Abstract
We utilized a multi-biomarker approach (Integrated Biomarker Response version 2, IBRv2) to investigate the scope and dispersion of groundwater contamination surrounding a rare earth mine tailings impoundment. Parameters of SD rat included in our IBRv2 analyses were glutathione levels, superoxide dismutase, catalase, and glutathione peroxidase activities, total anti-oxidative capacity, chromosome aberration, and micronucleus formation. The concentration of 20 pollutants including Cl−, SO4 2−, Na+, K+, Mg2+, Ca2+, TH, CODMn, As, Se, TDS, Be, Mn, Co, Ni, Cu, Zn, Mo, Cd, and Pb in the groundwater were also analyzed. The results of this study indicated that groundwater polluted by tailings impoundment leakage exhibited significant ecotoxicological effects. The selected biomarkers responded sensitively to groundwater pollution. Analyses showed a significant relationship between IBRv2 values and the Nemerow composite index. IBRv2 could serve as a sensitive ecotoxicological diagnosis method for assessing groundwater contamination in the vicinity of rare earth mine tailings. According to the trend of IBRv2 value and Nemerow composite index, the maximum diffusion distance of groundwater pollutants from rare earth mine tailings was approximately 5.7 km.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China is facing groundwater quality problems of enormous proportions from both industrial and other sources (Qiu 2011). Large areas such as the Northeastern plain including Inner Mongolia are affected (Rodríguez-Lado et al. 2013). Groundwater pollution due to mine tailings leakage is a serious environmental problem (Qiu 2011; Huang et al. 2014). Inadequate maintenance of mine tailings impoundments has contributed to the migration of pollutants into adjacent groundwater. These pollutants can seep into aquifers, disseminating them over large distances, contributing to environmental detriment, and decreasing biological diversity (Si et al. 2011). Many pollutants are toxic to plants and animals and can reach humans via the food chain (Kraus and Wiegand 2006; Si et al. 2011, 2015). In order to evaluate the mine environmental concerns, many pollutants have been previously determined and reported in mining areas and smelting sites, such as Cu, As, Al, Ni, Co, Cd, Cr, Pb, Mo, Fe, Ag, and Mn (Boularbah et al. 2006; Conesa et al. 2006; Edinger et al. 2008; Da Pelo et al. 2009). Several studies have addressed soil and vegetation contamination following closure of mines, both within and in the perimeter zones of former mine sites (Bruce et al. 2003; Si et al. 2015). “However, there was little research using bio-monitoring method to investigate the pollution level in the groundwater in the vicinity of tailings.”
Aquatic systems can contain complex mixtures of contaminants arising from many distinct anthropogenic discharges. Traditional measurements of water quality give limited information on the threat of these discharges to organisms that inhabit these ecosystems. Moreover, chemical analyses are expensive and cannot measure all classes of chemicals in groundwater. Biochemical effects analyses can provide relevant data to predict ecological changes arising from anthropogenic discharges (Oliveira et al. 2009). The validity of this approach depends on the selection of an appropriate biomarker set, as environmental contamination complexity may induce a variety of responses that are not necessarily correlated (Oliveira et al. 2009). Levels of superoxide dismutase (SOD), catalase (CAT); glutathione peroxidase (GPx); glutathione (GSH), glutathione S-transferase, glutathione reductase, metallothionein, lipid peroxidation, DNA damage including chromosome aberration (CA) and micronuclei (MN) frequency can be measured as biomarkers of xenobiotic-mediated oxidative stress under both field and laboratory conditions. (Ahmad et al. 2004; Gravato et al. 2006; Chesman et al. 2007; Jha 2008; Si et al. 2014). This type of approach is now being used increasingly to evaluate environmental genotoxicity effects of industrial chemicals, biocides, agrochemical, food additives, and pharmaceuticals (Nwani et al. 2011; Ginzkey et al. 2014). SD rats have been widely used in pharmacology, toxicology, efficacy, and GLP experiment. So it was chosen as experimental animals in this study.
