Abstract
The objective of this study was to monitor a newly constructed wetland (CW) in north Wales, UK, to assess whether it contributes to an improvement in water quality (nutrient removal) of a nearby drinking water reservoir. Inflow and outflow of the Free Water Surface (FWS) CW were monitored on a weekly basis and over a period of 6 months. Physicochemical parameters including pH, conductivity and dissolved oxygen (DO) were measured, as well as nutrients and dissolved organic and inorganic carbon (DOC, DIC) concentration. The CW was seen to contribute to water quality improvement; results show that nutrient removal took place within weeks after construction. It was found that 72 % of initial nitrate (N03 −), 53 % of initial phosphate (PO4 3−) and 35 % of initial biological oxygen demand (BOD) were removed, calculated as a total over the whole sampling period. From our study, it can be concluded that while inorganic nutrients do decline in CWs, the DOC outputs increases. This may suggest that CWs represent a source for DOC. To assess the carbon in- and output a C budget was calculated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands function as sinks for nutrients and are therefore often used to improve water quality. The ability of wetlands to sequester nutrients is due to two processes; the uptake and immobilisation of nutrients by plant, microorganisms and soil matrix (Corbitt and Bowen 1994) and suppressed rates of decomposition of organic matter and re-release of nutrients in anaerobic wetland sediments (Clymo and Reddaway 1971). Therefore, CWs offer a natural solution for removing nutrient pollution in aquatic ecosystems. Such low-tech treatment systems are often more economically favourable than energy-intensive engineered treatment plants, are easier to operate and can provide numerous secondary benefits such as improved biodiversity. CWs are increasingly used to reduce concentrations of nitrate and phosphate concentrations in surface waters to prevent eutrophication (Kadlec 2012). In England and Wales, two-thirds of drinking water comes from surface water (reservoirs, lakes and rivers) while one-third is taken from groundwater. In addition to nutrients, surface waters can contain naturally high concentrations of DOC. The removal of DOC is a costly and energy-intensive stage of water treatment (Jones et al. 2015) as DOC compounds persisting until the disinfectant stage can react with the disinfectant (usually chlorine) to produce carcinogenic disinfection by-products (DBPs) (Chow et al. 2003). Another recent concern is the increasing amount of DOC in our rivers and streams. A study by Evans et al. (2005) estimated an increase in DOC in UK waters by 91 %. The worry about rising DOC concentration is due to effect it can have on surface water pH and acid neutralising capacity (Kullberg et al. 1993). In addition, high DOC concentrations reduce light penetration in water by giving it a brownish shade. This affects the aquatic productivity, which has implications for the aquatic food chain and lake stratification (Keller et al. 2003). High DOC concentrations in surface waters that feed reservoirs are linked to catchment properties, hydrological conditions, land management characteristics and climatic conditions (Pacheco et al. 2013). DOC originates from either autochthonous or allochthonous sources; the autochthonous organic carbon pool is mainly controlled by algae and plant production and the allochthonous by climate and processes within the catchment (Junlong et al. 1997). Eutrophication, which affects many reservoirs, may present a major problem as the resulting enhanced algal growths may also increase the input of DOC, thereby increasing the potential risk of DBP formation (Gough et al. 2015). Only a few studies about C budgets of CWs exist, and their results are variable but confirm that CWs often act as a net source for DOC (Kovacic et al. 2000, 2006). The objective of this study is to monitor a newly constructed wetland on a weekly interval for its physiochemical parameters at the inflow and outflow, to evaluate the water quality improvement. We hope to make a statement about how long it takes for a new CW to remove nutrients and evaluate the removal efficiency on a high resolution time span.
Material and methods
Site description
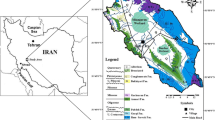
The surface flow CW (Fig. 1) is located on one of the inflow streams to a drinking water reservoir in north Wales. It is a forested area, with mainly coniferous trees. The reservoir in this study is eutrophic due to agricultural practices within the catchment and the streams flowing into the reservoirs have moderate to high DOC concentrations (∼10 mg/l). The area around the reservoir is used for cattle and sheep farming aided by modern agro-chemicals (Hughes et al. 2013). The bedrock at the site consists dominantly of Schist, and the aquifer has a limited yield for groundwater resources. The primary aim of the CW is the removal of nitrogen and phosphorus, to minimise the growth of algae in the reservoir and the formation of DBPs at the treatment plant. Phosphorus and nitrogen are high due to significant agricultural influence.
