Abstract
Bauxite residue is a hazardous solid waste produced during the production of alumina. Its high alkalinity is a potential threat to the environment which may disrupt the surrounding ecological balance of its disposal areas. China is one of the major global producers of alumina and bauxite residue, but differences in alkalinity and associated chemistry exist between residues from China and those from other countries. A detailed understanding of the chemistry of bauxite residue remains the key to improving its management, both in terms of minimizing environmental impacts and reducing its alkaline properties. The nature of bauxite residue and the chemistry required for its transformation are still poorly understood. This review focuses on various transformation processes generated from the Bayer process, sintering process, and combined Bayer-sintering process in China. Problems associated with transformation mechanisms, technical methods, and relative merits of these technologies are reviewed, while current knowledge gaps and research priorities are recommended. Future research should focus on transformation chemistry and its associated mechanisms and for the development of a clear and economic process to reduce alkalinity and soda in bauxite residue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nonferrous metals industry of China has been developing rapidly under the guidance of the scientific development concept policy (Xu et al. 2012). However, these rapid developments have caused numerous environment issues (Clark et al. 2015; Wu et al. 2015) especially as China is the largest producer of alumina in the world (Si et al. 2013; Liu et al. 2014b). Bauxite residue (red mud) is an alkaline solid generated during the process of alumina production. The accumulative inventory of bauxite residue reached an estimated 0.6 billion tons with an annual growth of more than 70 million tons in China, and currently, almost all bauxite residue is stored on land (Xue et al. 2016). Bauxites alkaline nature is detrimental to the environment, and the dust formed from the surface of disposal areas is harmful to the surrounding biology (Zhang et al. 2008; Johnston et al. 2010; Banning et al. 2014; Lockwood et al. 2015). Furthermore, due to breaching of the dams containing the residue, leaching of alkaline waste from the slurry is a potential problem (Zhou and Song 2004; Pulford et al. 2012; Samal et al. 2013; Zhu et al. 2016a). Nevertheless, some innovative technical options have been developed for the utilization of bauxite residue, but have yet to be applied to industrial processes (Tuazon and Corder 2008; Gräfe and Klauber 2011; Klauber et al. 2011; Kirwan et al. 2013; Pontikes and Angelopoulos 2013). Additionally, with increasing alumina production and the gradually decreasing grade of bauxite, annual production of bauxite residue appears to have increased, which makes its management more prominent in China.
These reasons highlight the need for effective alkalinity transformation of bauxite residue and to manage the environmental impacts of alumina production and contribute to industry sustainability. In recent decades, significant developments on alkalinity transformation of bauxite residue produced from the Bayer, sintering, and Bayer-sintering processes have been made in China (Liu et al. 2014a, b; Zhang 2014b; Pan et al. 2015; Zhu et al. 2016a, b). These processes can be classified in accordance with the conversion medium including alkaline solid transformation, waste acid synergy, acid gas sequestration, chloride salt neutralization, and bio-driven amelioration. Their current status, transformation mechanisms, and potential issues are presented in this review. Furthermore, existing knowledge gaps and research priorities are discussed and recommended.
Resources and distribution of bauxite
Reserves and distribution of bauxite
Bauxite is the main mineral resource for alumina production, and 98 % of alumina is obtained from bauxite in the world. It is composed of one or more aluminum hydroxide minerals including gibbsite (Al(OH)3)), boehmite (γ-AlO(OH)), and diaspore (α-AlO(OH)), major impurities such as quartz (SiO2), hematite (α-Fe2O3) and rutile (TiO2), and other impurities in minor or trace amounts (Bi 2006; MEC 2013).