China’s Inner Mongolia autonomous region is mostly arid or semiarid, and groundwater is the predominant source of domestic water. However, little has been done to develop effective monitoring of groundwater pollution, and there is essentially no biomarker monitoring literature on the scope of groundwater pollution surrounding rare earth (RE) tailings. In this study, we investigated the distribution of major pollutants in the groundwater in the vicinity of RE tailings, focusing on antioxidant defense responses, including GSH, SOD, CAT, GPx and total antioxidant capacity (T-AOC), as well as DNA damage responses including CA and MN frequency in Sprague Dawley (SD) rats. The Integrated Biomarker Response version 2 (IBRv2) was used to assess groundwater pollution level in the vicinity of RE tailings. We evaluated groundwater surrounding an RE tailings site in Inner Mongolia. We assessed the biological effects of contaminated groundwater, and estimated the scope of pollution downstream of the RE tailings resulting from tailing impoundment leakage.
Materials and methods
Study areas
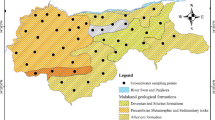
The focus of this study is an RE tailings site located 12 km to the west of Baotou city, near the border between the Kundulun and Jiuyuan districts. The reservoir was officially put into use in 1965 as a ground-type reservoir, with a size of 3.2 km from east to west, 3.5 km from south to north, and an area of 11 km2. Its effective capacity is approximately 68.83 million m3 (Li et al. 2014). The Baotou area has an inland arid climate, experiencing low temperatures and little rain. The main soil type is chestnut soil. The RE ore from the Bayan Obo mine is transported by railway and pipelines to the Baotou Dressing Plant. After the dressing and smelting process at Baotou Steel RE and Baotou Huamei RE, two hi-tech companies, remaining pulp is stored in Baotou RE tailings reservoir (Fig. 1). Local phreatic groundwater flows from north to south. The aquifer permeability coefficient was 3.75 m/d on average, and the longitudinal velocity was about 0.05 m/d (Tu et al. 1988). With the development of industry, the emissions and reservoir of waste water increased year by year, as well as the dam altitude and water pressure. As a result, the diffusion velocity maybe increased.
Groundwater sampling and analysis
The groundwater flows from north to south under the RE tailings reservoir. Therefore, thirteen groundwater monitoring sites were chosen to the south of the RE tailings. In increasing distance from the RE tailings, the sites were designated as S1–S13 as shown in Fig. 1. We obtained phreatic stratum groundwater by digging wells of depths between 15 and 30 m. In order to mitigate contamination during storage, all groundwater samples were collected using high-density polyethylene bottles and stored in a refrigerator at 4 °C prior to analysis. Based on our preliminary assessment and other studies (Tu et al. 1988; Si et al. 2015), the main components of the pollution in the groundwater surrounding the rare tailings are heavy metals and salt ions, while the rare earth element contents is very low. Therefore, in this study, 20 pollutants including Cl−, SO4 2−, Na+, K+, Mg2+, Ca2+, TH, CODMn, As, Se, TDS, Be, Mn, Co, Ni, Cu, Zn, Mo, Cd, and Pb were comprehensive selected. The concentrations of these contaminations in groundwater samples were determined in the analytical laboratory of the China National Nuclear Corporation (Beijing Research Institute of Uranium Geology) according to established technical specifications for environmental monitoring of groundwater (HJ/T164-2004).
Three water samples were collected at each site (N = 3), and each water sample was analyzed for contaminants three times. The certified standard solutions of elements and ions were measured simultaneously, and the standard addition recoveries were in the range of 100 ± 10 %.
The groundwater environmental quality standard (III Grade) (National Standard Bureau of PR China, GB/T 14848–93) was adopted as the evaluation standard. Groundwater quality was evaluated using the established Nemerow composite index (NCI calculated using an F-distribution) (Nemerow 1991). The NCI value was estimated using an F-distribution. Water quality was ranked into five pollution grades: very clean (F < 0.80), clean (0.80 ≤ F ≤ 2.50), lightly polluted (2.50 ≤ F ≤ 4.25), moderately–highly polluted (4.25 ≤ F ≤ 7.20), and heavily polluted (F > 7.20).
