ingestion (Wilford et al. 2005). Much attention has been focused recently on the significance of indoor dust (settled dust) as a pathway to human exposure to PBDEs and PAHs due to the large specific surface areas that act as a reservoir for pollutants released into the home and as a repository of contaminates over a long period of exposure (Cao et al. 2014; Shin et al. 2014; Butte and Heinzow 2002). Human exposure to these chemicals is a major problem in modern society, and babies and younger children may be especially at risk due to a combination of physiological and behavioral factors, such as small body mass, less ability to detoxify, rapid growth, development and differentiation of their vital organ systems, engaging in activities close to the floor, and hand-to-mouth behavior (Hwang et al. 2008; Butte and Heinzow 2002; Roberts and Ott 2007).
The extent of exposure from contaminated matrix (such as dust or soil) has been estimated for several studies of PBDEs (Bennett et al. 2015; Abafe and Martincigh 2015; Fromme et al. 2014), PAHs (Man et al. 2013, Kamal et al. 2015), PCBs (DellaValle et al. 2013; Harrad et al. 2009), phthatalate (Bamai et al. 2014; Fromme et al. 2013), phthatalate, and novel BR Fromme et al. 2014; Hassan and Shoeib 2015). These studies generally evaluated exposure to one pollutant group, and therefore, predicted the risk associated with this certain group. Hence, making a risk estimation for one group of POPs may lead to an underestimation of exposure to dust. All persistent organic pollutants adsorb on the same dust matrix and humans are exposed to all these pollutants in the contaminated dust via inhalation, ingestion, and dermal pathway. To make a sound evaluation of the risks associated with the contaminated matrix of POPs, the measured pollutants in the same dust matrix should cover all POP groups to the greatest possible extent. To the best of the author’s knowledge, there are only limited studies assessing the health risks associated with exposure to more than one POPs group (Harrad et al. 2010; Ali et al. 2012), and this can be considered as the first study to make estimations of the health risks associated with simultaneous exposure to PBDEs and PAHs from indoor dust in Turkey.
In summary, the main objectives of this research study are: (1) to determine the concentrations of 14 PBDEs and 16 PAHs on the same dust matrix; (2) to understand the sources of PBDEs and PAHs contributing to the measured POPs concentrations in the indoor environment from the questionnaire results; (3) to estimate the exposure and cancer risks associated with the ingestion, inhalation, and dermal contact of PBDEs and PAHs in indoor dust using site-specific parameters obtained from time-activity data for the cohort population; and (4) to compare the risks due to exposure to PBDEs and PAHs in the indoor dust.
Materials and methods
Study area and sampling
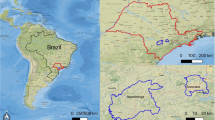
The indoor and outdoor dust sampling was performed in 40 homes within the Kocaeli region (29° 22′–30° 21′ E, 40° 31′–41° 13′ N). Kocaeli is host to one of the most important industrial zones in Turkey, which includes petrochemical plants, hazardous, and clinical waste incineration and storage facilities, as well as textile (185 industrial enterprises), machinery (112), mine (94), metal (64), food (67), automotive (53), paper (42), chemical (44), wood (38), petrol (19), leather (4), coal (53), and other (457) industrial plants. In addition, the proximity of the Government Highway-100 (D-100; 74,312 vehicles/day), Anatolia Highway of Transit European Motorway (TEM; 61,854 vehicles/day−1), and D-130 (49,451 vehicles/day−1) state highways ensures a steady and extensive flow of traffic. The general layout of the study area and locations of the houses are shown in Fig. 1.
The home to be sampled was first swept by the owner and then dust was left to deposit for subsequent 7 days. After 1 week, composite dust samples were collected from bedrooms, kitchens, hall, and living rooms of the floors (excluding wet surfaces) of 40 homes of volunteers using a Nilfisk Elit Plus 1600W vacuum cleaner (specified to capture 99.5 % of all particles 0.3 μm and larger). The volunteers were recruited through a personal communication method, and the location of the homes are also illustrated in Fig. 1. The mass of dust that deposited during 1 week in the homes was between 0.1 and 6 g. If the mass of the dust were lighter than 2 g, it was continued to revisit until the total masses of the collected dust reached 2 g. The natural ventilation rate by opening windows affects indoor air concentration from outdoor to a great extent (Wallace 1987; Woodruff et al. 2000). The shortest ventilation rates might be expected in days of winter. Sampling was conducted in winter from December 2015 to January 2016 to eliminate the effects of outdoor pollution on indoor pollution as much as possible. By this way, the collected indoor dust samples could represent indoor air quality. To avoid cross contamination, the vacuum cleaner equipment was cleaned carefully with solvent and a new bag was inserted for each new sampling. After the sampling, the bags were placed into individual amber glass bottles, pre-washed with isooctane, and stored in a freezer at −20 °C until sieving. Field blanks consisting of anhydrous sodium sulfate, “sampled” using the same equipment and procedures described above for the real samples, were also analyzed to determine if any contamination had occurred during the sample handling and preparation (Abdallah et al. 2013).
Detailed questionnaires were completed by all of the volunteer home owners to garner any information that may impact the concentrations of PBDEs and PAHs in the dust samples, such as home characteristics (e.g., year of construction, type of flooring, renovations, new furniture, ventilation and heating system, etc.) and any activity that may affect chemical loading (e.g., hobbies, cooking styles, and smoking/non-smoking). The house characteristics are listed in Table S3 in the supporting information. Besides the time-activity data corresponding to different inhalation rates, body weights, exposure frequency, and exposure time were obtained through the questionnaires, which were applied on the population living in the area. Details on the risk assessment and the questionnaires are provided in SI and in Table S4. All of these factors were used to study the source-receptor relationship of PBDEs and PAHs in indoor dust and to estimate the risk to health posed by exposure to these pollutants within indoor dust.
Chemicals
The PAH stock solution (100 μg ml−1 naphthalene (Nap), acenaphthylene (Acy), acenapthene (Ace), flourene (Flue), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flt), pyrene (Pyr), benzo(a)anthracene (BaA), chrysene (Chr), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenzo(a,h)anthracene (DahA), benzo(g,h,i)perylene (BgP), and indeno[1,2,3-cd]-pyrene (Ind) in acetone) and recovery standard (500 μg ml−1 acenaphthene-d10, chrysene-d12, perylene-d12, and phenanthrene-d10) was obtained from Absolute Standards, Inc. (Hamden, CT, USA). PBDEs calibration standards each 5 μg ml−1 of BDE-17, BDE-28, BDE-47, BDE-66, BDE-71, BDE-85, BDE-99, BDE- 100, BDE-138, BDE-153, BDE-154, BDE-183, BDE-190, and 25 μg ml−1 BDE-209, and isotopic labeled recover standards each 50 μg ml−1 of 13C12-BDE-47 and 13C12-BDE-203 were purchased from Accustandard Inc. (New Haven, CT, USA). Silica gel 60 (0.063–0.200 mm) and Alumina 90 used for column chromatography were obtained from Merck Co., Germany. All the chemicals used for extraction, column clean-up, and volume reduction processes, such as acetone(Ace), n-hexane(Hex), dichloromethane(DCM), and petroleum ether (PE), were GC analysis grade (Merck Co., Germany).