Bauxite resources are estimated to be as follows: Africa (32 %), Oceania (23 %), South America and the Caribbean (21 %), Asia (18 %), and elsewhere (6 %). Figures 1 and 2 show the reserves and bauxite production in China and other producing countries (USGS 2015). Mine reserves in China occupy sixth place with 830 million tons, while mine production reached 47 million tons during 2014; China is fast becoming the second largest producer in the world (USGS 2015). Almost all of China’s reserves are distributed between 7 provinces; Shanxi, Henan, Guangxi, Guizhou, Yunnan, Chongqing, and Shandong, the latter holding a 99 % share of the domestic reserves. Diaspore contains the high content of Al2O3 and SiO2 and is the major type of bauxite in China; therefore, the cost of alumina production is much higher than using imported bauxite. Additionally, many mines are still at the early stages of exploitation due to the limitation of mining costs, traffic conditions, and environmental protection issues. Hence, the struggle between the expansion of alumina production and the supply of domestic bauxite will become more apparent.
Reserves distribution of bauxite in China and other countries in 2014 (USGS 2015)
Bauxite mine production in 2013 and 2014 in China and other producing countries (USGS 2015). Date for bauxite production from USA in 2013 and 2014 was not available
Production processes and distribution of alumina
The production process of alumina, depending on the resource characteristics of bauxite ores in China, is the modified Bayer process for diaspore. Various modifications to the traditional Bayer process have been conducted in an attempt to resolve the problem of high silica concentrations in some ores. The modified processes including Bayer process with dressing, lime Bayer process, and Bayer-sintering combined process have been applied successfully in dealing with Chinese middle/low grade bauxite widely used in Shanxi, Henan, Shandong, and Guizhou (Table 1). The Bayer process with dressing is dedicated to resolve the problem of high silica or high sulfur levels in some bauxite ores. Firstly, bauxite is dressed for pre-desilication and desulfurization, then processed by the Bayer process. Lime Bayer process converts sodium aluminosilicate hydrate to hydrated calcium aluminosilicate by excessive addition of lime in the digestion step of the Bayer process. This reduces the consumption of chemical alkali and achieves improved economic efficiency. During the combined Bayer-sintering process (Fig. 3), high silica bauxite is firstly subjected to a traditional Bayer caustic leach with the resulting bauxite residue containing sodium aluminum silicates which is then sintered with limestone and soda ash. The sintered mass is then leached with water to recover alumina and soda.
Schematic of Bayer-sintering combined process (mixed combination process) (modified from Zhang 2014b)
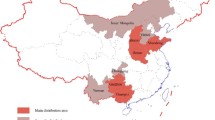
The production of alumina in China has been steadily increasing in recent years and approximately half of the alumina produced is distributed in Henan and Shandong; the others are located in Shanxi, Guangxi, Guizhou, Chongqing, Yunnan, and Neimenggu. The distribution of alumina production is presented in Fig. 4.
Management of bauxite residue
Generation of bauxite residue
The alumina extraction process requires a caustic liquor of Na and Ca hydroxides (pH >13) and produces two streams, a green liquor for alumina precipitation and an insoluble solid waste extract, the bauxite residue, for disposal. For each ton of alumina, 0.5–2.0 tons of bauxite residue are produced depending on the bauxite grade and the efficiency of alumina extraction process (Liu et al. 2014a, b; Borra et al. 2015; Zhu et al. 2015). In China, annually, 70 million tons of bauxite residue is produced from the combined extraction processes (Gu 2014; Xu et al. 2015), and the accumulative inventory of bauxite residue has reached an estimated 0.6 billion tons based on its current rate of production.
Disposal of bauxite residue
Globally, the disposal of bauxite residue is a serious concern and considerable research is being directed towards managing this environmental problem; management, environmental legacy, and land conservation are just some of the issues (Klauber et al. 2011; Liu et al. 2014a, b). Almost all bauxite residue generated in China is disposed on land and include methods such as wet processing, dry processing, and semi-dry processing.