The CW system consists of 414 m2 of treatment area in addition to 81 m2 of open water. The nominal hydraulic retention time (nHRT) is 3 days. Average inflow flow rate is 1.02 L/s. The length of the system is 33 m, width at the inflow is 12 and 18 m at the outflow. Furthermore, 1.8 m was added as open water zone, which allows oxygenation, increased retention time and provides mixing, which can enhance removal processes. The treatment area comprises of a range of naturally occurring reeds, like phragmites. Only material from the site was used for construction of the walls and a series of baffles, and a plastic outflow pipe was added. The flow worked as a natural system through gravity, no additional pumping was used. In this study, we aim to investigate the time in which it takes a newly constructed wetland to improve and enhance the water quality of an inflow into a eutrophic drinking water reservoir. Therefore, physicochemical parameters at the inflow and outflow were measured on a weekly basis over a period of 6 months and a C budget was calculated.
Field and laboratory techniques
Sampling was undertaken every week between 27/03/2014 and 24/10/2014. Sampling of the CW outflow started 11 days later from the 07/04 onwards. Mean temperature over the sampling period was 13.4 °C and average rainfall was 3.0 mm/day. In situ measurements of temperature and DO (Milwaukee Instruments MW-600 Smart DO Meter) were made each time. At the inflow and outflow, three samples were collected using a 1-L glass bottle, a 100-ml plastic bottle and a 50-ml amber glass bottle (to minimise UV influences on samples). The 1-L glass bottle was used to measure particulate organic carbon (POC) by high temperature combustion (550 °C for 2 h) of the sample on a GF/F Whatman glass fibre filter. The 100-ml plastic bottle was completely filled and left unfiltered to measure pH (SevenEasy pH meter, Mettler Toldeo), conductivity (Orion 5 probe), bicarbonate and dissolved greenhouse gas concentrations. Bicarbonate was measured by taking 10 ml of unfiltered sample and titrating to pH 4.3 with 0.1 M HCl. Dissolved gases were determined using a similar headspace equilibrium method as that described by Dawson et al. (2002), with the gases analysed using a Varian 450 GC. The remaining sample was filtered (0.45 μm Whatman Glass-fibre filters) and analysed for DOC and DIC concentrations (Thermalox TC/TN, Analytical Sciences Ltd.), specific ultraviolet absorbance (SUVA) (Spectromax M2e Spectrophotometer, Molecular Devices) and nutrients (850 IC, Metrohm). The SUVA value gave information about the aromaticity of the water sample and is calculated with the DOC (mg/l) and the UV absorbance at 254 nm. The 50-ml amber bottle was incubated at room temperature for 5 days, so biological oxygen demand (BOD) could be calculated. To assess carbon processing, the following mass balance equation was applied (slightly altered from Pacheco et al. 2013):
where TOC is total organic carbon, OC is organic carbon, the subscripted “in” signifies the inflow from the catchment, “dep” is atmospheric wet deposition, “out” is outflow from the lake, “s” is permanent burial in sediment, and CO2 is the concentration of CO2 in the water. Additionally, CH4 was calculated as a CO2 equivalent and added to the CO2. ΔTCst is the change in the pool of C in the lake over each sampling time period (1 week). POCin, DOCin, DICin, POCout, DOCout, DICout were calculated from weekly measured concentrations in streams.
To assess the effectiveness of the wetland nutrient removal efficiency (R) was calculated as follow
Nutrient concentration influent (Ci) was measured at the inflow and the nutrient concentration effluent (Co) was sampled at the outflow both on a weekly basis and for calculation of R the average mean value was used. Pearson correlation factors for all parameters were calculated with SPSS.
Results
Dissolved and particulate carbon
Mean DOC concentration of the inflow was 7.22 ± 0.17 mg/l and at the outflow it was 11.43 ± 0.21 mg/l, with the concentration of the outflow in general higher than the inflow and both exhibiting the same approximate seasonal trend (Fig. 2). DOC accumulation in the CW was 60 % as an average over the entire sampling period. DIC concentration at the inflow was 18.07 ± 0.21 and 16.24 ± 0.37 mg/l at the outflow. Removal of DIC was approximately 10 %. POC concentration at the inflow was 0.82 ± 0.04 mg/l and 2.00 ± 0.09 mg/l at the outflow. Pearson correlation coefficients for DIC, removal DIC, DOC, removal DOC, removal POC, SUVA and removal SUVA were calculated with SPSS (Table 1).