Sample preparation and analysis
Non-dust particles, hair, and debris were hand-picked from all samples. Driver et al. (1989) indicated that particles exceeding 150 μm in size do not adhere easily or efficiently to hands or skin and are, thus, less relevant in the context of exposure via ingestion or dermal pathways (Ma and Harrad 2015; Driver et al. 1989). Likewise Besis and Samara (2012) indicated that the collection of fine particles is also increased by a weaker circulation of indoor air, and it is more likely for PBDEs to be adsorbed onto the surface of fine particles rather than coarse particles (Besis and Samara 2012; Wei et al. 2009; Zhang et al. 2009; Mandalakis et al. 2009). Therefore, the samples were homogenized by sieving them through a 100-μm stainless steel sieve and analyzed following the methods suggested by Cetin et al. (2016) and Aydin et al. (2014). Briefly, approximately 1-g dust samples were extracted ultrasonically for 60 min with a 10-ml mixture of 1:1 acetone to hexane ratio. The mixing and extraction were repeated a second time with the addition of 10 ml of fresh solvent. Prior to extraction, all the samples were spiked with PAH (acetanapthalene-d10, chyresene-d10, perylene-d10, phenantherene-d10) and PBDEs (isotopic labeled internal standards 13C12-BDE-47 and 13C12-BDE-203 surrogate standards). The extract volumes were reduced and transferred into hexane (2 ml) using a rotary evaporator and a high purity N2 stream. A 30-cm × 1-cm glass column was packed with 0.1 g of glass wool and 3 g of silicic acid (deactivated with 5 % deionized water) and 2 g of alumina (deactivated with 6 % deionized water) and topped with 1 g of anhydrous sodium sulfate. The column was pre-washed with 20 ml of dichloromethane (DCM) followed by 20 ml of petroleum ether (PE). The sample in 2 ml hexane was then added to the column and PBDEs were eluted with 60 ml of PE and kept as elute 1. The column was further eluted with 40 ml of DCM and kept as elute 2. Firstly, the volumes of the elute 1 and 2 were separately reduced to approximately 2 ml in a rotary evaporator. The final extracts were solvent-exchanged into hexane by adding 2~4 ml of hexane and evaporating the extract to 2 ml under a stream of high purity nitrogen. Addition of the fresh solvent and the evaporating processes were repeated a third time. Finally, the elutes were purged till the final volume was reached (200 μl) and transferred into insert vials. Half volume of elute 1 and half volume of elute 2 were mixed (200 μl) and analyzed for PAHs since some lighter PAHs are eluted partly with fraction 1 (Aydin et al. 2014). The rest of elute 1 (100 μl) were analyzed for PBDEs. The schematic diagram of the extraction and column clean-up procedure was depicted in Fig. S1.
Elute 1 was analyzed for 14 PBDEs and after which equal volumes of elute 1 and 2 were combined and analyzed for 16 PAHs. For the analysis of PBDEs and PAHs, an Agilent 7890N GC coupled with an Agilent 5977A mass spectrometer system EI mode equipped with a DB-5HT capillary column (15 m × 0.25 mm i.d., 0.10 μm film thickness, J&W Scientific) was employed. PBDEs were detected in the system with the oven temperature program: 100 °C for 1 min, 8 °C min−1 to 320 °C, and held for 6 min. The temperatures were 280, 230, 150, and 320 °C for the injector, quadrupole, ion source, and interface, respectively. For the analyzed PAH, the initial oven temperature was held at 50 °C for 1 min, raised to 200 °C at 25 °C min−1, and raised to 300 °C at 8 °C min−1, and held for 8 min. The injector, ion source, quadrupole, and interface temperatures were 295, 290, 180, and 320 °C, respectively. (0.94 psi, 50 cm s−1 flow rate, velocity).
Quality assurance and quality control
For the method blank, dust samples were replaced with anhydrous sodium sulfate and passed through all the analytical procedures carried out for real samples. The masses of PBDE and PAH isomers detected in the blanks did not exceed the 4 % of average masses collected on the real dust samples. Field blank samples were collected (n = 6) by spreading anhydrous sodium sulfate on a pre-cleaned floor and analyzed following the same sampling protocol as for real samples. The traces of Flt + Pyr and BaA were found in the field blanks and blank correction was performed by subtracting the mean blank amount measured in the blank samples from the amount measured in the sample. Average recoveries for the surrogate standards were 76 ± 14 % (acetanapthalene-d10), 77 ± 12 % (perylene-d10) and 79 ± 17 % (chrysene-d10), 76 ± 19 % (phenantherene-d10), 63.2 ± 11 % (BDE-47), and 61.7 ± 6 % BDE-203. Recoveries were also performed with 14 PBDEs and 16 PAHs congeners mixed in spiked sodium sulfate and ranged from 59.7 to 78.2 % and from 72.3 to 94.5 %, respectively. The limit of detection (LOD) were determined from the lowest concentration analytical standard that could be integrated and corresponds to a chromatographic peak with a signal/noise ratio of 3/1 (Kefeni et al. 2014; Zhang et al. 2011). For 1-μl injection, the quantifiable amounts were 0.02–0.59 and 0.06–0.16 ng g−1 for PAHs and PBDEs except for BDE-209, respectively. LOD values for BDE-209 was 0.59 ng g−1. Quality control parameters of the 14 PBDEs and 16 PAHs can be found in Table S1 and S2 in the Supporting Information.
All glasswares were cleaned following the United States Environmental Protection Agency methods 1614 (US EPA 2007). Briefly, the glasswares were rinsed with solvent and washed in detergent solution through sonication. After detergent washing, the glasswares were rinsed with methanol and then with hot tap water. The tap water rinse was followed by another methanol rinse, and then acetone. Finally, the glasswares were baked in a kiln furnace at 450 °C. Direct ultraviolet light and plastic ware were avoided throughout the analysis.
Exposure scenarios and risk assessment
Human exposure to dust was estimated with the following assumptions: (1) Humans are exposed to dust through three main pathways: ingestion of dust particles, inhalation of dust particles, and dermal contact with dust particles (Deng et al. 2014; Kurt-Karakus 2012); (2) people are exposed to indoor dust while in their house; (3) some exposure parameters of those in the observed areas are similar to those of the reference populations; (4) the total non-carcinogenic risk may be calculated for each congener (BDE-47, BDE-99, BDE-153, and BDE-209, B2 carcinogenic PAHs) by totaling the individual risks associated with the three means of exposure (Man et al. 2013; Lim et al. 2014); (5) the total carcinogenic risk may be computed for each pollutant (BDE-209, B2 carcinogenic PAHs) by totaling the individual risks calculated for the three means of exposure (Lim et al. 2014; Kang et al. 2011b); and (6) humans are exposed to dust in their homes over their entire lifetime.
The formulas used to calculate the dose received through each of above-listed exposure pathways are given below (US EPA 1989; US EPA 2009; Kurt-Karakus 2012). All of the equations used in this research refer to US EPA methods and other documents (US EPA 2003 and 2006; Liu et al. 2015; Deng et al. 2014; Lim et al. 2014; Kamal et al. 2014; Man et al. 2013; Kang et al. 2011b).
where.
- \( {D}_{ingi},{D}_{inhi},{D}_{dermali} \) :
-
The chemical daily intake for the age-group i via ingestion; inhalation or dermal contact routes (mg kg−1 day−1)
- Cdust :
-
The measured dust concentration (mg kg−1; human exposed these pollutants in their homes)
- \( {R}_{\mathrm{ing}i} \) :
-
Daily ingestion rate for the age-group i (mg dust day−1)
- \( {R}_{\mathrm{inh}i} \) :
-
Daily inhalation rate or the age-group i (m3 day−1)
- Eti :
-
Exposure time per day in homes for the age-group i (hours day−1)
- EF :
-
Exposure frequency (day year−1)
- ED:
-
Exposure duration (years) for the age-group i
- BWi :
-
The mean body weight for the age-group i (kg)
- Tavrg :
-
The averaging lifespan in Turkey for the age-group i (days)
- CF :
-
Unit conversion factor
- \( {A}_{\mathrm{skin}i} \) :
-
The exposure skin area for the age-group i (cm2)
- PEF :
-
the particle emission factor (m3 kg−1)
- SAF:
-
The skin adherence factor (mg cm−2 h−1),
- DAF :
-
The dermal absorption factor (unitless),
The PEF parameter relates the contaminant concentration in soil with the concentration of respirable particles in the air due to fugitive dust emissions from surface contamination sites (USEPA 1996). Since there is no study conducted to determine the PEF parameters of inhalation of dust contaminated with PBDEs, PAHs or other pollutants in indoor environment, the default PEF value of 4.63 × 109 m3/kg was used (USEPA 2001). The values of the exposure parameters for children and adults with reference are provided in Table 1.