Wet processing is the conventional disposal method where residue slurry containing 15–30 % solids is deposited on the land surface in depressions contained by dams. This presents advantages such as low cost land-based disposal, potentially minimizing re-entrainment of wind-blown dust particulates (Zhu and Qi 2009; Rao 2010). Conversely, dry processing is a developing method where residue slurry contains 50–65 % solids; this provides advantages such as reduced surface area, less potential for leakage to groundwater, easy to manage de-liquoring, and the recovery of solids for alternative use (Wang and Xiu 2011; Liu et al. 2014a, b; Zhang 2014a, b). Semi-dry processing on the other hand results from the joint disposal of bauxite residue from the Bayer and sintering processes; the requirement of clay is reduced, and bauxite residue from the Bayer process can be pumped to the dam directly without dewatering, which takes full advantage of the reservoir capacity (Wang 2005; Sun 2009).
Utilization of bauxite residue
Bauxite residue has been used for building materials, application in environment materials, and recovery of valuable metals. However, due to its high alkalinity, high water content, and complex composition, few processes have been applied commercially. For example, its use in building materials is limited due to excessive Na and demands are small for its use in environment materials. Any recovery of valuable metals would generate secondary residues, which would then require further disposal (Klauber et al. 2011; Samal et al. 2013; Liu et al. 2014a, b). With respect to these challenges, the alkalinity and complex composition of bauxite residue requires further investigation. Currently bauxite residue is stored in bauxite residue disposal areas (BRDAs), and a focus on the stabilization of their surfaces to prevent wind and water erosion, minimize environmental pollution, and promote organic matter accumulation should remain a focus (Gherardi and Rengel 2003; Mendez and Maier 2008; Courtney et al. 2013).
Alkalinity and associated chemistry
Chemical and mineral compositions of bauxite residue
The main chemical components of bauxite residue are Na2O, Al2O3, SiO2, Fe2O3, CaO, and TiO2, their percentage distribution depending on the bauxite source and extraction process (Table 1; Fig. 3). The main minerals include sodalite, cancrinite, hydrogarnet, tri-calcium aluminate (TCA), calcite, perovskite, hematite, goethite, and diaspore, their concentrations depending on ore source and digestion process. The chemical and mineral characteristics of bauxite residue are fundamental to its alkalinity and are presented in Tables 2 and 3, respectively.
Alkalinity of bauxite residue
The alkaline mineral compositions of bauxite residue from Bayer process, sintering process, and Bayer-sintering combined process consist of sodalite ([Na6Al6Si6O24] · [2NaX or Na2X]), cancrinite ([Na6Al6Si6O24] · 2[CaCO3]), hydrogarnet (Ca3Al2(SiO4) x (OH)12−4x ) and tricalcium aluminate (TCA, Ca3Al2(OH)12) (Table 3). The following dissolution reactions result in bauxite residue becoming extremely alkaline: sodalite ([Na6Al6Si6O24] · [2NaX or Na2X] → 8Na+ + 6Al(OH)3 + 6H4SiO4 + 8X, X can be OH−, Cl−, CO3 2−, or SO4 2−) (Whittington 1996; Whittington et al. 1998), cancrinite ([Na6Al6Si6O24] · 2[CaCO3] + 26H2O → 6Na++2Ca2+ + 6Al(OH)3 + 6H4SiO4 + 8OH− + 2HCO3 −), hydro-garnet (Ca3Al2(SiO4) x (OH)12-4x → 3Ca2+ + 2Al(OH)3 + xH4SiO4 + (6−4x)OH−) (Paradis et al. 2007) and tri-calcium aluminate (Ca3Al2(OH)12 → 3Ca2+ + 2Al(OH)3 + 6OH−) (Suryavanshi et al. 1999).
The dissoluble alkalinity substances in bauxite residue are NaOH, Na2CO3, NaHCO3, NaAl(OH)4, KOH, K2CO3. These dissoluble alkalinities can be separated by water in the washing process but still remain in part in the residue. Therefore, the alkaline anions in bauxite residue solution are OH−, CO3 2−, HCO3 −, Al(OH)4 −, H2SiO4 2− and H3SiO4 −. Part of OH−, CO3 2−, HCO3 −, and Al(OH)4 − in the residue solution are derived from the dissoluble alkalinity substances. Most of OH−, CO3 2−, HCO3 − and Al(OH)4 −, and all of H2SiO4 2− and H3SiO4 − are the dissolution products of sodalite, cancrinite, hydrogarnet, and tri-calcium aluminate. The solution pH is buffered by these alkaline anions, ranging from 9.2 to 12.8 with an average value of 11.3 ± 1.0 (Thornber and Binet 1999). Residue pH will therefore not change until these solids are completely dissolved and their reaction products removed.