SD rats sampling and analysis
One-month-old male SD rats were procured from the animal experimental research center of Inner Mongolia University. The average length and average weight of animals at the beginning were 11.33 ± 2.08 cm and 18.53 ± 2.19 g, respectively. There were no significant differences across groups. All of the SD rats were randomly divided into 14 groups, including the 13 experimental groups and 1 control group, and 8 rats per group. All the experimental SD rats were approved by the Institutional Animal Ethics Committee and our work complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. SD rats were kept under controlled environmental conditions of temperature (22 ± 2 °C), relative humidity (50 ± 5 %) and a 12 h light/dark cycle. Standard rat chow and experimental water (groundwater for the experiment group, and high-purity water for control group) were provided to the animals ad libitum. The groundwater was from the well water at each site (13 sites), and high-purity water was made from tap water by water making systems (DBRO-SYS). Rat body weight was assessed frequently.
After exposure for 1 month, six SD rats in each group were randomly selected in each group on the basis of their size respectively, having an average length of 14.5 ± 2.5 cm and weighing 26.6 ± 3.7 g. When each SD rat was euthanized, three tissues were obtained for oxidative stress measurements. Liver tissue was assessed for protein content, SOD, CAT, and GPx activities and GSH content, bone marrow cells were obtained for CA frequency assays, and hemocyte cells were obtained for MN frequency assays. Among them, SOD, CAT, and GPx activities and GSH content were obtained using test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the appropriate guidelines. SOD and CAT activities were analyzed using xanthine oxidase and ammonium molybdate colorimetric method respectively. Both GPx activity and GSH content was analyzed using 5,5′-dithio-bis-(2-nitrobenzoic acid) colorimetric method with different experiments steps. CA (colchicines cultivation) and MN frequencies were analyzed using Giemsa stain microscopy method. Total protein content was measured in independent aliquots, according to the Coomassie Brilliant Blue method using bovine serum albumin as a standard. All of the indicators were measured three times, and two rats were used for each time each group.
Integrated Biological Responses version 2
The Integrated Biomarker Response (IBR) was described by Beliaeff and Burgeot (2002), and utilizes a star plot of standardized biomarker responses. This method has been used extensively to evaluate the biological effects of pollution in field and laboratory studies (Oliveira et al. 2009. Arzate-Cárdenas and Martínez-Jerónimo 2011; Serafim et al. 2012; Zheng et al. 2013). The original IBR has subsequently been updated in the form of the “Integrated Biological Responses version 2 (IBRv2)” based on the reference deviation concept. IBRv2 can be calculated without specific software and combines a mathematical value and a graphical result to conserve the specific responses of investigated biomarkers. The integrated values were expressed as a sum of each area by star-plot analysis, representing a value of IBRv2 (Sanchez et al. 2013). IBRv2 was applied in this study based on the assessment of a set of biomarker responses in SD rats. The IBRv2 data processing was as follows.
A i , Z i , Z 0 , μ, s, X i , and X 0 represented biomarker deviation index, the mean of standardized biomarker response, mean of reference biomarker data, general mean of Y i , standard deviation of Y i , individual biomarker data, and mean reference data, respectively.
Statistical analyses
Results were presented as means ± standard deviation (SD). Statistical analyses were carried out using SPSS 17.0 statistics software. Assumptions of normality and homogeneity of data were verified. One-way Analysis of Variance followed by Duncan’s Multiple Comparisons were used to detect differences. P < 0.05 was considered to be statistically significant, P < 0.01 was considered to be extremely statistically significant.
Results
Groundwater pollution and F value (NCI)
Twenty types of pollutants including Cl−, SO4 2−, Na+, K+, Mg2+, Ca2+, TH, CODMn, As, Se, TDS, Be, Mn, Co, Ni, Cu, Zn, Mo, Cd, and Pb were determined (Table 1). Many pollutant concentrations at sites S1–S5, S8, and S10–S13 had levels exceeding those in the China National Quality Grade III standard for groundwater (GB/T 14848–93).