A hazard quotient (HQ) for no-cancer risk was calculated for the PBDEs and PAHs having RfD values (Eq. 4), whereas the additional lifetime cancer risk due to exposure to the pollutant via inhalation, ingestion, and dermal contact pathways of the dust was estimated through Eqs. 5, 6, and 7 (US EPA 2009). Based on the information from the US EPA IRIS, BDE-209 and BaP are the only PBDEs and PAHs associated with cancer risk in humans with neurobehavioral effects, respectively (Chou et al. 2016; Kang et al. 2011a). Generally, in health risk studies, BaP is used as a marker substance, having been considered in most relevant studies as responsible for 50 % of the carcinogenic potential of PAHs (according to the most recent data (Hanedar et al. 2014; Petry et al. 1996). Seven carcinogenic PAHs concentrations and their respective toxic equivalency factors (TEFs) including BaP (1), BaA (0.1), BbF (0.1), BkF (0.01), Chr (0.001), DahA (1), and Ind (0.1) were used in the cancer risk assessment applying BaP toxicity equivalence (Man et al. 2013; US EPA 1993).
where RfDi is the reference dose via ingestion, dermal contact, and inhalation pathways, D is the average daily exposure dose of individual PBDEs or PAHs in dust through ingestion, dermal contact, and inhalation pathways (mg/kg/day); SF is the slope factor (mg/kg/day)−1 via ingestion, dermal contact, and inhalation pathways; and GIABS is the gastrointestinal absorption factor. In the absence of data on the percentage of PBDEs and PAHs adsorbed to dust and, in turn, available for absorption, as well as gastrointestinal absorption data, we assumed the total absorption of contaminants for all congeners, and the GIABS was taken as 1 (De Wit et al. 2012; Kamal et al. 2014; Hassan et al. 2015). HQi is the hazard quotient used to estimate the non-carcinogenic effects of the pollutants through ingestion, dermal contact, and inhalation pathways, while the hazard index (HI) is equal to multiple routes (US EPA 2009). The reference dose (RfD) and slope factor (SF) used in the health risk estimations are provided in Table S6 in the supporting information.
Result and discussion
Levels and sources estimation of PBDEs and PAHs in indoor dust
All of the dust samples collected from the homes (n = 40) contained PBDEs, except for BDE-17 (88 % detected) and BDE-85 (93 % detected). The mean and median concentrations (ng g−1) of 14 measured congeners with minimum and maximum values are summarized in Table 2. Generally, PBDEs were detected with relatively high variability in all the samples.
The total concentrations of PBDEs (Σ14PBDEs) ranged from 29.32 to 4790 ng g−1, with a median of 316.1 ng g−1. This relatively high difference between minimum and maximum values is an indication of the degree of variability in the presence of physical condition and the availability of different numbers of flame retardant furniture items or electronic devices in the homes. This variation is more pronounced for BDE-85 and BDE-99, the concentration of which ranged from <LOD to 419.8 and from 0.75 to 254.4 ng gr−1, respectively. The difference between the minimum and maximum values of these two congeners represents around three orders of magnitude difference. BDE-209 (median, 138.32 ng g−1) was the most dominate congener, followed by BDE-183 (median, 20.52 ng g−1), BDE-71 (median, 15.84 ng g−1) and BDE-154 (median, 15.21 ng g−1), while BDE-28 (median, 4.01 ng g−1) was the least abundant congener for all the samples due to its relatively high volatility (Li et al. 2015). The four most dominate congeners varied in quantities, from 40 to 70 % of all congeners.
Overall, 16 PAHs were detected in all of the dust samples, except for acenapthene and anthracene, with a detection rate of 92.5 and 95 %, respectively. The concentration of PAHs is listed in Table 3. The total indoor dust concentrations of 16 PAHs (Σ16PAHs; in ng g−1) extend over three to four orders of magnitude, ranging from 85.91 to 40,359 with a median value of 2489 ng g−1. The concentrations of Phe, Flt + Pyr, Chry, Bkf, Bbf, and Naph were comparatively higher among the 16 individual PAHs at all the locations, accounting for 15.7, 10.4, 9.8, 8.7, 7.5, and 7.3 %, respectively. The total concentrations of B2 group seven PAHs were comparable to those of the other nine PAHs, accounting for 49.6 and 50.4 % of the total PAHs. The concentration of BaP, the most potent carcinogenic, ranged between 5.72 and 3940 ng g−1 with a median of 156.1 ng g−1.
It has been observed that PAH concentrations in indoor environment are related to PAHs sources emitted from human activities such as cigarette smoking and cooking (Ren et al. 2006; Zhu and Wang 2003; Chuang et al. 1999). Qi et al. (2014) also observed a significant positive correlation between PAHs concentrations in indoor dust and based on location (latitude and longitude). Cao et al. (2012) indicated that the PBDEs results obtained by applying different analytical procedure and different dust fraction in the literature effected on the results varied up to tenfold. Based on the findings in literature, the dust concentrations of PBDEs and PAHs found in any study depends very strongly on the dust particle size, the sampling season (winter or summer) and methodology, the sampling years, the distance between the sampling location and pollutant sources (such as roads or point emissions from industries), the level of social-economic development, ventilation rate, and human activity (Abafe and Martincigh 2015; Qi et al. 2014; Mannino and Orecchio 2008; Allen et al. 2008; Cao et al. 2012). Nevertheless, to roughly see the pollution level of the indoor dust contaminated with PBDE and PAH in Turkey compared with that in other countries, the reported data in literature for different parts of the world is discussed in Supporting Information and given in Tables S7 and S8, together with the data generated in this study.
Congener profiles
PBDEs
The composition patterns of PBDE congeners with the same numbers of bromine atoms can provide an indication of potential sources of PBDEs. BDE-209 was the predominant congener, accounting between 13 and 83 % of the total 14 PBDEs (median, 138.32 ng g−1). The dominance of BDE-209 present in deca-BDE products is not surprising, as most other studies have found this to be the most prolific congener (Abafe et al. 2015; Vorkamp et al. 2011), which can be attributed to two possible reasons. Firstly, BDE-209 is extremely hydrophobic (log KOW ≈ 10) and has been widely expected to possess low bioavailability and a strong tendency to bind to soil (Wu et al. 2015). Secondly, there is widespread usage of commercial deca-BDE products, such as Saytex 102E and Bromkal 82-0DE (Yu et al. 2012, Kang et al. 2011b). According to the Bromine Science Environmental Forum (BSEF), the deca-BDE mixture is the most widely used of these, accounting for approximately 83 % of the total PBDEs production worldwide (Besis 2012). This value falls within the ranges measured in Toronto, Canada by Harrad et al. (2008) and in Hong Kong, China by Kang et al. (2011b), although these levels are not as high as those observed in London, UK (Sjödin et al. 2008) and the USA (Johnson-Restrepo and Kannan 2009), which can be attributed to the greater use of these chemicals as a flame retardant in these countries. It has been reported that 95 % of all upholstery materials in the UK are flame retardant to comply with the fire safety regulations, and that approximately 1000–1200 tons/year of BDE-209 were used in textile applications in the country (European Chemicals Bureau 2002; Sjödin et al. 2008).
The second most predominant congener was BDE-183 (a hepta-BDE congener), one of the primary congeners found in commercial octa-BDE products, which contains about 80 % of the total octa-BDE (La Guardia et al. 2006). BDE-183 accounted for 2 to 16 % of all Σ14PBDEs in house dust samples. This congener is known to be used as a flame retardant in plastic computer monitors and television housings (Abafe and Martincigh 2015; Labunska et al. 2013) and in textiles (coverings and furniture; Krol et al. 2012). In this regard, BDE-183 is correlated significantly (r = 0.54, r = 0.73; p < 0.5) with the number of electronics in the home.
The other dominant congener was BDE-154 (present in penta-BDE products), which accounted for between 2 and 10 % of all congeners. The high level of BDE-154 observed may be linked with either the debromination of BDE-209 (Yang et al. 2013) or emissions in the home from insulation materials (Wu et al. 2015). The second of these is supported by the positive correlation of BDE-154 with newly (<2 years old) insulated homes (r = 0.69 p < 0.05). The existing and new buildings should be insulated according to the environmental conditions and in line with the requirements of the regulation in Turkey (MEU 2008), according to which most of the buildings sampled in the study have been insulated in the last 6 years.