Alkalinity transformation of bauxite residue
Alkaline solid transformation
Alkaline solids (lime and hydrated lime) transform alkaline substances by replacing Na2O of sodalite and cancrinite with CaO or Ca(OH)2 (Eqs. (1) and (2)), precipitating CO3 2− and Al(OH)4 − as CaCO3 and hydrocalumite at the same time. When the additive CaO reaches saturated state in the low concentration liquid phase, the equilibrium system of CaO–Na2O–Al2O3–SiO2–H2O is reached, and Ca2+ of CaO or Ca(OH)2 partly replaces Na+ of sodalite and cancrinite under the conditions of appropriate temperature, the mass ratio of CaO/bauxite residue and appropriately stirring strength. Moreover, β-CaO · SiO2 and Na2O · Al2O3 can emerge into a steady solid solution containing some portion of Na2O, and this solid solution alkali cannot be precipitated by the common processes. With hydration of CaO, Na2O can be precipitated effectively as Eq. (3). The soluble product of the reactions (Eqs. (1), (2), and (4)) is NaOH which increases the pH of the residue solution comparatively, which can then be leached with water and be recovered to alumina extraction process.
The dealkalization of bauxite residue by alkaline solids was developed rapidly and formed the hydrothermal process (hydrothermal alkali process) (Mei et al. 1997; Wang et al. 2010a; Zheng et al. 2010), atmospheric lime process, and lime-soda sintering process (Jiang 2009; Li et al. 2009; Liu et al. 2012) in China. The lime-soda sintering process is different from the other processes as CaO, Na2CO3, and bauxite residue are mixed and sintered at a temperature above 1273 K. The oxides in the residue are transformed into calcium silicate, sodium aluminate, calcium ferrite, and calcium titanate. After leaching with water, calcium silicate, calcium ferrite, and calcium titanate remain in the residue and sodium aluminate enters into the leached liquor and is then recovered. The hydrothermal lime process and atmospheric lime process have both been used to dealkalize bauxite residue generated from the sintering process and Bayer-sintering combined process, achieving favorable results.
The processes of alkalinity transformation of bauxite residue by alkaline solids, lime, and hydrated lime are suitable additives, but Al2O3 still remains in bauxite residue. In order to achieve dealkalization of the residue and recover Al2O3, some researchers have proposed a new approach, the combined process of calcification-carbonization (Fig. 5) (Zhang 2011). During this process (Fig. 5), the equilibrium phase of bauxite residue (sodium aluminosilicate) was firstly converted to hydrogarnet (3CaO · Al2O3 · xSiO2 · (6−2x)H2O, x < 1). Obtained intermediates which can react with CO2 are changed into calcium silicate, calcium carbonate and aluminum hydroxide. Calcium silicate and calcium carbonate remain in the residues. These generated residues can then be used directly in the production of cement. Aluminum hydroxide can also be leached with green liquor from the Bayer process and be recycled.
Schematic of the combined process of calcification-carbonization for dealing with bauxite residue (modified from Zhang 2014b)
Waste acid synergy
Hydroxides and oxides in bauxite residue can be easily leached with waste acids (H2SO4, HCl, HNO3 and H3PO4) (Zhu 2012; Sushil and Batra 2012). Alkali of bauxite residue including attached alkali, free alkali, and chemistry alkali react with these waste acids. The mechanisms of these reactions (taking H2SO4 as an example) are summarized as Eqs. (5)–(8) (Lu et al. 2010; Zhu 2012). Insoluble sodalite is leached and the product of sodium sulfate can be crystallized and recycled.