SUVA
Mean SUVA value at the inflow was 3.49 ± 0.06 and for the outflow 3.13 ± 0.04. After initially high (>4) SUVA values at the outflow during start-up phase, SUVA concentration stabilises to values lower than four at the end of April. Outflow waters have a higher DOC concentration and higher UV254 absorbance.
Nutrients
Mean nitrate concentrations were 4.5 ± 0.15 mg/l (inflow) and 1.2 ± 0.09 mg/l (outflow) (Fig. 3). Nitrate removal was measured 2 months after construction and the removal rate over the whole sampling period were 72 %. Nitrate removal rates were significantly lower in autumn, with an average of 30–40 %. MMR of nitrate was 0.70 g · m–2 day–1. Removal of DIC and O2 was negatively correlated with NO3 removal rates (Table 2).
Mean phosphate concentration was 0.04 ± 0.0018 mg/l (inflow) and 0.02 ± 0.0021 mg/l (outflow). Phosphate removal was 53.4 % as an average over the sampling period and positively correlated with Nitrate removal (Table 2). Phosphate MMR was 0.0043 g m–2 day–1. Phosphate concentrations are much lower at the outflow than at the inflow (Fig. 4)
Mean bromide concentration (Fig. 5) at the inflow was 0.07 ± 0.0007 and 0.1 ± 0.0012 mg/l at the outflow. MMR was calculated as −0.0049 g m–2 day–1. Nutrient removal efficiency was −31.5 %.
Dissolved oxygen, BOD5, temperature and pH
The average DO value at the inflow was 8.26 ± 0.12 mg/l, and at the outflow, it was 7.89 ± 0.22 mg/l. Dissolved oxygen increased by 4.5 % from inflow to outflow, and the mean BOD5 was reduced by 46 %. The average temperature at the inflow was 12.99 ± 0.08 °C. Outflow temperature was 19.5 ± 0.2 °C on average, and the wetland was by average 6.6° warmer than the inflow. Mean inflow pH was 7.7 ± 0.2, and at the outflow, it was 7.6 ± 0.3. By average, the pH was 0.1 lower at the outflow than at the outflow. Table 3 shows significant correlations between the discussed parameters.
Greenhouse Gases
The concentrations of dissolved greenhouse gases (GHGs), especially CH4, were substantially higher in the outflow compared to inflow (Table 4). Average CH4 emission at the inflow was 2.11 ± 0.16 μg/l, and at the outflow, it was 20.11 ± 0.56 μg/l. CH4 concentration was especially high in the summer when the water was warmer (Fig. 6). The CO2 concentration was 737.89 ± 20.94 mg/l (inflow) and 1128.67 ± 25.15 mg/l (outflow). Removal efficiency for CO2 was approximately −53 %. The outflow concentration increased steadily until the end of September; afterwards, it declined and dropped under the concentration of the inflow (Fig. 7).
Carbon budget
The carbon budget (Fig. 8) shows that, over the entire sampling period, 17.2 % less DIC, 11.5 % more DOC and approximately twice as much POC flowed into the reservoir compared to the amounts that are expected without the presence of the CW. Of the carbon, 7.5 % is retained in the wetland through sedimentation and plant uptake. Nutrient removal efficiency is −60.7 %. Mass removal rate (MRR) was −0.92 g m–2 day–1 calculated as mean average over the whole sampling period.
Discussion
This study aimed to investigate the nutrient removal capability of a new CW, calculate its carbon budget and determine its impact on DOC loading to a reservoir.
Nitrate removal is expected to be controlled by three main mechanisms: (1) denitrification, (2) plant uptake and (3) mineralization (Vymazal 2007). The higher nitrate concentration during the startup phase is most likely due to the growth of the vegetation around the area and may partly be due to the wash off of nutrients during the construction phase. The CW functions as a nitrate source in autumn; this may be related to plant senescence and rapid mineralization of organic N within the system, while very high removal rates in summer can be explained by active denitrification. For denitrification, DOC is a key factor as well as DO, as an anaerobic environment is needed. As found in previous research, denitrification rates are mainly constrained by environmental conditions such as temperature, pH and carbon availability (Song et al. 2011 and Bachand and Horne 2000).