The Pearson correlations between PBDEs congeners were calculated to identify any species that may originate or not from similar sources (Hassan et al., 2015). Some of the penta-PBDE congeners (BDE-47, −85, −99, −100 and −138) were significantly correlated with each other (r = 0.76, p < 0.05), and a closer examination of the data set indicated that the highest concentration measured for each of the BDE-47, BDE-85, BDE-99, BDE-100, and BDE-138 congeners originated from the same homes, all of which were installed with new electronic equipment. The abundance of these congeners demonstrates the source contribution of the penta-BDE commercial formulation (Law et al. 2003). The ratios of BDE-47/BDE-99 in the home dust samples were also investigated and compared to those ratios in the commercial penta-BDE. The ratio of BDE-47/BDE-99 in the commercial penta-BDE mixtures indicated that PBDE emissions from materials were treated with either DE-71 or Bromkal 70-5DE (Besis et al. 2014). The ratios for DE-71 and Bromkal 70-5DE was 0.79 and 0.96, respectively (La Guardia et al. 2006). Eight samples of the study had BDE-47 to BDE-99 ratios ranging from 0.57 to 0.88 with an average of 0.72. However, most of the dust samples (n = 30) had a ratio of BDE-47/BDE-99 ranging from 0.92 to 1.28 with an average value of 0.97, which may be due to the presence of more materials embedded with Bromkal 70-5DE compared to DE-71 in these sampling houses.
However, the penta-BDE congeners were shown to be uncorrelated with PBDE-154, suggesting different sources. On the other hand, these penta-BDE formulations, namely the BDE-47, BDE-85, BDE-99, BDE-100, and BDE-138 congeners, were significantly correlated with BDE-183 and 190 concentrations (r = 0.69, p < 0.05), which indicates similar sources. The BDE-209 congeners were statistically significantly correlated with all the PBDEs congeners except for BDE-154, suggesting similar applications of technical mixtures and/or occurrence in dust. While an abundance of BDE-47, BDE-99, and BDE-100 demonstrates the source contribution of penta-BDE commercial formulations, an abundance of BDE-183 was indicative of the presence of Octa-BDE commercial formulation (Law et al. 2003). Although deca-PBDE products (BDE-209) were the principal source of PBDEs contamination in the homes, the correlation in the homes is indicative of a similar source for both the commercial penta- and deca-PBDE formulas.
PAHs
The high molecular weight (HMW; four + five + six-ring PAHs) PAHs ranged from 29 to 85 % of the total PAH concentrations, with an average value of 61.9 %. Although LMW PAHs (2- and 3-ring PAHs) are relatively more volatile, which results in less abundance in settled dust, and are more abundant in the air (Qi et al. 2014), LMWs were found to be the main PAH isomers in nine homes, all of which were smoke-free residences with central natural gas heating systems. LMWs (Ant, Pyr, Ace, Flue, and Nap) all originate from a natural gas (NG) combustion source (Callen et al. 2013; Li et al. 1999; Lee et al. 2004), and these homes could be directly impacted from NG combustion emissions.
PAH diagnostic ratios have been widely used to predict PAH sources in ambient air (Katsoyiannis et al. 2011; Martellini et al. 2012; Katsoyiannis and Breivik 2014) or indoor dust (Kamal et al. 2014; Qi et al. 2014, Ma et al. 2015; Yunker et al. 2002. Although fewer calculations are required in this method, it should be used with caution because it is often difficult to discriminate between emissions from combustion of coal and other biomass fuels or those emissions from vehicles using different fuel types (such as gasoline and diesel) (Ravindra et al. 2006). These ratios show intra-source variability, but inter-source similarity (Hanedar et al. 2014). Even so, for the identification of indoor source in particular, it is important to distinguish between combustion and vehicular emissions. The diagnostic ratios Ant/(Ant + Phe), BaP/BgP, and Ind/(Ind + BgP) are often used as indicators of whether the PAH in a sample originates from pyrogenic or petrogenic sources in dust (Kamal et al. 2014; Qi et al. 2014; Ma et al. 2015; Yunker et al. 2002). The diagnostic ratios and their possible sources are tabulated in Table 4 together with the data generated in this study. Aside from four homes, all 40 homes in the sample have an Ant/(Ant + Phe) ratio of >0.1, reflecting combustion emissions, while most of the measured indoor dust had a Ind/(Ind + BgP) ratio of between 0.2 and 0.46 in the homes burning biomass/coal, or with a central natural gas heating system. In most of these homes, natural gas was also used for cooking purposes, and these Ind/(Ind + BgP) ratios indicate the potential role of cooking emissions. Mannino and Orecchio (2008) reported kitchen dust as displaying the highest PAHs concentrations in their study of indoor dust from a range of microenvironments (Mannino and Orecchio 2008). The high Ind/(Ind + BgP) ratio values (0.71–0.90) were observed in smoker’s house, while the BaP/BgP ranged from 1.25 to 1.50, in the homes burning coal/biomass in home furnaces, and between 3.1 and 3.87 in smokers’ homes. These results indicate that the main sources of PAHs measured in the indoor samples are coal/biomass combustion, smoking, and cooking emissions.
Table 4: Diagnostic ratios for PAHs in this study, as well as previously reported findings for major emission sources.
No significant correlation (r = 0.21, p > 0.01) was observed between PAH levels and the number of inhabitants in the houses. The highest total PAH concentrations (22,411 ng g−1) were detected in the houses of both smokers and coal burners, while the house with lowest PAH concentration (457 ng g−1) was newly constructed (3 years old). Previous studies have found that PAHs concentrations in the indoor environment are related to PAH source activities such as cigarette smoking (Kang et al. 2011a; Ren et al. 2006).
Estimation of daily intake of PBDEs and PAHs from indoor dust and risk assessment
Exposure
The concentration data of the measured PAHs and PBDEs reported in Tables 2 and 3 were used for internal environments to estimate the contributions of inhalation, ingestion and dermal contact with dust to the exposure of adults and children to Σ14PBDEs and Σ16PAHs. The children and adults with occupational exposure to PBDEs and PAHs via indoor dust ingestion, inhalation and dermal contact were assessed (see Table 5) to determine non-dietary daily intake, non-cancer risk and cancer risk related to indoor house dust. The average daily intakes of ∑14PBDEs through dust ingestion (2.67 × 10−1 ng kg−1 day−1 for children and 6.64 × 10−2 ng kg−1 day−1 for adults) and dermal absorption (2.04 × 10−2 ng kg−1 day−1 for children and 1.96 × 10−1 ng kg−1 day−1 for adults) were three to four orders of magnitude higher than the exposure from inhalation (3.68 × 10−5 ng kg−1 day−1 for children and 2.99 × 10−5 ng kg−1 day−1 for adults). For children and adults, the contributions to ∑14PBDEs exposure were approximately 93 and 25 % for the ingestion of indoor dust and 7 and 75 % for dermal contact, whereas exposure via inhalation of indoor dust was negligible for both groups. This finding is not surprising when the higher dust ingestion rates of children is considered, as well as the lower body weight (Karakus 2012). Moreover, Stapleton et al. (2012) suggest that PBDE levels in hand wipes are associated with serum PBDEs, and claim that hand-to-mouth activity may be a significant source of exposure to PBDEs among children (Lim et al. 2014). Wang et al. (2014) came to the same conclusion, claiming that dust ingestion (69.3–96.1 %) was the predominant PBDE exposure route for toddlers living in different households in two major urban centers of the Pearl River Delta (PRD). Other recent studies have come to the same conclusion, finding that the ingestion of indoor dust is a significant exposure route of PBDE pollutants, especially among children (Hassan et al. 2015; Lim et al. 2014; Dirtu and Covaci 2010), occurring in their homes (approximately 80 %; Lim et al. 2014). The exposure to dust via dermal contact was the most significant pathway for adults, since it has been estimated that 51 mg of indoor dust is likely to be adsorbed through the hands of adults, while 28 mg of indoor dust is likely to be adsorbed through children’s hands (Kang et al. 2011b; Hawley 1985). With respect to exposure to Σ16PAH, the average daily intake of ∑16PAHs through dermal contact (20.3 ng kg−1 day−1 for children and 187 ng kg−1 day−1 for adults) and ingestion (2.11 ng kg−1 day−1 for children and 4.88 × 10−1 ng kg−1 day−1 for adults) was four to five orders of magnitude higher than exposure from inhalation (2.64 × 10−4 ng kg−1 day−1 for children and 2.19 × 10−4 ng kg−1 day−1 for adults). The average daily intake of ∑16PAHs were comparatively higher than that of ∑14PBDEs, since the PAHs isomer concentrations were measured high when compared to the PBDE isomer levels in the same dust matrix. Exposure to ∑16PAHs through dermal contact was the dominant route for both children (90.6 %) and adults (99.7 %), while exposure via inhalation of indoor dust was negligible for the two groups due to the presence of higher DAF values of PAH isomers than PBDEs isomers (Man et al. 2013; US EPA 2012). Inhalation exposure to dust contaminated with both PBDE and PAH were lower level since inhalable (<10 μm) and respirable (<2.5 μm) particles constitute the greatest risks for airborne particle-associated PAH and PBDE exposure due to their long suspension duration and ability to penetrate deeply into the lungs (USEPA 1999).
