Abstract
The capacity of Ulva australis Areschoug to tolerate and accumulate selenium (Se) supplied in the form of selenate or selenite was investigated. The macroalga was provided for 3 and 7 days with concentrations of selenate (Na2SeO4) or selenite (Na2SeO3) ranging from 0 to 400 μM. U. australis exhibited the highest ability to accumulate selenium when fed with 100 μM selenate and 200 μM selenite after 7 days, and accumulation values were respectively 25 and 36 ppm Se. At the same concentrations, stimulation of the synthesis of chlorophylls and carotenoids was observed. Elevated doses of selenate or selenite decreased Se accumulation inside algal cells, perhaps through repression of membrane transporters. This effect was more pronounced in thalli cultivated with selenate. There were no morphological and ultrastructural alterations in thalli exposed to Se. However, selenite induced the increase of the oxidized fraction of glutathione (GSSG), perhaps because of its capacity to bind the thiol group of reduced glutathione (GSH). In conclusion, this study highlights the capacity of U. australis to resist to very high concentrations of selenite and selenate, which are normally toxic to other organisms. Also, the lack of bioconcentration in U. australis indicates that this alga does not facilitate delivery of Se in the food chain and remains safe for consumption when it grows in water bodies contaminated with Se. Its potential for the removal of excess Se from water bodies appears limited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is a trace element that is essential for many normal biologic functions in humans, animals, and microorganisms (Rayman 2000; Novoselov et al. 2002). In microalgae, the essential requirement for selenium has been reported at least in 33 species belonging to six phyla, but its biochemical significance is still largely unclear (Araie and Shiraiwa 2009).
Selenium can be beneficial, but it can also be toxic. For humans and animals, the concentration range of Se required as a nutrient and the amount that causes toxicity are quite narrow (50–70 μg Se day−1, USDA 2012; Pilon-Smits and LeDuc 2009; Zhu et al. 2009). Low selenium intake can result in cancer promotion, dysfunction of the immune system, cardiovascular diseases, decreased fertility, and hypothyroidism, whereas excessive dietary selenium can induce adverse cardiometabolic effects as well as a number of naturally occurring chronic Se poisoning symptoms generally known as “selenosis” (Rayman 2012).
Because of anthropogenic activities, Se concentration is increasing in many areas of North America, Australia, New Zealand, and China, thus posing a risk to human and animal health. In addition, while in aquatic environments Se level is known to be usually very low (10−8–10−10 mol L−1) (Robberecht and van Grieken 1982), there are a number of water bodies in which Se levels are becoming elevated and Se impact on aquatic population and whole ecosystem could occur (Hartikainen 2005; Chapman et al. 2010).
In aquatic ecosystems, Se is present in two main oxidation states, Se(IV) selenite and Se(VI) selenate, which dominate the inorganic dissolved fraction of Se under oxidizing conditions (Cutter and Brulan 1984; Plant et al. 2004). Selenate is highly soluble and thus more bioavailable than selenite to aquatic organisms, suggesting that selenate may be the main dissolved species (Plant et al. 2004; Chapman et al. 2010). However, selenate and selenite can interchange depending on water chemical and physical characteristics.
Inorganic Se uptake by microalgae is a key process in understanding how Se affects aquatic ecosystems (Hamilton 2004). The toxicity of Se on marine algae strongly depends upon the algal species (Abdel-Hamid and Skulberg 2006; Wheeler et al. 1982), Se concentration, and oxidation state (Pastierova et al. 2009; Umisová et al. 2009). Selenite, for instance, is reported to be less toxic than selenate in many microalgae (Wheeler et al. 1982).
The capacity to take up selenate and selenite by algae is known to vary as a function of pH over the range 5 to 9 (Tuzen and Sari 2010; Riedel and Sanders 1996). In Chlamydomonas reinhardtii, the maximum uptake of selenate occurred at pH 8 whereas selenite absorption significantly increased at the lower pH values (Riedel and Sanders 1996). In addition, Se accumulation in algae is influenced by certain macronutrients, especially sulfur (S) and phosphorus (P) (Lee and Wang 2001). Sulfur in the form of sulfate, in particular, is a well-known antagonist of selenate for the active transport inside cells mediated by sulfate permeases (Simmons and Emery 2011; Fournier et al. 2010). On the contrary, selenite transport activity was not inhibited by sulfate ions in the microalga Emiliania huxleyi (Araie and Shiraiwa 2009), and two mechanisms for selenite uptake have been proposed in this species: an ATP-dependent active transport process with a high affinity for selenite and a passive transport process with a low affinity for selenite (Araie et al. 2011).