Initial phosphate concentration at the inflow most likely originates from PO4 3− in the soil, which was released during construction work. Later, increasing phosphate concentrations were probably due to agricultural nutrients leaching into the surface water, which is beneficial for plant growth. According to Verhoeven and Meuleman (1999), phosphate removal in most CWs does not exceed more than 50 %. A review study by Vymazal (2007) stated total phosphate removal rates between 40 and 60 % and total nitrogen removal rates between 40 and 55 %, for various types of CWs. Therefore, the removal rates, phosphate 53 % and nitrate 73 %, achieved during the startup phase of this CW show a high performance quality.
While inorganic nutrients were sequestered in this CW, achieving the main aim of their installation, the DOC outputs increased. Since only one site was monitored during this study, broad conclusions about the impacts of CWs on DOC cannot be made, but the enhanced DOC loading to the reservoir is a significant water quality issue at this site because the reservoir as used as a source of drinking water. DOC can be leached into water flowing through wetlands as plants, algae, and bacteria grow, die and decay (Pinney et al. 2000; Villa et al. 2014). A study by Xenopoulos et al. (2003) investigated the amount of DOC loadings from different catchment areas; the results showed that forested wetlands, especially those with coniferous vegetation, have the highest leaching of DOC into the environment.
Particulate organic carbon causes the formation of anaerobic microsites, supporting simultaneous nitrification and denitrification. Therefore, it may play a dual role in denitrification, since it supports the heterotrophic metabolism of denitrifying bacteria as well as the O2 consumption which creates anaerobic microsites necessary for denitrification (Hamersley and Howes 2002). The accumulation of twice as much POC in the constructed wetland enhances denitrification processes. Bioavailable POC acts not only as a C substrate for denitrifiers, but also depletes DO levels within particles via aerobic respiration, supporting denitrification within aerobic wastewaters. According to Moran and Hodson (1994), higher POC additions results in higher respiration rates, and the faster creation of anaerobic microsite volume to support denitrification.
According to Lin et al. (2002), macrophytes present species-specific nitrate removal efficiency, depending on their ability to produce carbon for denitrification. DOC and bromide accumulation increases the likelihood of DB production in the form of trihalomthanes (THMs) and haloacetonitriles (HANs) during water treatment. Some of these DBPs are suspected to be mutagens, carcinogens or developmental toxicants if ingested over extended periods of time (Villanueva et al. 2004). Bromide is a major inorganic DBP precursor that results in formation of brominated DBPs, which are generally more toxic than chlorinated DBPs (Richardson et al. 2007). According to Ingersoll and Baker (1998), when nitrate removal efficiencies increase, dissolved organic carbon in the effluent also increases, as does chloroform formation potential. Bacterial decomposition of plant detritus has been shown to convert POC into dissolved form and cause the release of humic substances into the bulk DOC pool (Moran and Hodson 1994).
SUVA is a reliable indicator of DOC aromaticity and liability of natural organic matter (NOM) to coagulation, and it is known that the NOM in high-SUVA waters tends to have lower alkalinities and hardness and higher TOC concentrations and is therefore more amenable to removal by coagulation at the treatment works (Archer and Singer 2006). Results show that despite the rise in DOC concentration at the outflow, SUVA values decrease. This indicates the added DOC will be harder to treat, but the CW will ultimately reduce algae in the reservoir, which will lead to higher SUVA values (Nguyen et al. 2005) and easier to treat water. The rather high (>4) SUVA values, during the first few weeks, could be due to disturbance effect of the CW construction.
Dissolved oxygen increased through the CW, probably due to the growing vegetation in the startup phase. A survey conducted by Puigagut et al. (2007) stated that BOD5 removal generally ranged from 80 to 95 %. Even though BOD removal in the studied CW has a much lower removal rate, we assume that much oxygen was added through the free water area and cannot find a negative impact on other removal parameters. Kadlec and Wallace (2009) stated that BOD removal rates in CWs are always lower in summer.