Non-carcinogenic risk
The hazard index (HI) is the sum of calculated hazard quotients (HQ). An HQ value greater than 1 indicates that adverse effects may be possible, while an HQ value lower than 1 indicates no significant risk of non-carcinogenic effects (US EPA2011; Deng et al. 2014). Since the reference dose value (Rfd) for the inhalation of PBDEs is only available for 4 PBDEs congeners (BDE-47, BDE-99, BDE-153, and BDE-209) in US EPA, a hazard quotient (HQ) for these chemicals was considered and depicted in Table 6. Although the average daily intakes of BDE-209 through dust ingestion were one to two orders of magnitude higher than the other congeners studied, the HQ for BDE-47 was the highest among the 4 PBDE congeners. The contributions of the four BDEs were ranked in the order BDE-47 (45 %) > BDE-99 (35 %) > BDE-153 (19 %) > BDE-209 (~0 %). The HI for all four non-carcinogenic PBDEs via dust exposure (4.4 × 10−8 for children and 3.3 × 10−8 for adults) did not exceed the critical value of 1.00, indicating that the ingestion of indoor dust containing these four PBDEs was not a lifetime cause of non-cancer neurobehavioral disease.
Carcinogenic risk
As presented in Table 6, for children, the mean carcinogenic levels were 9.2 × 10−15, 6.44 × 10−11, and 4.88 × 10−12 for inhalation, ingestion and dermal exposure of BDE-209, respectively, while for adults, the mean levels were 7.5 × 10−15, 1.6 × 10−11, and 4.9 × 10−11. Similar to the findings of earlier studies, the cancer risk from the ingestion exposure pathways was found to be the highest in children (Shy et al. 2015; Deng et al. 2014), while in the case of adults, the cancer risk levels via ingestion were within the same order of magnitude (10−11 to 10−12) as through dermal contact, indicating that both ingestion and dermal contact contribute to the cancer risk in adults. Considering the three exposure pathways, the mean lifetime cancer risk of BDE-209, which is 6.9 × 10−11 and 6.5 × 10−11 for adults and children, respectively, is significantly lower than the threshold level (10−6), which indicates that the cancer risk from BDE-209 due to exposure to indoor dust falls within the acceptable levels. Li et al. (2015) and Shy et al. (2015) reported similar safe limits related to exposure to BDE-209 from indoor dust in Shanghai (China) and Pingtung and Kaohsiung (Taiwan).
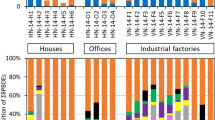
The cancer risk among adults and children exposed to B2 carcinogenic PAHs via ingestion, inhalation and dermal contact were evaluated depending on the toxic Equivalence Factor (TEF) and carcinogenic slope factor of BaP. As depicted in Table 6, the mean B2 carcinogenic levels via inhalation, ingestion and dermal contact pathways were 1.1 × 10−10, 4.4 × 10−7, and 5.5 × 10−4 for adults, while the levels were 1.2 × 10−10, 2.0 × 10−6, and 5.9 × 10−5 for children. For the two groups, dermal exposure to B2 carcinogenic levels posed the highest risk, whereas the risks associated with inhalation pathways were negligible among the three pathways (see Fig. 2). The mean risk values in the present study were consistent with those reported in previous literature (Kang et al. 2011a; Wang et al. 2011; Maertens et al. 2008) at below 10−6, indicating that B2 group PAH exposure via indoor dust is not harmful to human health.
The carcinogenic risk level of B2 PAHs is five to six orders of magnitude higher than that of BDE-209, indicating that the exposure of the B2 group PAH contributed greatly to the risk of cancer for both groups when compared to BDE-209 exposure. Most regulatory programs state that an excess cancer risk of between 10−6 and 10−4 indicates a potential risk, whereas a cancer risk greater than 10−4 suggests a high potential health risk (Kamal et al. 2015; Chen and Liao 2006). In this regard, a cancer risk of lower than 10−4 is generally considered to be significant (Kurt-Karakus 2012; Hu et al. 2011). Considering only ingestion + dermal contact, the carcinogenic risk level of both B2 PAHs and BDE-209 for adults and children (6.2 × 10−5 and 5.6 × 10−4) were within acceptable levels. Despite the assumptions made here (GIABS = 1), which may lead to an overestimation of the exposure, the total carcinogenic risk from exposure to both PBDEs and PAHs were within safe limits. If there are any health effect risks posed by the determined levels of both B2 PAHs and BDE-209 in indoor dust in the present study, then the health risk for adults from both B2 PAHs and BDE-209 resulting from exposure to indoor dust should be higher compared to children. Aside from inhalation, dermal contact with dust would seem to be the main pathway of exposure to PBDEs and PAHs from indoor dust, followed by ingestion.
Limitations
It should be noted that due to the relatively small sample size used in this study and limitations in the sampling procedures, the results cannot be taken as representative of all the indoor environments in these regions, and so further studies are required to validate these results.
In the study, the data has been obtained from indoor dust with a particle size of <100 μm. Cao et al. (2012) stated that the selection of dust fractions is significant and will have a high impact on exposure risk calculation result. In this regard, further studies are recommended to investigate a wider range of pollutants of different particle size fractions in indoor dust to provide a better measure of exposure.
Moreover, the exposure analysis and risk estimation was based on the exposure parameters of the US EPA, although these parameters may differ from one country to another where lifestyles may be different, along with pollution characteristics and exposure mode. Although some exposure parameters (such as body weight, inhalation rate, and exposure duration) were produced from the study, other important parameters (such as skin adherence factor, exposure skin area, dermal absorption, and particulate emission factor) were obtained from the literature. To date, no study has been conducted to determine the parameters of specific pollutant groups in indoor dust, and so more specific and accurate parameters should be used for human exposure models in the future.
Conclusion
The concentration levels of 14 PBDE and 16 PAH congeners in dust samples collected from 40 different homes in Kocaeli were reported. The total concentrations of PBDEs (Σ14PBDEs) ranged from 29.32 to 4790 ng g−1, with a median of 316.1 ng g−1, while the total indoor dust concentrations of 16 PAHs (Σ16PAHs) extended over three to four orders of magnitude, ranging from 85.91 to 40,359 with a median value of 2489 ng g−1. The main sources of PAHs measured in the indoor samples could be attributed to coal/biomass combustion, smoking and cooking emissions, while penta- and deca-PBDE isomers may be emitted from the available materials in the home in which they are embedded.