Uptake studies indicate that both selenite and selenate can be incorporated into algal cells (Schiavon et al. 2012a; Wheeler et al. 1982) and affect growth in a dose-dependent manner (Umisová et al. 2009). Selenium at low dosages can stimulate the growth of some algae, such as the diatom Thalassiosira pseudonana (Price et al. 1987) and Chysochromulina breviturrita belonging to Haptophyceae (Wehr and Brown 1985). However, Se at high concentration becomes toxic to algae causing inhibition of growth, alterations of cell ultrastructure, and decrease of storage products (Fournier et al. 2010; Umisová et al. 2009; Pelah and Cohen 2005; Wheeler et al. 1982). Furthermore, since Se is metabolized through the S assimilation pathway, its accumulation at high levels in algal cells may interfere with the synthesis of several S-containing compounds (Umisová et al. 2009).
In the unicellular green alga C. reinhardtii, both selenate (Geoffroy et al. 2007) and selenite (Morlon et al. 2006) caused ultrastructural damages to chloroplasts resulting in impaired photosynthesis. On the contrary, Chlorella sp. could tolerate selenite up to a concentration of 100 mg L−1 (Pelah and Cohen 2005) and Chlorella vulgaris was shown to produce higher amounts of stress-related S compounds (phytochelatins and glutathione) in response to toxic selenate concentrations (Simmons and Emery 2011).
While the effects of Se in microalgae are well documented, information from the literature concerning the capacity of macroalgae to accumulate different inorganic forms of Se is scarce. In a recent investigation, the capacity of Ulva sp. to accumulate Se was reported to be strictly dependent on selenate concentration in the growth medium (Schiavon et al. 2012a). The significant increase of superoxide dismutase (SOD) and catalase (CAT) activities as well as the content of antioxidant molecules such as phenolic compounds, even at low external selenate concentration, suggested the existence of multiple mechanisms in Ulva sp. to cope with Se-induced oxidative stress (Schiavon et al. 2012a).
The aim of the current investigation was to estimate the capacity to accumulate and tolerate Se, either in the selenate or selenite form, by a green laminar seaweed Ulva australis naturally growing in the Venice Lagoon (Italy). Additionally, S content in the macroalga was determined as a potential factor interacting with Se accumulation in U. australis. The effects of Se accumulation on algal physiology and structure were assayed by measuring the content of photosynthetic pigments as well as by evaluating ultrastructural and morphological changes in the macroalga. Furthermore, the amount of glutathione (total and oxidized) was measured as Se is known to induce reactive oxygen species (ROS) in both plants and Ulva spp., an event that may partially cause an imbalance in the levels of glutathione (GSH) crucial for Se assimilation.
Materials and methods
Experimental conditions
Thalli of U. australis collected on March 2013 from the Venice Lagoon (Italy) were thoroughly rinsed in seawater and cleaned by a soft brush to eliminate any epiphytes present on their surface. Subsequently, thalli were cut in 15-mm-diameter disks, placed in flasks containing 1 L of filtered seawater (Millipore GF/C, 1–2 μm pore size) and kept for 3 days to acclimate inside a climate chamber with a 12-h light/12-h dark cycle, at a temperature of 20 °C and a photon flux density of 80 μmol m−2 s−1. The initial pH of the seawater in the flasks was 7.2.
After acclimation, Se either in the form of sodium selenate (Na2SeO4, Sigma-Aldrich, Steinheim, Germany) or sodium selenite (Na2SeO3, Sigma-Aldrich, Steinheim, Germany) was added to the seawater at the following concentrations: 0 (control) 50, 100, 200, and 400 μM. This wide range of selenate and selenite concentrations was used to determine the relationship between Se dosage and physiological and ultrastructural changes.
Thalli were sampled at the beginning of the experiment and at the 3rd and 7th day of treatment. Before analyses, thalli were carefully washed with distilled water. For each tested Se concentration, five replicates were performed, each one consisting of one flask with 100 disks.
Elemental analysis of Se and S
Samples from thalli (100 mg) were dried for 48 h at 80 °C and then digested in nitric acid as described by Zarcinas et al. (1987). Inductively coupled plasma atomic emission spectroscopy (ICP-AES) was used as described by Fassel (1978) to determine each digest’s Se and S concentrations. The values obtained were expressed in milligrams of the element per kilogram dry weight.
Quantification of pigments
Chlorophyll and carotenoids were extracted from control and selenate/selenite-treated thalli with N,N-dimethylformamide (Moran and Porath 1980). The extracts were kept in the dark for 1 day at 4 °C (Wellburn 1993) and then analyzed spectrophotometrically (124, PerkinElmer, Norwalk, CT, USA) at 664 for chlorophyll a, 647 for chlorophyll b and 480 for carotenoids. The concentrations of chlorophylls and carotenoids were calculated using the extinction coefficients according to Inskeep and Bloom (1985) and expressed in milligrams per gram fresh weight.