However, the leached liquor and residue has always been a complex problem to deal with. This process will produce large amounts of waste liquor and bring about additional environment pollution. Research on alkalinity transformation of bauxite residue by waste acid is limited in China. Unless it is to recover rare and precious metals, bauxite residue will be processed by waste acid (Wang and Li 2007; Gao 2014). If the leachate and leaching residue can be resolved, the alkalinity transformation of bauxite residue by waste acid warrants further research.
Acid gas sequestration
Acid gasses (CO2 and SO2) transform the alkaline substances of bauxite residue by the reaction of CO2 and SO2 with hydroxide to form bicarbonate and sulfite which can then be oxidized to sulfate under atmospheric conditions. Alkaline transformation with CO2 also capitalizes on the reversibility of key alkalinity reactions between hydroxide, bicarbonate, and carbonate (Eqs. (9)–(11)) (Wang et al. 2013). Then the hydroxide component of bauxite residue may be converted to carbonate and bicarbonate. Sodium metaaluminate and sodium silicate can also be precipitated by CO2 as Eqs. (12) and (13) if they are present. Additionally, much of the hydroxide is involved in the solubilization of residual aluminate (Al(OH)4 −). With consumption of free hydroxides from the aluminate anion by CO2, this will lead to alumina precipitation (Eqs. (14) and (15)) (Johnston et al. 2010). As a result, bicarbonate and carbonate should be the dominant products in this process. However, some work on transformation by CO2 suggests that the precipitated product is dawsonite by either Eq. (16) or Eq. (17) (Cooling et al. 2002; Guilfoyle et al. 2005). This requires further research.
Transformation reactions with hydroxide, sodium metaaluminate, and sodium silicate by SO2 are somewhat distinct with transformation by CO2 (Eqs. (18)–(21)) (Chen and Nie 2007; Liu and Li 2015). Furthermore, prolonged treatment of bauxite residue with SO2 exhausts the free Na+ and increases the H+ concentration and, thereby, accelerates the dissolution of sodalite ([Na6Al6Si6O24] · [2NaOH]) (Eq. (22)) (Smith 2009). Generated sulfite oxidizes further to sulfate in the presence of O2 (Eq. (23)).
Alkaline transformation of bauxite residue by acid gas has been studied particularly by thermodynamics and kinetics (Nan et al. 2010; Pang et al. 2012; Ji et al. 2014). Usually, bauxite residue can be roasted at different temperatures due to its characteristics prior to transformation with acid gas. Almost all of the attached alkali and/or free alkali in bauxite residue can be neutralized by CO2 and SO2 (Wang et al. 2009). It is, however, impossible to change the alkali by acid gas excluding the dissolution of sodalite by SO2 according to previous literature.
Though the effect of acid gas is not clear, the innovative nature of this transformation is the stable mineral sequestration of CO2 and SO2 to reduce atmospheric and industrial carbon dioxide and sulfur dioxide emissions. Meanwhile, it may provide additional benefits such as trapping metal and metalloid contaminants (Yang et al. 2010; Yi and Han 2012; Lin et al. 2013; Liu and Naidu 2014). This process achieves the synergistic action of acid gas and bauxite residue, which has a strong positive role in environmental protection.