The hazard index (HI) values for BDE-47, BDE-99, BDE-153, and BDE-209 were lower than the safe limit of 1, suggesting that none of the population groups would be likely to experience a potential health risk due to exposure to PBDEs from indoor dust in the study area. Considering only ingestion + dermal contact, the carcinogenic risk level of both B2 PAHs and BDE-209 for adults and children (6.2 × 10−5 and 5.6 × 10−4) were in the range of the EPA’s safe limits (1 × 10−6 and 1 × 10−4). On the basis of our findings, it can be said that exposure to PBDEs and PAHs in the indoor environment did not have a negative impact on the people when only the non-carcinogenic and carcinogenic effects on neurological behavior were considered. Although only a limited polluted group was investigated—PAHs and PBDEs—these findings are important due to the scarcity of reported data related to the exposure of children and adults to both PAHs and PBDEs in house dust via inhalation, non-dietary ingestion, and dermal contact that would contribute to lifetime risk of cancer.
References
Abafe OA, Martincigh BS (2015) Polybrominated diphenyl ethers and polychlorinated biphenyls in indoor dust in Durban, South Africa. Indoor Air 25:547–556
Abdallah MA, Drage D, Harrad S (2013) A one-step extraction/clean-up method for determination of PCBs, PBDEs and HBCDs in environmental solid matrices. Environ Sci Processes Impacts 15:2279–2287
Ali N, Van den Eede N, Dirtu AC, Neels H, Covaci A (2012) Assessment of human exposure to indoor organic contaminants via dust ingestion in Pakistan. Indoor Air 22:200–211
Allen JG, McClean MD, Stapleton HM, Webster TF (2008) Critical factors in assessing exposure to PBDEs via house dust. Environ Int 34:1085–1091
Aydin YM, Dumanoglu Y, Elbir T, Kara M, Odabasi M (2014) Source apportionment of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in ambient air of an industrial region in Turkey. Atmos Environ 97:271–285
Bamai YA, Shibata E, Saitoc I, Araki A, Kanazawa A, Morimoto K, Nakayama K, Tanaka M, Takigawa T, Yoshimura T, Chikara H, Saijo Y, Kishi R (2014) Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci Total Environ 485–486:153–163
Bennett DH, Moran RE, Wu XM, Tulve NS, Clifton MS, Colon M, Weathers W, Sjödin A, Jones R, Hertz-Picciotto I (2015) Polybrominated diphenyl ether (PBDE) concentrations and resulting exposure in homes in California: relationships among passive air, surface wipe and dust concentrations, and temporal variability. Indoor Air 25:220–229
Besis A, Samara C (2012) Review polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments e a review on occurrence and human exposure. Environ Pol 169:217–229
Besis A, Katsoyiannis A, Botsaropoulou E, Samara C (2014) Concentrations of polybrominated diphenyl ethers (PBDEs) in central air-conditioner filter dust and relevance of non-dietary exposure in occupational indoor environments in Greece. Environ Pol 188(6):4–70
Betts KS (2008) Unwelcome guest e PBDEs in indoor dust. Environ Health Perspect 116:A202–A208
Butte W, Heinzow B (2002) Pollutants in house dust as indicators of indoor contamination. Rev Environ Contam Toxicol 175:1–46
Callén MS, López JM, Iturmendi A, Mastral AM (2013) Nature and sources of particle associated polycyclic aromatic hydrocarbons (PAH) in the atmospheric environment of an urban area. Environ Pol 183:166–174
Cao ZG, Gang Y, Chen YS, Cao QM, Fiedler H, Deng SB, Huang J, Wang B (2012) Particle size: a missing factor in risk assessment of human exposure to toxic chemicals in settled indoor dust. Environ Int 49:24–30
Cao Z, Xu F, Covaci A, Wu M, Wang H, Yu G, Wang B, Deng S, Huang J, Wang X (2014) Distribution patterns of brominated, chlorinated, and phosphorus flame retardants with particle size in indoor and outdoor dust and implications for human exposure. Environ Sci Technol 48:8839–8846
Cetin B, Odabasi M, Bayram A (2016) Wet deposition of persistent organic pollutants (POPs) in Izmir. Turkey Environ Sci Pollut Res:6183–6186
Chen SC, Liao CM (2006) Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci Total Environ 366:112–123
Chou HM, Kao CC, Chuang KP, Lin C, Shy CG, Chen RF, et al. (2016) Levels of Polybrominated diphenyl ethers in air-conditioner filter dust used to assess health risks in clinic and electronic plant employees. Aerosol Air Qual Res 16:184–194
De Wit CA, Björklund JA, Thuresson K (2012) Tri-decabrominated diphenyl ethers and hexabromocyclododecane in indoor air and dust from Stockholm microenvironments 2: indoor sources and human exposure. Environ Int 39:141–147
DellaValle CT, Wheeler DC, Deziel NC, De Roos AJ, Cerhan JR, Cozen W, Severson RK, et al. (2013) Environmental determinants of polychlorinated biphenyl concentrations in residential carpet dust. Environ Sci Technol 47:10405–10414
Deng J, Guo J, Zhou X, Zhou P, Fu X, Zhang W, Lin K (2014) Hazardous substances in indoor dust emitted from waste TV recycling facility. Environ Sci Pollut Res 21:7656–7667
Dirtu A, Covaci A (2010) Estimation of daily intake of Organohalogenated contaminants from food. Environ Sci Technol 44:6297–6304
Driver JH, Konz JJ, Whitmyre GK (1989) Soil adherence to human skin. Bull Environ Contam Toxicol 43:814–820
European Chemicals Bureau (2002) IEuropean union risk assessment report: bis(pentabromophenyl) ether. Office for Official Publications of the European Communities, Luxembourg
Fromme H, Hilger B, Kopp E, Miserok M, Volkel W (2014) Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany. Environ Int 64:61–68
Fromme HT, Lahrz M, Kraft L, Fembacher S, Dietrich S, Sievering R, Burghardt R, Schuster R, Bolte G, Völkel W (2013) Phthalates in German daycare centers: occurrence in air and dust and the excretion of their metabolites by children (LUPE 3). Environ Int 61:64–72
Hanedar A, Alp K, Kaynak B, Avşar E (2014) Toxicity evaluation and source apportionment of polycyclic aromatic hydrocarbons (PAHs) at three stations in Istanbul, Turkey. Sci Total Environ 488–489:437–446
Harrad S, Abdallah MAE, Oluseyi T (2016) Polybrominated diphenyl ethers and polychlorinated biphenyls in dust from cars, homes, and offices in Lagos. Nigeria, Chemosphere 146:346–353
Harrad S, Goosey E, Desborough J, Abdallah MA-E, Roosens L, Covaci A (2010) Dust from U.K. primary school classrooms and daycare centers: the significance of dust as a pathway of exposure of young U.K. children to brominated flame retardants and polychlorinated biphenyls. Environ Sci Technol 44:4198–4202
Harrad S, Ibarra C, Abdallah ME, Boon R, Neels H, Covaci A (2008) Concentrations of brominated flame retardants in dust from United Kingdom cars, homes, and offices: causes of variability and implications for human exposure. Environ Int 34:1170–1175
Harrad S, Ibarra C, Robson M, Melymuk L, Zhang X, Diamond M, Douwes J (2009) Polychlorinated biphenyls in domestic dust from Canada, New Zealand, United Kingdom and United States: implications for human exposure. Chemosphere 76:232–238
Hassan Y, Shoeib T (2015) Levels of polybrominated diphenyl ethers and novel flame retardants in microenvironment dust from Egypt: an assessment of human exposure. Sci Total Environ 505:47–55
Hawley JK (1985) Assessment of health risk from exposure to contaminated soil. Risk Anal 5:289–302
Hu X, Zhang Y, Luo J, Wang T, Lian H, Ding Z (2011) Bioaccessibility and health risk of arsenic, mercury and other heavy metals in urban street dusts from a mega-city, Nanjing, China. Environ Pollut 159:1215–1221
Hwang HM, Park EK, Young TM, Hammock BD (2008) Occurrence of endocrine-disrupting chemicals in indoor dust. Sci Total Environ 404:26–35