Light and electron microscopy
Samples from control and selenate/selenite-treated thalli were fixed overnight at 4 °C in 3 % glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 6.9) and post-fixed at 4 °C for 2 h in 1 % osmium tetroxide in the same buffer. The specimens were dehydrated in a graded series of ethyl alcohol and propylene oxide and embedded in araldite. Sections were cut using an ultramicrotome (Ultracut S, Reichert-Jung, Vienna, Austria). For light microscopy, thin sections (1 μm) stained with toluidine blue (1 % basic toluidine and 1 % Na tetraborate, 1:1 v/v) were observed by a DMR 5000 Leica (Sweden) microscope, equipped with a digital image acquisition system. For transmission electron microscopy, ultrathin sections (600 Å) stained with uranyl acetate and lead citrate were observed with a transmission electron microscope (TEM 300, Hitachi, Tokyo, Japan) operating at 75 kV.
Quantification of total GSH and oxidized glutathione
For the quantification of GSH and oxidized glutathione (GSSG), three samples consisting of multiple disks each were washed with PBS and deproteinized with 1.5 ml of 6 % meta-phosphoric acid. After two cycles of freezing and thawing in liquid nitrogen, the samples were centrifuged at 15,800×g for 20 min and the supernatant was neutralized with 15 % Na3PO4. The reaction for total glutathione determination was performed spectrophotometrically at 412 nm (Tietze 1969). Furthermore, for each sample, aliquots of 400 μL were treated with 8 μL of 2-vinylpyridine, in order to derivatize reduced glutathione, for 40 min. Oxidized glutathione was then estimated according to Anderson (1985).
Statistical analysis
Two-way analysis of variance (ANOVA) was applied to the data. Statistical analysis was performed using SPSS 10.0 (Norusis 1993). All probabilities were two-tailed. Data were checked for normality and homogeneity of variance (Levene’s test) and are presented as mean ± SD of five replicates. Differences between means were evaluated for significance by using Duncan’s multiple range test (DMRT). Statistically significant differences (P < 0.05) are reported in the tables and figures.
Results
Determination of selenium and sulfur in U. australis
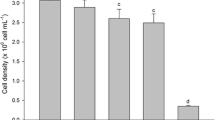
Selenium accumulation in thalli of U. australis strongly correlated with the culture medium concentration of selenate up to 100 μM (y = 7.76x − 4.27, R 2 = 0.99 for the 3-day period and y = 10.71x − 6.38, R 2 = 0.97 for the 7-day period) (Fig. 1a). Supplying the seaweed with higher selenate dosages (200 or 400 μM) resulted in a marked decrease of Se accumulation (roughly −63 and −71 % than values measured at 100 μM selenate after 3 and 7 days of Se treatment, respectively). Se accumulation was also time-dependent when selenate was furnished at 50 and 100 μM, as values increased with the duration of the experiment. At higher doses of selenate, no differences in Se accumulation were evident between thalli exposed to the short (3 days) and long (7 days) periods to Se.
A different pattern of Se accumulation was observed in thalli of U. australis treated with selenite (Fig. 1b). In this case, the level of Se in the seaweed increased almost linearly with increasing selenite dosages (y = 5.79x − 2.74, R 2 = 0.95) during the 3-day period. After 7 days, the linear correlation between selenite dose and Se accumulation in thalli was evident only in the range from 0 to 200 μM Se (y = 10.19x − 5.40, R 2 = 0.96). Indeed, the concentration of Se in thalli treated with 400 μM selenite was about 60 % lower than that measured in the seaweed cultivated with 200 μM. Generally, the content of Se increased in thalli with the duration of the experiments, even though Se accumulated more in thalli grown for 3 days with 400 μM selenite than in thalli harvested after 7 days and exposed to the same selenite concentration.
With respect to S accumulation, while no differences were observed between thalli of the control condition and those exposed to selenate for 3 days, an increase of S levels was evidenced in the seaweed cultivated with 50 and 100 μM selenate for 7 days (Fig. 2a).
The enhancement of S accumulation in U. australis from 3 to 7 days of cultivation was also observed in thalli exposed to selenite, although no differences were evident between thalli of the various experimental conditions (Fig. 2b). Interestingly, the level of S was lower than in thalli cultivated in the presence of selenate, regardless of the Se concentration in the culture medium and the duration of the experiment.