Chloride salt neutralization
Calcium chloride and magnesium chloride can also be used to precipitate soluble hydroxides, aluminates and carbonates as insoluble solids. By addition of CaCl2 and/or MgCl2, soluble alkaline substances can be converted to calcite (CaCO3), hydrocalumite (Ca4Al2(OH)12 · CO3), aluminohydrocalcite (CaAl2(CO3)2(OH)4 · 3H2O), brucite (Mg3(OH)6) and hydrotalcite (Mg6Al2(CO3)(OH)16 · 4H2O) (Eqs. (24)–(27)) (Hanahan et al. 2004; Menzies et al. 2004; Palmer et al. 2012). The insoluble TCA existing in bauxite residue will participate in forming soluble Al(OH)4 − ions (Alekseev et al. 1984; Alekseev 1985; Blenkinsop et al. 1985; Whittington et al. 1997; Palmer et al. 2011). It is thought that a combination of two predominant reactions (Eqs. (28) and (29)) involving Na2CO3 and NaOH take place. The initial dissolution step of TCA involves a combination of these reactions occurring simultaneously. Equation (28) releases NaOH into bauxite reside until the Na2CO3 concentration is depleted, and the pH increases as 6 mol of NaOH are released. Nevertheless, the consumption of 2 mol of NaOH in Eq. (29) reduces the rate at which the pH increases. It is also believed that the dissolution of Ca(OH)2 formed from Eq. (29) causes an increase in OH− ions. The dissolution of Ca(OH)2 occurs once the majority of NaOH has been neutralized by the dissolution of TCA. The increased concentration of OH− ions in bauxite residue is then believed to cause further dissolution of TCA, removing OH− ions by the formation Ca(OH)2. It is not until the majority of TCA is dissolved that the dissolution of Ca(OH)2 becomes dominant. The presence of carbonate also promotes Ca(OH)2 dissolution through the precipitation of calcite, resulting in the additional release of OH− ions into bauxite residue. The presence of Ca(OH)2 in the liquor promotes the formation of hydrocalumite during the calcium chloride and magnesium chloride neutralization processes (Palmer et al. 2012). Therefore, the concentration of Ca(OH)2 affects the efficiency of the calcium chloride and magnesium chloride neutralization process for bauxite residue both directly and indirectly.
Ammonium chloride transforms alkaline substances in bauxite residue into chloride, in which free NH4 + bonds with metal ions as Eqs. (30), (31), (32), and (33). Products of NH3 and CO2 are collected and returned to the cyclic utilization process of NH4Cl, achieving reuse of ammonium chloride (Eqs. (34) and (35)). This novel technology for sodium elimination was studied by the Chinese Academy of Sciences (Wang et al. 2010b). Bauxite residue leached (hot pressure) with NH4Cl produces residues that contain insoluble hydroxides formed from hydrolysis, and these residues can be used in the production of cement (Wang et al. 2010c).
Chloride salt including calcium chloride, magnesium chloride, and ammonium chloride precipitate soluble hydroxides, aluminates, and carbonates as insoluble substances, lowering liquor pH and alkalinity and allowing discharge. The residue can be disposed of more safely or be reused in other applications. Calcium chloride and magnesium chloride neutralization (seawater neutralization) of bauxite residue is used by some coastal refineries in Australia (Menzies et al. 2009; Santini et al. 2011; Couperthwaite et al. 2014). Though an excess of NH4Cl is required by ammonium chloride neutralization, this technology produces no secondary pollutants. Successful implementation of this technology would be a significant milestone in the development of the chloride salt transformation technology.
Revegetation-driven amelioration
Revegetation-driven amelioration is regarded as a promising way forward in an attempt to ameliorate bauxite residue (Wong and Ho 1994; Jones et al. 2011; Courtney et al. 2014; Santini et al. 2015). The focus is on the potential application of tolerant plants and suitable microorganisms as further amendments to ameliorate the alkalinity and salinity of the restored bauxite residue. For in situ revegetation of the amended bauxite residue disposal areas (BRDAs), it is necessary to understand the application of plants and establishment of microbes and investigate the transfer of sodium and mitigation of pH (Banning et al. 2011; Schmalenberger et al. 2013; Krishna et al. 2014).
Using salt-resistant halophytes may be a potential means of ameliorating the high saline and sodic condition on bauxite residue surface, according to the similar and parallel works in saline-sodic soils (Gräfe and Klauber 2011). Yang et al. (2009) evaluated the effects of alkaline buffering capacity on the performance (relative growth rates, photosynthesis, and Na uptake) of Chloris virgata Swartz. in solutions ranging from pH 9.5 to 9.7 with an acid neutralizing capacity (ANC) between 0 and 316 mmol H+L−1. Yang et al. (2009) revealed that relative growth rate and photosynthetic activity declined with increasing buffering capacity, while the accumulation of Na+ in tissues was the highest (4.6 wt.%) from solutions buffered at 316 mmol H+L−1 ANC. Li et al. (2010) reported that the effects of various alkali and salt stresses on growth, organic solutes, and cation accumulation in Spartina alterniflora Loisel. and demonstrated that the plant was capable of surviving in all treatments under pH ≥8.3 regardless of salinity level.