GHGs were monitored to assess the carbon budget and to provide additional insight into the CW functionality. The concentration pattern of GHGs can be explained by vegetation growth phases over the sampling period. GHG concentration in CWs can vary significantly, depending on the kind of wetland, its location and various other parameters (Kang and Freeman 2002). CH4 concentration showed high concentrations over spring; during the startup phase and a decline over the summer months and an increase during autumn, these results are the similar with the findings of Kang and Freeman 2002. The study suggests that higher CH4 emissions from swamps in autumn were linked with litterfall production, which indicates a coupling between carbon input and methanogenesis. We found a positive correlation between DOC and CH4 increases (Table 1). The high CH4 concentration over spring in the outflow is mostly likely due to the rapidly growing vegetation around the wetland and the leaching of DOC from plants. Plants are the main source of carbon for microorganisms in CWs. This carbon is further transformed to gaseous forms and increases the loading of CO2 and CH4 into the wetland. Due to increased litterfall during autumn, the high CH4 and CO2 concentrations in September can be explained. Furthermore, plants increase the efficiency of nitrogen removal by supporting denitrifying microorganisms with easily decomposable organic matter (Picek et al. 2007). In a study conducted by Liikanen et al. (2006), it was estimated that even if all global wastewaters were treated in constructed wetlands, their share in atmospheric liability would be less than 1 % in total.
Conclusion
As newly CWs have not been previously monitored during startup phase, weekly monitoring over a 6-month period was meant to give insight into the early stage functionality of a new CW. This study showed that newly constructed wetlands contribute to water quality improvement within a few weeks after construction. Nitrate and BOD5 removal started showing an effect within 4 weeks after construction. Nitrate removal was the fastest and most efficient process, probably boosted by the very rapidly establishing vegetation around the CW. The early denitrification process could also be initiated by the carbon content of the soil or water. The plants leached organic matter into the wetland, which leads to enhanced DOC loading to the reservoir. By the beginning of September, nitrate had accumulated in the CW, possibly because vegetation started to decrease and could not take up nitrate as efficiently as over the spring/summer months. Evergreen plants for vegetation might be a valid solution to produce less organic matter and take up nitrate all year around. Overall, spring was a good time to set up the CW, since natural vegetation developed fastest and promoted the CW’s productivity.
References
Archer AD, Singer PC (2006) An evaluation of the relationship between SUVA and NOM coagulation using ICR database. J Am Water Works Assoc 98(7):110–123
Bachand PA, & Horne AJ (2000) Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol Eng 14:17–32
Chow AT, Tanji KK, Gao S (2003) Production of dissolved organic carbon (DOC) and trihalomethane (THM) precursor from peat soils. Water Res 37:4475–4485
Clymo RS, Reddaway EJ (1971) Productivity of Sphagnum (bog-moss) and peat accumulation. Hydrobiologia 12:181–192
Corbitt RA, Bowen P (1994) Applied wetlands science and technology (p. Chapter 10). In: Kent DM (ed) Applied Wetlands Science and Technology. CRC Press, Florida
Dawson JJ, Billett MF, Neal C, Hill S (2002) A comparison of particulate, dissolved and gaseous carbon in two contrasting upland streams in the UK. J Hydrol 257:226–246
Evans CD, Monteith DT, Cooper DM (2005) Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environ Pollut 137:55–71
Gough R, Holliman PJ, Cooke GM, Freeman C (2015) Characterisation of algogenic organic matter during an algal bloom and its implications for trihalomethane formation. Sustain Water Qual Ecol 6:11–19
Hamersley MR, Howes BL (2002) Control of denitrification in a septage-treating artificial wetland: the dual role of particulate organic carbon. Water Res 39:4415–4427
Hughes DD, Holliman PJ, Jones T, Freeman C (2013) Temporal variations in dissolved organic carbon concentrations in upland and lowland lakes in North Wales. Water Environ J 27:275–283