Effect of selenate and selenite on chlorophyll and carotenoid content
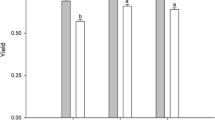
The content of chlorophylls (a + b) and carotenoids in thalli of U. australis cultivated for 3 days in the presence of 50, 200, or 400 μM selenate was not significantly different from that measured in thalli cultivated without Se (Fig. 3a). However, an increase of these pigments was observed in thalli exposed to 100 μM selenate. A similar trend of chlorophyll and carotenoid contents was observed in thalli after 7 days of cultivation with selenate (Fig. 3b).
Effect of different selenate and selenite concentrations on the level of chlorophyll (Chl) (a, c, respectively) and carotenoids (Car) (b, d, respectively). The measurements were performed after 3 and 7 days of Ulva australis cultivation in the presence of Se. Different letters indicate significant differences between treatments (P < 0.05, ±SD)
When thalli were grown for 3 days in the presence of selenite, values of chlorophylls (a + b) (Fig. 3c) and carotenoids (Fig. 3d) were generally comparable to those determined in the controls, although a weak increase in the content of these pigments was detected in thalli treated with 200 μM selenite. After 7 days, the content of chlorophylls (a + b) and carotenoids in the seaweed treated with the lowest dosages of selenite (50 and 100 μM) was similar to that measured in the control thalli, whereas higher selenite concentrations (200 and 400 μM) caused an evident increase of these pigments.
Effects of selenate and selenite on thallus morphology and ultrastructure
After 3 days of algal cultivation, the presence of either selenate (Fig. 4(a–e)) or selenite (Fig. 5(a–e)) did not affect the morphology of thalli, as they exhibited similar thickness and bilayered morphology to the control thalli. Similarly, treating the seaweed with either selenate (Fig. 4(a–e)) or selenite (Fig. 5(a–e)) for 7 days did not cause any alteration of the thalli morphology compared to the controls.
With respect to cell ultrastructure, no significant alterations were observed between control thalli (Fig. 6a) and those treated with selenate (Fig. 6b) or selenite (Fig. 6c) for 3 days. Cells of U. australis thalli incubated with Se showed a normal organization, with as only difference that the plastids were characterized by an increase in starch granules.
Transmission electron microscope details of the cell of control (a) and 7-day selenate-treated (b) and 7-day selenite-treated (c) thalli of Ulva australis. The ultrastructure of thalli treated with 100 μM selenite or selenate was reported as representative example. Note the chloroplasts with starch (arrows) in the stroma. No evident alterations of ultrastructure are visible in cells. Bar = 1 μm (a); bar = 1 μm (b); bar = 2 μm (c)
Effects of selenate and selenite on the synthesis of GSH and GSSG
Selenate supply did not significantly affect the content of total GSH in Ulva thalli after 3 days of treatment but stimulated GSH synthesis after a 7-day period (Fig. 7a). The fraction percentage of the total glutathione that was present in the oxidized form (GSSG) was slightly reduced in the presence of 400 μM selenate after 3 days as well as at selenate concentrations ranging from 50 to 200 μM after 7 days (Fig. 7b).
Effect of different selenate concentrations on total glutathione (a) and oxidized glutathione (b). The measurements were performed in thalli of Ulva australis after 3 and 7 days of cultivation in the presence of sodium selenate. Effect of different selenite concentrations on total glutathione (c) and oxidized glutathione (d). The measurements were performed in thalli of U. australis after 3 and 7 days of cultivation in the presence of sodium selenite
The exposure of the seaweed to increasing selenite concentrations resulted in a pronounced increase in total glutathione level (Fig. 7c). This effect was most evident after 3 days of selenite treatment. In addition, supply with selenite resulted in an increased fraction of the total glutathione being present in the oxidized form; this effect depended on the Se concentration of the treatment (Fig. 7d) and was especially pronounced in thalli grown in the presence of 400 μM selenite for 7 days.
Discussion
The essentiality of Se for macroalgae has not been established yet, even though many phytoplankton species require this element at low concentration (Araie and Shiraiwa 2009). In a previous study, Ulva spp. growing in the Venice Lagoon were shown to accumulate and tolerate Se when furnished as selenate up to 100 μM (Schiavon et al. 2012a). Selenate supply to these algae led to accumulation of ROS, as well as upregulation of enzymatic antioxidant defenses.