The main pollutants exceeding the Grade III standard comprise Cl−, SO4 2−, Na+, TH, CODMn, TDS, Mn, and Zn. Groundwater salination was a significant issue with samples collected at most of the sites. The groundwater qualities at the 13 sites were synthetically analyzed using the Nemerow Index Method, and the results are depicted in Fig. 2.
The results showed that significant water pollution was observed at S1, S2, S3, S4, S5, S11, and S13. The F values at S4, S5, S12, and S13 were between 2.50 and 7.20, indicating that the water was lightly or moderately polluted. Groundwater collected at S6, S7, S8, S9, and S10 contained low levels of pollutants, indicating that these sites were not adversely affected by the RE tailings.
Biomarkers and IBRv2
Non-enzymatic defenses
GSH
The GSH content of SD rats first increased and then decreased along with the increase of the distance from tailings (Fig. 3). The values for S6–S8 were significantly higher than those in other sites (P < 0.05), and the lowest values appeared at sites S1–S3. Compared with the control group, S1, S2 and S13 showed no significant difference, but S3 was significantly lower than the control group, while other sites were significantly higher than the control group.
Enzymatic defenses
SOD
Figure 4 shows the relationship between SOD activities in SD rats and the locations of the sources of groundwater they were provided with. Generally, the activities first moderately increased and then decreased with the distance of the groundwater sources from the tailings. The values at S5, S7, and S9 were significantly higher than the values at the other sites (P < 0.05), and the maximum inhibition was observed at S12. Compared with the control group, S1, S2, S6, and S8 showed no significant difference, S3, S4, S10, and S12 were significantly lower than the control group, while other sites were significantly higher than the control group.
GPx
GPx activities peaked at S4 and S10 (Fig. 5). The values at the heavily polluted S2 and S13 sites were significantly lower than the values at other sites (P < 0.05). Compared with the control group, S2, S7, and S13 showed no significant difference, while other sites was significantly higher than the control group.
CAT
The CAT activities were first decreased and then significantly increased (P < 0.05) with increasing distance from the tailings (Fig. 6). Activities were highest at S2 and S11, where the groundwater samples were heavily polluted. Compared with the control group, S7 showed no significant difference, while other sites were significantly higher than the control group.
T-AOC
T-AOC first increased and then decreased along with the increase of the distance from tailings (Fig. 7). Values reached a maximum at S6, which had the cleanest water quality. Conversely, T-AOC values were lower in S4, S11, S12, and S13 where the groundwater was polluted. T-AOC as well as GSH and SOD activities showed a similar pattern of response. Compared with the control group, S6, S7, and S9 were significantly higher than the control group, while other sites were significantly lower than the control group.
Genetic damage
CA and MN
CA and MN frequencies in SD rats showed a similar pattern of responses among the different groundwater collection sites (Figs. 8 and 9). Both the CA and MN frequencies first decreased and then increased with increasing distance from the tailings, indicated by a higher frequency in rats provided with groundwater from S1–S3 and S11–S13 when compared to the other sites. CA frequency reached a minimum in SD rats provided with groundwater from S5–S8 and S10, and the MN frequency reached a minimum at S7–S10. Compared with the control group, CA frequency in S4, S5, S6, S9, and S10 showed no significant difference, S7and S8 were significantly lower than the control group, while other sites were significantly higher than the control group. MN frequency in S7, S8, and S9 showed no significant difference, while other sites were significantly higher than the control group.
Integrated Biomarker Response
The results of IBRv2 analysis and the star plots for SD rats at the thirteen groundwater collection sites are shown in Figs. 10 and 11.
The IBRv2 values were between 1.46 and 13.77 (Fig. 11). Clear differences were observed between the various sites. Clean sites (S6–S9) presented IBRv2 values between 1.46 and 3.27, and polluted sites (S1–S4 and S11–S13) exhibited values between 7.04 and 13.77. Thus, a clear distinction was observed between clean and polluted sites. The star plots obtained from each site reflected a diversity of contamination patterns across the sampled sites. From Figs. 6, 8, 9, and 10, we can see that the patterns of CAT, CA, MN, and IBRv2 values were all very similar. Correlation analyses showed a statistically significant correlation between IBRv2 and F (NCI) (R = 0.868, P < 0.01).