Johnson-Restrepo B, Kannan K (2009) An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 76:542–548
Kamal A, Malik RN, Martellini T, Cincinelli A (2014) Cancer risk evaluation of brick kiln workers exposed to dust bound PAHs in Punjab province (Pakistan). Sci Total Environ 493:562–570
Kamal A, Malik RN, Martellini T, Cincinelli A (2015) Source, profile, and carcinogenic risk assessment for cohorts occupationally exposed to dust-bound PAHs in Lahore and Rawalpindi cities (Punjab province, Pakistan). Environ Sci Pollut Res 22:10580–10591
Kang Y, Cheung KC, Wong MH (2011a) Mutagenicity, genotoxicity and carcinogenic risk assessment of indoor dust from three major cities around the Pearl River Delta. Environ Int 37:637–643
Kang Y, Wang HS, Cheung KC, Wong MH (2011b) Polybrominated diphenyl ethers (PBDEs) in indoor dust and human hair. Atmos Environ 45:2386–2393
Katsoyiannis A, Breivik K (2014) Model-based evaluation of the use of polycyclic aromatic hydrocarbons molecular diagnostic ratios as a source identification tool. Environ Pollut 184:488–494
Katsoyiannis A, Sweetman AJ, Jones KC (2011) PAH molecular diagnostic ratios applied to atmospheric sources: a critical evaluation using two decades of source inventory and air concentration data from the U.K. Environ Pollut 45:8897–8906
Kefeni KK, Okonkwo JO, Botha BM (2014) Concentrations of polybromobiphenyls and polybromodiphenyl ethers in home dust: relevance to socio-economic status and human exposure rate. Sci Total Environ 470–47:1250–1256
Kemmlein S, Herzke D, Law RJ (2009) Brominated flame retardants in the European chemicals policy of REACH-regulation and determination in materials. J Chromatogr A 1216:320–333
Krol S, Zabiegala B, Namiesnik J (2012) Review: PBDEs in environmental samples: sampling and analysis. Talanta 93:1–17
Kurt-Karakus PB (2012) Determination of heavy metals in indoor dust from Istanbul, Turkey: estimation of the health risk. Environ Int 50:47–55
La Guardia MJ, Hale RC, Harvey E (2006) Detailed Polybrominated diphenyl ether (PBDE) congener composition of the widely used Penta-, Octa-, and Deca-PBDE technical flame-retardant mixtures. Environ Sci Technol 40:6247–6254
La Guardia MJ, Hale RC, Harvey E (2009) Detailed Polybrominated diphenyl ether (PBDE) congener composition of the widely used Penta-, Octa-, and Deca-PBDE technical flame-retardant mixtures. Environ Sci Technol 40:6247–6254
Labunska I, Harrad S, Santillo D, Johnston P, Brigden K (2013) Levels and distribution of polybrominated diphenyl ethers in soil, sediment and dust samples collected from various electronic waste recycling sites within Guiyu town, southern China. Environ Sci Process Impacts 15:503–511
Law RJ, Alaee M, Allchin CR, Boon JP, Lebeuf M, Lepom P, Stern GA (2003) Levels and trends of polybrominated diphenylethers and other brominated flame retardants in wildlife. Environ Int 29:757–770
Lee JH, Gigliotti CL, Offenberg JH, Eisenreich SJ, Turpin BJ (2004) Sources of polycyclic aromatic hydrocarbons to the Hudson River Airshed. Atmos Environ 38:5971–5981
Legler J, Brouwer A (2003) Are brominated flame retardants endocrine disrupters. Environ Int 29:879–885
Li CT, Mi HH, Lee WJ, You WC, Wang YF (1999) PAH emission from the industrial boilers. J Hazard Mater 69:1–11
Lim YK, Kim HH, Lee CS, Shin DC, Chang YS, Yang JY (2014) Exposure assessment and health risk of poly-brominateddiphenyl ether (PBDE) flame retardants in the indoor environment of elementary school students in Korea. Sci Total Environ 470–471:1376–1389
Ma Y, Harrad S (2015) Review article: spatiotemporal analysis and human exposure assessment on polycyclic aromatic hydrocarbons in indoor air, settled house dust, and diet. Environ Int 84:7–16
Maertens RM, Yang X, Zhu J, Gagne RW, Douglas GR, White PA (2008) Mutagenic and carcinogenic hazards of settled house dust I:polycyclic aromatic hydrocarbon content and excess lifetime cancer risk from preschool exposure. Environ Sci Technol 42:1747–1753
Mandalakis M, Besis A, Stephanou EG (2009) Particle-size distribution and gas/particle partitioning of atmospheric polybrominated diphenyl ethers in urban areas of Greece. Environ Pol 157:1227–1233
Mannino MR, Orecchio S (2008) Polycyclic aromatic hydrocarbons (PAHs) in indoor dust matter of Palermo (Italy) area: extraction, GC-MS analysis, distribution and sources. Atmos Environ 42:1801–1817
Martellini T, Giannoni M, Lepri L, Katsoyiannis A, Cincinelli A (2012) One year intensive PM 2.5 bound polycyclic aromatic hydrocarbons monitoring in the area of Tuscany, Italy, concentrations, source understanding and implications. Environ Pol 164:252–258
MEU (Republic of Turkey Ministry Ministry of Environment and Urban Planning) (2008) Regulation of building insulation. Ankara, Official Gazette No: 27019
NAP (National Application Plan) (2014), Republic of Turkey Ministry of Environment and Urbanization. http://csb.gov.tr/db/cygm/editordosya/UUP_Taslak.pdf
Pandey PK, Patel KS, Lenicek J (1999) Polycyclic aromatic hydrocarbons: need for assessment of health risks in India—study of an urban-industrial location in India. Environ Monit Assess 59:287–319
Park SS, Kim YJ, Kang CH (2002) Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos Environ 36:2917–2924
Petry T, Schmid P, Schlatter C (1996) The use of toxic equivalency factors in assessing occupational and environmental health risk associated with exposure to airborne mixtures of PAHs. Chemosphere 32:639–648
Qi H, Li WL, Zhu NZ, Ma W, Liu LY, Zhang F, Li Y (2014) Concentrations and sources of polycyclic aromatic hydrocarbons in indoor dust in China. Sci Total Environ 491–492:100–107
Ravindra K, Wauters E, Taygi SK, Mor S, Van Grieken R (2006) Assessment of air quality after the implementation of CNG as fuel in public transport in Delhi, India. Environ Monit Assess 115:405–417
Ren Y, Cheng TT, Chen JM (2006) Polycyclic aromatic hydrocarbons in dust from computers: one possible indoor source of human exposure. Atmos Environ 40:6956–6965
Rudel RA, Perovich LJ (2009) Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ 43:170–181
Shin HM, Bennett DH, Croen LA, Fallin MD, Hertz-Picciotto I, McKone TE, Newschaffer CJ, Nishioka MG (2014) Determining source strength of semivolatile organic compounds using measured concentrations in indoor dust. Indoor Air 24:260–271
Shy CG, Hsu YC, Shih S, Chuang KP, Lin CW, Wu CW, Chuang CY, Chao HR (2015) Indoor level of polybrominated diphenyl ethers in the home environment and assessment of human health risks. Aerosol Air Qual Res 15:1494–1505
Sjödin A, Päpke O, McGahee E, Focant JF, Jones RS, Mulloli TP, Toms LM, Herrmann T, Müller J, Needham LL, Patterson DG Jr (2008) Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere 73:131–136
Söderström G, Sellström U, de Wit CA, Tysklind M (2004) Photolytic debromination of decabromodiphenyl ether (BDE 209). Environ Sci Technol 38:127–132
Stapleton HM, Eagle S, Sjödin A, Webster TF (2012) Serum PBDEs in a north Carolin at oddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Persp 120(7):1049–1054
U.S EPA (United States Environmental Protection Agency (2007) Method 1614: brominated diphenyl ethers in water, soil, sediment and tissue by HRGC/HRMS. United States Environmental Protection Agency, Office of Science and Technology, Washington, DC
U.S. EPA (United States Environmental Protection Agency) (1989) Risk assessment guidance for superfund, Vol. I: human health evaluation manual. EPA/540/1–89/002. Office of Solid Waste and Emergency Response, Washington, DC
U.S. EPA (United States Environmental Protection Agency) (1993) Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons. EPA/600/R-93/089. Office of Health and Environmental Assessment, Washington, DC