The accumulation of Se in U. australis thalli varied depending on the concentration and the inorganic form of Se provided in the growth medium. Interestingly, selenate led to dose-dependent accumulation of Se in the seaweed until 100 μM, which was then repressed at 200 and 400 μM, regardless of the duration of Se exposure. A possible explanation is that U. australis faced selenate concentrations as high as 200 and 400 μM through a mechanism of Se exclusion, possibly involving the downregulation of at least one sulfate transporter. In plants and microalgae, for comparison, selenate and sulfate can be transported over membranes by the activity of sulfate transporters, which display different affinities for selenate or sulfate depending on the relative concentration of the two anions in the growth medium (Riedel and Sanders 1996; Terry et al. 2000; Neumann et al. 2003; White et al. 2004; Umisová et al. 2009; Fournier et al. 2010; Schiavon et al. 2012b, 2015). Repression of Se accumulation was also evident in U. australis thalli grown in the presence of 400 μM selenite for 7 days. The decrease of selenite absorption by U. australis could be due to the inhibition of the expression or activity of one or more transporters that mediate selenite influx, perhaps phosphate transporters (Zhang et al. 2014), as a mechanism of resistance to selenite toxicity, according to what was assumed in selenite-resistant strains of the microalga Scenedesmus communis E. Hegewald (ex Scenedesmus quadricauda; Umisová et al. 2009).
Variations of Se accumulation in the seaweed after 3 days of treatment with either selenate or selenite were not tightly associated with changes in S concentration. However, a very slight increase in S levels were observed in thalli grown with selenate at 50 and 100 μM on the 7th day, indicating a weak positive relationship between selenate and S uptake. In the case of U. australis supplied with selenite, a less clear association between Se and S accumulation was evident. There was no substantial variation in S concentration among the thalli exposed to different doses of selenite after 7 days, although a weak increase in S level occurred between 3 and 7 days of treatment.
With respect to the potential of U. australis to accumulate Se, values were lower than those reported for microalgae, but in line with those previously reported by Schiavon et al. (2012a). Microalgae generally exhibit a stronger capacity for Se bioaccumulation compared to macroalgae, and as a result, their cellular levels of Se are usually high enough to induce toxicity (Tuner 2013; Fournier et al. 2010; Umisová et al. 2009; Geoffroy et al. 2007; Reunova et al. 2007). For example, S. communis E. Hegewald (ex S. quadricauda) cells accumulated 3730 mg Se kg−1 dry weight when exposed to 50 mg L−1 (about 600 μM) Se and cultivated in the presence of 40 mM S (Umisová et al. 2009).
The high S concentration measured in the seawater derived from the Venice Lagoon (10–20 mM) (Schiavon et al. 2012a) that was used as a culture medium could also affect the capacity of Ulva spp. to accumulate Se. Indeed, S in the form of sulfate may reduce selenate absorption by algae because of competition for membrane transporters (Fournier et al. 2010). In C. vulgaris and C. reinhardtii, for instance, the uptake rate of selenate was low in the presence of high sulfate concentrations in the growth medium (Fournier et al. 2010; Riedel and Sanders 1996; Shrift 1954). Similarly, high phosphate in the seawater could inhibit selenite uptake (Zhang et al. 2014). In the Venice Lagoon, the average phosphate concentration reported in the seawater was 0.79 μM (Scarponi et al. 1998).
Interestingly, the trend of Se concentration in U. australis thalli supplied with selenate highly correlated with the amounts of chlorophyll and carotenoids. Similar to Se, the content of these pigments significantly increased in thalli grown with 50 and 100 μM, in agreement with a previous study (Schiavon et al. 2012a). The observation that elevate Se concentration in thalli did not injure chlorophyll levels indicates that the synthesis of these pigments is not a target of Se toxicity in U. australis. With respect to carotenoids, their increase in response to high Se accumulation could represent one mechanism to withstand selenate toxicity because of their protective effects on membrane integrity. Indeed, carotenoids can protect chloroplast membranes from damage caused by ROS produced under stress (Young 1991; Havaux 1998).
When thalli were fed with high selenite concentrations (200 and 400 μM), the levels of chlorophylls and carotenoids increased. A similar trend was observed for glutathione (GSH), which is known to play a pivotal role in S/Se assimilation in plants via its role in selenite reduction and inhibition of sulfur transport and assimilation (Dixon et al. 1998; Anderson and McMahon 2001; Schiavon et al. 2012b). GSH can directly scavenge oxidants and free radicals and indirectly remove H2O2, as a component of glutathione systems (Noctor et al. 2002; Hernández et al. 2015). Selenite at high concentrations resulted in a strong oxidation of glutathione, up to 60 %. On the contrary, the fraction of oxidized glutathione was unaffected in the presence of selenate. The higher levels of total and oxidized GSH measured in U. australis thalli treated with selenite may be indicative of the lower capacity of the seaweed to tolerate selenite than selenate when supplied at high concentration. It is also possible that selenite, but not selenate, can be bound by the thiol group of reduced GSH, thereby making it undetectable in the GSH assay and leading to a higher fraction of oxidized GSH. Similarly, arsenite (and not arsenate) is known to be bound by reduced GSH (Mizumura et al. 2009). Furthermore, the progressive degradation and oxidation of the glutathione pool is known to occur upon strong stress impacts (Tausz et al. 2004). However, the low levels of Se in the seaweed and the lack of ultrastructural alterations appear to discard the hypothesis that Se induced a significant stress condition to U. australis.