Discussion
The F (NCI) results showed that higher levels of contamination were located near the RE tailings (S1–S5), but were also observed relatively far from the RE tailings (S11–S13). The overall results show that pollution levels in groundwater decreased initially with distance from tailing (S1–S6), and then irregularly increased from S7–S13. This result can explain the variability of the contaminations from the RE tailings. It is not clear what causes the increase of pollution levels from S7–S13.
In the past century, much mining has concentrated on the extraction of RE metals, for example, RE, Au, Ag, Cu, Zn, Pb, Cd, Cr, Fe, Se, Hg, and As. Having access to these metals is necessary for the economic or industrial development of societies (Huang et al. 2014). Disposal of mine waste in tailings has led to environmental contamination due to impoundment leakage. Pollutants include cation contaminants such as metals and some anion contaminants such as chloride, sulfate, and nitrate (Qiu 2011; Rodríguez-Lado et al. 2013; Huang et al. 2014). The literatures showed that the pollution risk assessments on this rare tailing were mainly focused on heavy metals and radioactive element, such as Cu, Zn Pb, Cd, and Th (Si et al. 2011; Huang et al. 2014). However, the toxicological effects elicited by many anion contaminants have not been given enough attention. All these pollutants contribute to the worsening of groundwater contamination, and deterioration of the groundwater ecological environment.
In the present study, GSH content in SD rats was measured and showed higher values in S4–S10, especially in S6–S8, where the groundwater pollutant levels were lower. However, the GSH values of SD rats provided with groundwater from S1–S3 and S11–S13 were significantly lower than those found in other sites (P < 0.05). This result was inconsistent with the study results reported by Oliveira et al. (2009), who considered higher GSH value was a clear indication of the presence of contaminants challenging thiol-dependent cell defenses. This was mainly because water contaminants were believed to have consumed by the vast majority of the SD rats’ GSH, so that GSH values in S1–S3 and S11–S13 were diminished. Another possibility is that excess pollution impaired the production of SGH. As we know, contaminants can stimulate living organisms to synthesis more GSH to destroy free radicals and ROS in vivo. Metal metabolism involves formation of GSH-metal complexes, from which the metal is further transferred to metallothionein apoproteins (Oliveira et al. 2009).
SOD together with GPx and CAT are considered to be the three major antioxidant enzymes necessary to maintain an appropriate intracellular oxidant/antioxidant balance (Zheng et al. 2013). SOD and GPx activities presented similar patterns at the thirteen sites, and showed lower levels in S1–S3 and S11–S13, where groundwater was heavily polluted. This is a clear indication of the presence of contaminants challenging cell defenses depending on antioxidants. However in S7, the SOD activities were higher, but the GPx activities were lower. An increase in SOD and GPx activities and a simultaneous decrease in CAT activities were also observed. This dichotomy between antioxidant activities was also reported in previous studies (Zheng et al. 2013). It probably results from the excess amount of H2O2 provided by SOD, which in turn leads to increased CAT activities. SOD could catalyze dismutation of superoxide anions generated in peroxisomes and mitochondria to water and hydrogen peroxide (Velisek et al. 2011; Stara et al. 2012). Meanwhile, SOD and GPx might be inactivated by hydrogen peroxide. Similarly, CAT can also be inactivated by many free radicals. The three enzymes are vital first-line defenses against oxidative damage, and they also have a role in balancing mutually compensatory effects.
T-AOC represents the total antioxidant status of SD rats. It should be also noted that the T-AOC values were negatively associated with the pollution levels (NCI) of groundwater (R = −0.443, P > 0.05). The higher T-AOC values appeared in S6, S7, and S9 where groundwater qualities were clean. This means that the rats have good scavenging capacity for reactive oxygen species. It suggests T-AOC has the potential to be used as a sensitive biomarker for the risk assessment of groundwater pollution caused by RE tailings reservoir seepage (see Si et al. 2014).