U.S. EPA (United States Environmental Protection Agency) (1999) Integrated sampling of suspended particulate matter (SPM) in ambient air, Overvıew, EPA/625/R-96/010a, https://www3.epa.gov/ttnamti1/files/ambient/inorganic/overvw2.pdf
U.S. EPA (United States Environmental Protection Agency) (2001) Risk assessment guidance for superfund: volume III — part A, process for conducting probabilistic risk assessment. EPA 540-R-02-002. Washington, D.C. [Online] https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf
U.S. EPA (United States Environmental Protection Agency) (2002a) Child specific exposure factors handbook. EPA-600-P-00-002B. National Center for Environmental Assessment, Washington, DC [Online] https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=55145
U.S. EPA (United States Environmental Protection Agency) (2002b) Supplemental guidance for developing soil screening levels for superfund sites. OSWER 9355.4–24 [Online] http://www.epa.gov/superfund/health/conmedia/soil/index.htm
U.S. EPA (United States Environmental Protection Agency) (2003) Framework for cumulative risk assessment. EPA/630/P-02/001F. Washington,DC:Risk Assessment Forum. [Online] http://cfpub.epa.gov/ncea/raf/recordisplay.cfm?deid = 54944
U.S. EPA (United States Environmental Protection Agency) (2006) A framework for assessing health risks ofen vironmental exposures to children. EPA/600/R-05/093 A. Washington, DC: National Center for Environmental Assessment Office of Research and Development [Online] http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid = 158363
U.S. EPA (United States Environmental Protection Agency) (2009) Risk assessment guidance for superfund. Human health evaluation manual (part F, supplemental guidance for inhalation risk assessment) EPA/540/R/070/002, vol. I. Office of Superfund Remediation and Technology Innovation, Washington, DC [Online] http://www.epa.gov/oswer/riskassessment/ragsf/pdf/partf_200901_final.pdf
U.S. EPA (United States Environmental Protection Agency) (2011) Exposure factors handbook 2011 edition. EPA/600/R-09/052F. National Center for Environmental Assessment, Office of Research and Development U.S. Environmental Protection Agency, Washington, D.C. 20460 [Online] https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252
U.S. EPA (United States Environmental Protection Agency) (2012) Mid Atlantic risk assessment, Regional Screening Level (RSL) Summary Table. [Online] http://www.epa.gov/region9/superfund/prg/
U.S. EPA (United States Environmental Protection Agency) (2016a) Technology Transfer Network https://www3.epa.gov/airtoxics/hlthef/polycycl.html
U.S. EPA (United States Environmental Protection Agency) (2016b) Mid Atlantic risk assessment, Regional Screening Level (RSL) Summary Table, [Online] http://www.epa.gov/region9/superfund/prg/
U.S. EPA IRIS (United States Environmental Protection Agency Integrated Risk Information System) (2016a) 2, 2′,3,3′,4,4′,5,5′,6,6′-Decabromodiphenyl ether (BDE-209)(CASRN1163–19-5). US Environmental Protection Agency, Washington, DC [Online] http://www.epa.gov/iris/subst/0035.htm
UNEP/POPS/COP.4/17 (2009) Recommendations of the persistent organic pollutants review committee of the Stockholm convention to amend annexes A, B or C of the convention. Stockholm Convention on Persistent Organic Pollutants, 4 Feb
Vafeiadi M, Vrijheid M, Fthenou E, Chalkiadaki G, Rantakokko P, Kiviranta H, Kyrtopoulos AS, Chatzi L, Kogevinas M (2014) Persistent organic pollutants exposure during pregnancy, maternal gestational weight gain, and birth outcomes in the mother–child cohort in Crete, Greece (RHEA study). Environ Int 64:116–123
Vorkamp K, Thomsen M, Frederiksen M, Pedersen M, Knudsen LE (2011) Polybrominated diphenyl ethers (PBDEs) in the indoor environment and associations with prenatal exposure. Environ Int 37:1–10
Wallace LA (1987) Total exposure assessment methodology (TEAM) study: summary and analysis. U.S. Environmental Protection Agency, Vol:1, Springfield, Virginia
Wang W, Huang M, Kang Y, Wang H, Leung AOW, Cheung KC, Wong MH (2011) Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: status, sources and human health risk assessment. Sci Total Environ 409:4519–4527
Wang W, Zheng J, Chan CY, Huang MJ, Cheung KC, Wong MH (2014) Health risk assessment of exposure to polybrominated diphenyl ethers (PBDEs) contained in residential air particulate and dust in Guangzhou and Hong Kong. Atmos Environ 89:786–796
Wei H, Turyk M, Cali S, Dorevitch S, Erdal S, Li A (2009) Particle size fractionation and human exposure of polybrominated diphenyl ethers in indoor dust from Chicago. J Environ Sci Heal 44:1353–1361
Weschler C (2009) Changes in indoor pollutants since the 1950s. Atmos Environ 43:153–169
Weschler CJ, Nazaroff WW (2008) Semivolatile organic compounds in indoor environment. Atmos Environ 42:9018–9040
Wilford BH, Shoeib M, Harner T, Zhu J, Jones KC (2005) Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: implications for sources and exposure. Environ Sci Technol 39(18):7027–7035
Woodruff TJ, Caldwell J, Cogliano VJ, Axelrad DA (2000) Estimating cancer risk from outdoor concentrations of hazardous air pollutants in 1990. Environ Res 82:194–206
Wu MH, Pei JC, Zhieng M, Tang L, Bao Y, Xu BT, Sun R, Sun YS, Xu G, Lei JQ (2015) Polybrominated diphenyl ethers (PBDEs) in soil and outdoor dust from a multi-functional area of shanghai: levels, compositional profiles and İnterrelationships. Chemosphere 118:87–95
Yamei K, Minghui Z, Zhengtao L, Lirong G (2009) Distribution of polycyclic aromatic hydrocarbons in sediments from Yellow River estuary and Yangtze River estuary, China. J Environ Sci 21:1625–1631
Yang Q, Qiu X, Li R, Liu S, Li K, Wang F, Zhu P, Li G, Zhu T (2013) Exposure to typical persistent organic pollutants from an electronic waste recycling site in northern China. Chemosphere 91:205–211
Yu YX, Pang YP, Li C, Li JL, Zhang XY, Yu ZQ, Feng JL, Wu MH, Sheng GY, Fu JM (2012) Concentrations and seasonal variations of polybrominated diphenyl ethers (PBDEs) in in- and out-house dust and human daily intake via dust ingestion corrected with bioaccessibility of PBDEs. Environ Int 42:124–131
Yunker MA, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zhang X, Diamond ML, Robson M, Harrad S (2011) Sources, emissions, and fate of polybrominated diphenyl ethers and polychlorinated biphenyls indoors in Toronto, Canada. Environ Sci Technol 45:3268–3274
Zhang BZ, Guan YF, Li SM, Zeng EY (2009) Occurrence of polybrominated diphenyl ethers in air and precipitation of the Pearl River Delta, South China: annual washout ratios and depositional rates. Environ Sci Technol 43:9142–9147
Acknowledgments
Funding for this project under Project No. 115Y405 was obtained from the Scientific and Technological Research Council of Turkey (TUBITAK). Kocaeli University under Grant KOU-BAP-14/89 project financially supported to purchase the GC-MS used in the current study. The author is grateful to all volunteers who participated and contributed to this work by filling questionnaires and allowing the sampling of their homes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Constantini Samara
Electronic supplementary material
ESM 1
(DOCX 504 kb)
Rights and permissions
About this article
Cite this article
Civan, M.Y., Kara, U.M. Risk assessment of PBDEs and PAHs in house dust in Kocaeli, Turkey: levels and sources. Environ Sci Pollut Res 23, 23369–23384 (2016). https://doi.org/10.1007/s11356-016-7512-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7512-5