Both selenate and selenite did not affect the morphology of U. australis thalli, even at the highest tested concentrations. This is similar to results obtained for the microalga Arthrospira platensis Gomont (ex Spirulina platensis) treated with selenate (Belokobylsky et al. 2004). While the overall morphology was unaffected, Se added as either selenate or selenite did affect the ultrastructure of the chloroplasts, which contained more starch granules. The plastids are recognized as an important target of Se toxicity (Geoffroy et al. 2007; Vítová et al. 2011). The results on algal ultrastructure obtained from different microalgae showed that both selenate and selenite were able to impair the thylakoid membranes, causing an overproduction of starch granules that became larger as a consequence (Vítová et al. 2011). The greater amounts of starch in the chloroplasts of U. australis thalli treated with either selenite or selenate compared to the organelles of control thalli may represent a general response of these organelles to Se, as observed for a number of elicitors.
Conclusions
The macroalga U. australis accumulated more Se in the presence of selenite when the anion was supplied at a concentration higher than 200 μM. Elevated doses of selenite or selenate repressed Se influx into algal cells, perhaps through inhibition of gene expression and/or activity of one or more membrane transporters. This effect of repression was more evident in thalli grown with selenate over a short time period but comparable between selenate- and selenite-treated thalli in the longer period. The increased oxidation of the GSH pool in the seaweed fed with selenite could reflect binding of selenite to GSH, thereby blocking the thiol group. Indeed, considering the absence of algal morphological and ultrastructural changes and the low Se accumulation in thalli, a significant GSH production triggered by elevated Se toxicity should not be expected.
We conclude that U. australis is able to resist concentrations of selenite and selenate that are commonly toxic to organisms. The resistance mechanism may include exclusion of Se from accumulation inside thalli and imply a role for GSH. This study opens the way for further research on the effects of Se on macroalgae and has relevance for Se movement in the environment. The lack of bioconcentration in U. australis indicates that its potential for the removal of excess Se from water bodies is limited and this species does not facilitate movement of Se in the food chain. Thus, U. australis could be safe for consumption when water bodies get contaminated with low levels of Se. High levels of Se as those used in this study are indeed not commonly found in waters.
References
Abdel-Hamid M, Skulberg OM (2006) Effect of selenium on the growth of some selected green and blue-green algae. Lakes Res Manag 1:205–211. doi:10.1111/j.1440-1770.1995.tb00025.x
Anderson M (1985) Determination of glutathione and glutathione disulfide in biological samples. Method Enzymol 113:548–555. doi:10.1016/s0076-6879(85)13073-9
Anderson JW, McMahon PJ (2001) The role of glutathione in the uptake and metabolism of sulfur and selenium. In: Grill D, Tausz M, De Kok LJ (eds) Significance of glutathione to plant adaptation to the environment. Plant ecophysiology. Springer, Netherlands, pp 57–99
Araie H, Shiraiwa Y (2009) Selenium utilization strategy by microalgae. Molecules 14(12):4880–4891. doi:10.3390/molecules14124880
Araie H, Sakamoto K, Suzuki I, Shiraiwa Y (2011) Characterization of the selenite uptake mechanism in the coccolithophore Emiliania huxleyi (Haptophyta). Plant Cell Physiol 52:1204–1210. doi:10.1093/pcp/pcr070
Belokobylsky AI, Mosulishvili LM, Frontasyeva M, Kirkesali EI, Gundorina SF, Aksenova NG (2004) Accumulation of selenium and chromium in the growth dynamics of Spirulina platensis. J Radioanal Nucl Chem 259(1):65–68. doi:10.1023/B:JRNC.0000015807.53132.c0
Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw DP (2010) Ecological assessment of selenium in the aquatic environment. CRC, Boca Raton
Cutter GA, Brulan KW (1984) The marine biogeochemistry of selenium: a re-evaluation. Limnol Oceanogr 29:1179–1192
Dixon DP, Cummins L, Cole DJ, Edwards R (1998) Glutathione-mediated detoxification systems in plants. Curr Opin Plant Biol 1(3):258–266. doi:10.1016/S1369-5266(98)80114-3