This study showed that the CA and MN frequency in SD rats fed with different groundwater surrounding the RE tailings were positively correlated with the integrated pollution level (NCI) (CA: R = 0.845, P < 0.01; MN: R = 0.603, P < 0.05). This is in accordance with the results of previous studies such as those of Bufo raddei exposed to petrochemical contaminants (Huang et al. 2007), freshwater fish Channa punctatus exposed to atrazine herbicide (Nwani et al. 2011), and human primary parotid gland cells exposed to nicotine (Ginzkey et al. 2014). Although CA and MN frequency have similar varying tendencies, they also reveal differences between some sites, especially in S6 and S9. Furthermore, the values of MN frequency were significantly lower (P < 0.05) than CA in the heavily polluted sites (S1–3 and S11–13), and higher (P < 0.05) than CA in the lightly polluted sites (except S9). Our results agree with a previous study conducted by Nwani et al. (2011). A possible explanation for this phenomenon could be the influence of combined pollutants on the cell cycle and proliferation. High DNA damage is known to influence apoptosis and the cell cycle. A reduced proliferation rate or a loss of cells could be responsible for not achieving telophase, in which MN are scored (Argentin and Cicchetti 2004). The origin of MN is a chromosome breakage and/or chromosome loss (aneuploidy), followed by complete nuclear division. In contrast, the test detects CA during the metaphase stage of the cell cycle (Ginzkey et al. 2014). Cells containing CAs were expected to survive for a long time with the risk of inducing mutagenesis. Later experiments discovered malformed SD rats in the first filial generation at a heavily polluted site. However, at this point we cannot exclude other reasons, so further experiments are needed to verify such a teratogenic effect.
In order to comprehensively assess the overall stress of groundwater pollution induced in SD rats, the IBRv2 index was applied. This approach provides a simple tool to reveal the health risk state of a studied water environment, combining different biomarker signals (Sanchez et al. 2013). The majority of the available studies using the IBR index provided consistent indications, reflecting the pollution levels measured at different locations, regardless of the variability in the biomarker sets used for the index calculations (Oliveira et al. 2009; Serafim et al. 2012; Zheng et al. 2013; Kim et al. 2014). In this study, the IBRv2 results suggest that S1–S4, and S10–S13 were the more affected sites. The variation tendency of IBRv2 was highly consistent with F value (Nemerow Composite Index, NCI). Meanwhile, the trend of IBRv2 variability was smoother than that for F (NCI) with increasing distance from the tailings. This provides a convenient mechanism for us to estimate the scope of groundwater pollution caused by RE tailings impoundment leakage.
From the above discussion, the maximum diffusion distance of groundwater pollutions may be determined using the minimum inflection point of IBRv2 close to the RE tailing. In the present study, we can roughly estimate the maximum diffusion distance is already reached at S7 (5.7 km) located on the south side of RE tailings. And the later high pollution far away from RE tailings maybe caused by other reasons, and this principle was supported by F (NCI).
Although such trends are emerging, nevertheless, it should be emphasized that our analyses are based on limited data sets. The IBRv2 was more suitable for the qualitative and not for the quantitative evaluation of stress among the contaminated sites (Serafim et al. 2012). Therefore, further analyses on data sets and preferably more comprehensive experimental data sets are needed before an definitive conclusion can be drawn (Sanchez et al. 2013).
Conclusion
This study was the first to use multi-biomarker approach to assess the contamination of RE tailings impoundment leakage on the surrounding groundwater using SD rats. The selected oxidative and anti-oxidative responses biomarkers of SD rats exhibited the greatest biological effects and responded to groundwater contamination in the vicinity of the RE tailings after an exposure of 1 month. The IBRv2 could serve as a sensitive ecotoxicological diagnosis method for groundwater contamination caused by RE tailings impoundment leakage. Use of IBRv2 in this study preliminarily determined the pollution dispersion scope in groundwater on the south side of the RE tailings was over a distance of approximately 5.7 km (S7), and then inferred that the more distant pollution should be investigated for other reasons. The scope measured using IBRv2 basically agrees well with F (NCI) calculated value. Still, it deserves to be mentioned that more comprehensive experimental sites and data sets are necessary to generate more accurate estimates.