Ingersoll TL, Baker LA (1998) Nitrate removal in wetland microcosms. Water Res 32(3):667–684
Jones TG, Evans C, Freeman C (2015) The greenhouse gas (GHG) emissions associated with aquatic carbon removal during drinking water treatment. Aquat Sci. doi:10.1007/s00027-015-0458-8
Junlong Q, Sumin W, Bin X, Rusong C, Shanzhe K (1997) A method of quantitatively calculating amount of allochthonous organic carbon in lake sediments. Chin Sci Bull 42(21):1821–1823
Kadlec RH (2012) Constructed marshes for nitrate removal. Crit Rev Environ Sci Technol 42(9):934–1005
Kadlec RH, Wallace SD (2009) Treatment wetlands, 2nd edn. CRC Press, United States
Kang H, Freeman C (2002) The influence of hydrochemistry on methane emissions from two contrasting northern Wetlands. Water Air Soil Pollut 141:263–272
Keller W, Heneberry JH, Dixit SS (2003) Decreased acid deposition and the chemical recovery of Killarney, Ontario, lakes. Ambio 32:183–189
Kovacic DA, David MB, Gentry LE, Starks KM, Cooke RA (2000) Effectiveness of constructed wetlands in reducing nitrogen and phosphorus export from agricultural tile drainage. J Environ Qual 29(4):1262–1274
Kovacic DA, Twait RM, Wallace MP, Bowling JM (2006) Use of created wetlands to improve water quality in the Midwest—Lake Bloomington case study. Ecol Eng 28:258–270
Kullberg A, Bishop KH, Hargeby A, Jansson M, Petersen RC (1993) The ecological significance of dissolved organic carbon in acidified waters. Ambio 22:331–337
Liikanen A, Huttunen JT, Karjalainen SM, Väisänen TS, Nykänen H, Martikainen PJ (2006) Spatial and seasonal variation in greenhouse gas emissions from a constructed wetland purifying peat mining runoff waters. Ecol Eng 26:241–251
Lin Y-F, Jing S-R, Wang T-W, Lee D-Y (2002) Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environ Pollut 119:413–420
Moran MA, Hodson RE (1994) Dissolved humic substances of vascular plant origin in a coastal marine environment. Limnol Oceanogr 39(4):762–771
Nguyen M.-L, Westerhoff P, Baker L, Hu Q, Esparza-Soto M, Sommerfeld M (2005) Characteristics and reactivity of algae-produced dissolved organic carbon. J Environ Eng. doi:10.1061/ ASCE 0733-9372 2005 131:111574
Pacheco FS, Roland F, Downing JA (2013) Eutrophication reverses whole-lake carbon budgets. Inland Waters 4:41–48
Picek T, Cízková H, Dusek J (2007) Greenhouse gas emissions from a constructed wetland—plants as important sources of carbon. Ecol Eng 31:98–106
Pinney ML, Westerhoff PK, Baker L (2000) Transformations in dissolved organic carbon through constructed wetlands. Water Res 34:1897–1911
Puigagut J, Villasenor J, Salas JJ, Bécares E, García J (2007) Subsurface-flow constructed wetlands in Spain for the sanitatom of small communities: a comparative study. Ecol Eng 30:312–319
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636:178–242
Song K, Lee S-H, Kang H (2011) Denitrification rates and community structure of denitrifying bacteria in newly constructed wetland. Eur J Soil Biol 47:24–29
Verhoeven JT, Meuleman AF (1999) Wetlands for wastewater treatment: opportunities and limitations. Ecol Eng 12:5–12
Villa JA, Mitsch WJ, Song K, Miao S (2014) Contribution of different wetland plant species to the DOC exported from a mesocosm experiment in the Florida Everglades. Ecol Eng 71:118–125
Villanueva CM, Cantor KP, Cordier S, Jaakkola JJ, King WD, Lynch CF et al (2004) Disinfection byproducts and bladder cancer—a pooled analysis. Epidemiology 15(3):357–366
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380:48–65
Xenopoulos MA, Lodge DM, Frentress J, Kreps TA, Bridgham SD, Grossman E et al (2003) Regional comparisons of watershed determinants of dissolved organic carbon in temperate lakes from the Upper Great Lakes region and selected regions globally. Limnol Oceanogr 48:2321–2334
Acknowledgments
The authors thank the Wolfson Carbon Capture Laboratory Team for their support and the Natural Resources Wales and the MET office for providing data.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Scholz, C., Jones, T.G., West, M. et al. Constructed wetlands may lower inorganic nutrient inputs but enhance DOC loadings into a drinking water reservoir in North Wales. Environ Sci Pollut Res 23, 18192–18199 (2016). https://doi.org/10.1007/s11356-016-6991-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6991-8