Fassel VA (1978) Quantitative elemental analyses by plasma emission spectroscopy. Science 202:183–191. doi:10.1126/science.202.4364.183
Fournier E, Adam-Guillermin C, Potin-Gautier M, Pannier F (2010) Selenate bioaccumulation and toxicity in Chlamydomonas reinhardtii: influence of ambient sulphate ion concentration. Aquat Toxicol 97:51–57. doi:10.1016/j.aquatox.2009.12.003
Geoffroy L, Gilbin R, Simon O, Floriani M, Adam C, Pradines C, Cournac L, Garnier-Laplace J (2007) Effect of selenate on growth and photosynthesis of Chlamydomonas reinhardtii. Aquat Toxicol 83(2):149–158. doi:10.1016/j.aquatox.2007.04.001
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Tot Envir 326:1–31. doi:10.1016/j.scitotenv.2004.01.019
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18:309–318. doi:10.1016/j.jtemb.2005.02.009
Havaux M (1998) Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 3(4):147–151. doi:10.1016/S1360-1385(98)01200-X
Hernández LE, Sobrino-Plata J, Montero-Palmero MB, Carrasco-Gil S, Flores-Cáceres ML, Ortega-Villasante C, Escobar C (2015) Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J Exp Bot 66(10):2901–2911. doi:10.1093/jxb/erv063
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll-a and chlorophyll-b in N,N-dimethylformamide and 90-percent acetone. Plant Physiol 77(2):483–485. doi:10.1104/pp.77.2.483
Lee WY, Wang WX (2001) Metal accumulation in the green macroalga Ulva fasciata: effects of nitrate, ammonium and phosphate. Sci Total Environ 278(1-3):11–22. doi:10.1016/S0048-9697(00)00884-6
Mizumura A, Watanabe T, Kobayashi Y, Hirano S (2009) Identification of arsenite-and arsenic diglutathione-binding proteins in human hepatocarcinoma cells. Toxicol Appl Pharmacol 242(2):119–125. doi:10.1016/j.taap.2009.10.013
Moran R, Porath D (1980) Chlorophyll determination in intact tissue using N,N-dimethylformamide. Plant Physiol 77:483–485. doi:10.1104/pp.65.3.478
Morlon H, Fortin C, Adam C, Garnier-Laplace J (2006) Selenite transport and its inhibition in the unicellular green alga Chlamydomonas reinhardtii. Environ Toxicol Chem 25:1408–1417. doi:10.1897/2512039.1
Neumann PM, de Souza MP, Pickering IJ, Terry N (2003) Rapid microalgal metabolism of selenate to volatile dimethylselenite. Plant Cell Environ 26:897–905. doi:10.1046/j.1365-3040.2003.01022.x
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53(372):1283–1304. doi:10.1093/jexbot/53.372.1283
Norusis MJ (1993) SPSS for Windows: base system user’s guide, release 6.0. SPSS Inc, Chicago
Novoselov S, Rao M, Onoshko N, Zhi H, Kryukov G, Xiang Y, Weeks D, Hatfield D, Gladyshev V (2002) Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. EMBO J 21:3681–3693. doi:10.1093/emboj/cdf372
Pastierova J, Kramarova Z, Molnarova M, Fargasova A (2009) Comparison of the sensitivity of four freshwater macroalgae to selenate and selenite. Fresen Envir Bull 18(11):2029–2033
Pelah D, Cohen E (2005) Cellular response of Chlorella zofingiensis to exogenous selenium. Plant Growth Regul 45:225–232. doi:10.1007/s10725-005-3230-6
Pilon-Smits EAH, LeDuc DL (2009) Phytoremediation of selenium using transgenic plants. Curr Opin Biotech 20(2):207–212. doi:10.1016/j.copbio.2009.02.001
Plant JA, Kinniburgh DG, Smedley PL, Fordyce FM, Klinck BA (2004) Arsenic and selenium. In: Holland HD, Turekian KK (eds) Treatise on geochemistry, vol 9. Environ Geochem, Elsevier, Amsterdam, pp 17–66
Price NM, Thompson PA, Harrison PJ (1987) Selenium: an essential element for the growth of the coastal marine diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol 23:1–9. doi:10.1111/j.1529-8817.1987.tb04421.x
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241. doi:10.1016/S0140-6736(00)02490-9
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268. doi:10.1016/S0140-6736(11)61452-9
Reunova YA, Aizdaicher NA, Khristoforova NK, Reunova AA (2007) Effects of selenium on growth and ultrastructure of the marine unicellular alga Dunaliella salina (Chlorophyta). Russian J of Mar Biol 33(2):125–132. doi:10.1134/S1063074007020071
Riedel GF, Sanders GJ (1996) The influence of pH and media composition on the uptake of inorganic selenium by Chlamydomonas reinhardtii. Environ Toxicol Chem 15:1577–1583. doi:10.1002/etc.5620150922