Abbreviations
- TH:
-
Total hardness
- CODMn :
-
Permanganate index
- TDS:
-
Total dissolved solids
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- CA:
-
Chromosome aberration
- MN:
-
Micronuclei
- T-AOC:
-
Total antioxidant capacity
- RE:
-
Rare earth
References
Ahmad I, Pacheco M, Santos MA (2004) Enzymatic and nonenzymatic antioxidants as an adaptation to phagocyte-induced damage in Anguilla anguilla L. following in situ harbor water exposure. Ecotoxicol Environ Saf 57:290–302
Argentin G, Cicchetti R (2004) Genotoxic and antiapoptotic effect of nicotine on human gingival fibroblasts. Toxicol Sci 79:75–81
Arzate-Cárdenas MA, Martínez-Jerónimo F (2011) Age-altered susceptibility in hexavalent chromium-exposed Daphnia schodleri (Anomopoda: Daphniidae): integrated biomarker response implementation. Aquat Toxicol 105:528–534
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21(6):1316–1322
Boularbah A, Schwartz C, Bitton G, Morel JL (2006) Heavy metal contamination from mining sites in South Morocco. Use of a biotest to assess metal toxicity of tailings and soils. Chemosphere 63:802–810
Bruce SL, Noller BN, Grigg AH, Mullen BF, Mulligan DR, Ritchie PJ et al (2003) A field study conducted at Kidston Gold Mine, to evaluate the impact of arsenic and zinc from mine tailing to grazing cattle. Toxicol Lett 137:23–34
Chesman BS, O’Hara S, Burt GR, Langston WJ (2007) Hepatic metallothionein and total oxyradical scavenging capacity in Atlantic cod Gadus morhua caged in open sea contamination gradients. Aquat Toxicol 84:310–320
Conesa HM, Faz A, Arnaldos R (2006) Heavy metal accumulation and tolerance in plants from mine tailings of the semiarid Cartagena-La Unión mining district (SE Spain). Sci Total Environ 366:1–11
Da Pelo S, Musu E, Cidu R, Frau F, Lattanzi P (2009) Release of toxic elements from rocks and mine wastes at the Furtei gold mine (Sardinia, Italy). J Geochem Explor 100:142–152
Edinger EN, Azmy K, Diegor W, Siregar PR (2008) Heavy metal contamination from gold mining recorded in Porites lobata skeletons, Buyat-Ratototok district, North Sulawesi, Indonesia. Mar Pollut Bull 56:1553–1569
Ginzkey C, Steussloff G, Koehler C, Burghartz M, Scherzed A, Hackenberg S et al (2014) Nicotine derived genotoxic effects in human primary parotid gland cells as assessed in vitro by comet assay, cytokinesis-block micronucleus test and chromosome aberrations test. Toxicol in Vitro 28:838–846
Gravato C, Teles M, Oliveira M, Santos MA (2006) Oxidative stress, liver biotransformation and genotoxic effects induced by copper in Anguilla Anguilla L.—the influence of pre-exposure to β-naphth of lavone. Chemosphere 65:1821–1830
Huang D, Zhang Y, Wang Y, Xie Z, Ji W (2007) Assessment of the genotoxicity in toad Bufo raddei exposed to petrochemical contaminants in Lanzhou Region, China. Mutat Res Genet Toxicol Environ Mutagen 629:81–88
Huang X, Cao GL, Liu J, Prommer H, Zheng CM (2014) Reactive transport modeling of thorium in a cloud computing environment. J Geochem Explor 144:63–73
Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221
Kim JH, Yeom DH, An KG (2014) A new approach of Integrated Health Responses (IHRs) modeling for ecological risk/health assessments of an urban stream. Chemosphere 108:376–382
Kraus U, Wiegand J (2006) Long-term effects of the Aznalcóllar mine spill-heavy metal content and mobility in soils and sediments of the Guadiamar River Valley (SW Spain). Sci Total Environ 367:855–871
Li RY, Li Q, Chen S, Wu FC, Sun DZ, Liao HQ (2014) Distribution of thorium in soils surrounding the rare-earth tailings reservoir in Baotou, China. J Radioanal Nucl Chem 299:1453–1459