Robberecht H, van Grieken RV (1982) Selenium in environmental waters: determination, speciation and concentration levels. Talanta 29(10):823–844. doi:10.1016/0039-9140(82)80252-X
Scarponi G, Turetta C, Capodaglio G, Toscano G, Barbante C, Moret I, Cescon P (1998) Chemometric studies in the Lagoon of Venice, Italy. 1. The environmental quality of water and sediment matrices. J Chem Inf Comput Sci 8:552–562. doi:10.1021/ci980018h
Schiavon M, Moro I, Pilon-Smits EAH, Matozzo V, Malagoli M, Dalla Vecchia F (2012a) Accumulation of selenium in Ulva sp. and effects on morphology, ultrastructure and antioxidant enzymes and metabolites. 122–123:222–231. doi:10.1016/j.aquatox.2012.06.014
Schiavon M, Pittarello M, Pilon-Smits EH, Wirtz M, Hell R, Malagoli M (2012b) Selenate and molybdate alter sulfate transport and assimilation in Brassica juncea L. Czern: implications for phytoremediation. Environ Exp Bot 75:41–51. doi:10.1016/j.envexpbot.2011.08.016
Schiavon M, Pilon M, Malagoli M, Pilon-Smits EAH (2015) Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation—a comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front Plant Sci 6:2. doi:10.3389/fpls.2015.00002
Shrift A (1954) Sulfur-selenium antagonism. Antimetabolite action of selenate on the growth of Chlorella vulgaris. Am J Botan 41:223–230
Simmons DBD, Emery RJN (2011) Phytochelatin induction by selenate in Chlorella vulgaris and regulation of effect by sulfate levels. Environ Toxicol Chem 30(2):469–476. doi:10.1002/etc.392
Tausz M, Sircelj H, Grill D (2004) The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J Exp Bot 55:1955–1962. doi:10.1093/jxb/erh19
Terry N, Zayed AM, de Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432. doi:10.1146/annurev.arplant.51.1.401
Tietze F (1969) Enzymatic method for quantitative determination of nanograms amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522. doi:10.1016/0003-2697(69)90064-5
Tuner A (2013) Selenium in sediments and biota from estuaries of southwest England. Mar Pollut Bull 73(1):192–198. doi:10.1016/j.marpolbul.2013.05.023
Tuzen M, Sari A (2010) Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: equilibrium, thermodynamic and kinetic studies. Chem Eng J 158:200–206. doi:10.1016/j.cej.2009.12.041
U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, release 25. Nutrient Data Laboratory Home Page, 2012. http://warp.nal.usda.gov/fnic/etextA/000105.html
Umisová D, Vìtovà M, Douskovà I, Bisovà K, Hlavovà M, Cizkovà M, Machàt J, Doucha J, Zachleder V (2009) Bioaccumulation and toxicity of selenium compounds in the green alga Scenedesmus quadricauda. BMC Plant Biol 9:58–74. doi:10.1186/1471-2229-9-58
Vítová M, Bišová K, Hlavová M, Zachleder V, Rucki M, Čížková M (2011) Glutathione peroxidase activity in the selenium-treated alga Scenedesmus quadricauda. Aquatic Toxicol 102:87–94. doi:10.1016/j.aquatox.2011.01.003
Wehr JD, Brown LM (1985) Selenium requirement of a bloom-forming planktonic alga from softwater and acidified lakes. Can J Fish Aquat Sci 42:1783–1788. doi:10.1139/f85-223
Wellburn AR (1993) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. doi:10.1016/S0176-1617(11)81192-2
Wheeler AE, Zingaro RA, Irgolic K (1982) The effects of selenate, selenite and sulphate on the growth of six unicellular marine algae. J Exp Mar Biol Ecol 57:181–194. doi:10.1016/0022-0981(82)90191-5
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937. doi:10.1093/jxb/erh192
Young AJ (1991) The photoprotective role of carotenoids in higher plants. Physiol Plant 83(4):702–708. doi:10.1111/j.1399-3054.1991.tb02490.x
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Comm Soil Sci Plan Anal 18:131–146. doi:10.1080/00103628709367806
Zhang L, Hu B, Li W, Che R, Deng K, Li H, Yu F, Ling H, Li Y, Chu C (2014) OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol 201(4):1183–1191. doi:10.1111/nph.12596
Zhu Y-G, Pilon-Smits EAH, Zhao F-J, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trend Plant Sci 14(8):436–442. doi:10.1016/j.tplants.2009.06.006
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Schiavon, M., Pilon-Smits, E.A.H., Citta, A. et al. Comparative effects of selenate and selenite on selenium accumulation, morphophysiology, and glutathione synthesis in Ulva australis . Environ Sci Pollut Res 23, 15023–15032 (2016). https://doi.org/10.1007/s11356-016-6649-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6649-6