Nemerow NL (1991) Stream, lake, estuary, and ocean pollution, 2nd edn. Van Nostrand Reinhold, New York, pp 1–472
Nwani CD, Nagpure NS, Kumar R, Kushwaha B, Kumar P, Lakra WS (2011) Mutagenic and genotoxic assessment of atrazine-based herbicide to freshwater fish Channa punctatus (Bloch) using micronucleus test and single cell gel electrophoresis. Environ Toxicol Pharmacol 31:314–322
Oliveira M, Maria VL, Ahmad I, Serafim A, Bebianno MJ, Pacheco M et al (2009) Contamination assessment of a coastal lagoon (Ria de Aveiro, Portugal) using defence and damage biochemical indicators in gill of Liza aurata—an integrated biomarker approach. Environ Pollut 157:959–967
Qiu J (2011) Environmental science. China to spend billions cleaning up ground water. Science 334:745–745
Rodríguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q et al (2013) Ground water arsenic contamination throughout China. Science 341:866–868
Sanchez W, Burgeot T, Porcher JM (2013) A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ Sci Pollut Res 20:2721–2725
Serafim A, Company R, Lopes B, Fonseca VF, França S, Vasconcelos RP et al (2012) Application of an integrated biomarker response index (IBR) to assess temporal variation of environmental quality in two Portuguese aquatic systems. Ecol Indic 19:215–225
Si WT, Ji WH, Yang F, Lv Y, Wang YM, Zhang YM (2011) The function of constructed wetland in reducing the risk of heavy metals on human health. Environ Monit Assess 181:531–537
Si WT, Zhang WY, Lv Y, Yang F, Liu JM, Zhang YM (2014) Heavy metal removal in a constructed wetland and benefits for the development of the toad Bufo raddei. Pol J Environ Stud 23:2207–2215
Si WT, Liu JM, Cai L, Jiang HM, Zheng CL, He XY et al (2015) Health risks of metals in contaminated farmland soils and spring wheat irrigated with Yellow River water in Baotou, China. Bull Environ Contam Toxicol 94:214–219
Stara A, Machova J, Velisek J (2012) Effect of chronic exposure to simazine on oxidative stress and antioxidant response in common carp (Cyprinus carpio L). Environ Toxicol Pharmacol 33:334–343
Tu XZ, Liu XM, Wang ZB, Luo P, Zheng RK, Wu ZX (1988) The aquifer water dispersion test and water quality model of Baogang tailing. Eng Investig (Chinese) 4:28–32
Velisek J, Stara A, Kolarova Z, Svobodova Z (2011) Biochemical, physiological and morphological responses in common carp (Cyprinus carpio L.) after long-term exposure to terbutryn in real environmental concentration. Pestic Biochem Physiol 100:305–313
Zheng Q, Feng M, Dai Y (2013) Comparative antioxidant responses in liver of Carassius auratus exposed to phthalates: an integrated biomarker approach. Environ Toxicol Pharmacol 36:741–749
Acknowledgments
The project was supported by the National Natural Science Foundation of China (No. 31460142, 31560598). The Special Foundation for State Science and Technology Basic Work of China (No. 2014FY110800). The Natural Science Fund of Inner Mongolia (No. 2014BS0311). The Special Fund of Public Welfare Industry Scientific Research of China’s Ministry of Environmental Protection (No. 201309005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Xiaoying is a co-first author
Rights and permissions
About this article
Cite this article
Si, W., He, X., Li, A. et al. Application of an integrated biomarker response index to assess ground water contamination in the vicinity of a rare earth mine tailings site. Environ Sci Pollut Res 23, 17345–17356 (2016). https://doi.org/10.1007/s11356-016-6728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6728-8