Microorganisms have beneficial effects towards improving the chemical properties of bauxite residue amended with gypsum, organic wastes, and fertilizers. For example, in restored bauxite residues (amended with compost and gypsum) with different restoration histories, Schmalenberger et al. (2013) established bacterial communities and investigated their successional development and community structure. In a rehabilitated bauxite residue disposal area (amended with gypsum and organic matter), Courtney et al. (2013) and (2014) demonstrated that microbes decreased pH, reduced Na+ concentrations, and enhanced conditions for the further establishment of flora and fauna. Hamdy and Williams (2001) demonstrated that damaged bacterial cells actively grew following amelioration using nutrients or hay. The microorganisms grew from less than 10 to more than 109 cells per gram of bauxite residue and formed organic acids that lowered the pH from 13 to approximately 7.0.
Production of organic acids by fermentative metabolism is also of interest to bioremediation of bauxite residue because they may contribute to reducing pH by reaction of H+ with various sources of alkalinity in both pore water and solids (Santini et al. 2015). The mechanisms of organic acid generation may contribute to pH neutralization (Fig. 6). In addition, production of carbon dioxide, extracellular polymeric substances (EPS) that bind soil particles into stable aggregates, altering the ionic balance through solubilization of minerals, and selective uptake of ions from solution (Santini et al. 2015), may all contribute to in situ remediation of bauxite residue.
Mechanisms by which organic acid production possibly contribute to pH neutralization in bauxite residue (modified from Santini et al. 2015)
Nevertheless, the lack of the knowledge of solution composition, dissolution mechanisms, ion exchange processes, chemical speciation, the presence and form of macro and micronutrients, and a model for salt transportation and deposition present future challenges. Insufficient knowledge of the stress and tolerance levels of halophytes, glycophytes, and microbes (including resident, injured, and alkaliphilic microbes) to salinity and alkalinity are further problems. Furthermore, it is unclear how to assess the survivability of microbial inoculants and to understand the factors which influence survival and the extent to which microbial inoculants may influence amelioration of bauxite residue in the long term.
Conclusion
Bauxite residue is dominated by the presence of multiple alkaline solids and dissoluble alkaline substances. A diverse range of methods have been highlighted regarding alkalinity transformations from the view point of mechanisms, processes and deficiencies, all being highly complex and considerably different. The transformation processes being selected based on end-application requirement. Alkaline solid transformation, chloride salt neutralization and bio-driven amelioration primarily focus on the storage of bauxite residue in BRDAs while waste acid synergy and acid gas sequestration focus on the application of bauxite residue following the process. These methods can not completely remove alkalinity, but it must be alleviated for successful disposal, metal recovery, and bioremediation. Nevertheless, these transformation technologies do have drawbacks which limit their commercial application. Key knowledge gaps that require further advancement of understanding include the following:
-
The behavior of insoluble alkalinity of bauxite residue in transformation processes is poorly understood.
-
Dissolution behaviors of hydrogarnet, tri-calcium aluminate and sodalite are poorly understood.
-
Limited knowledge of dissolved salts and liquor dynamic transport within bauxite residue profiles.
-
Clear and low-cost technologies to reduce alkalinity and soda.
-
The synergistic application of waste acid and bauxite residue.
-
Lack of the knowledge of the stress and tolerance levels of halophytes, glycophytes, and microbes to high alkalinity.
Future progress on sustainable management and alkalinity transformation of bauxite residue will be dependent on the development of an improved understanding of the complex neutralization and transformation chemistry of bauxite residue. Continuing research into the chemistry and technology of alkalinity transformation is required. Current research priorities are therefore recommended as follows:
-
Development of reaction thermodynamics of acid gas-alkaline substances in bauxite residue and optimize thermodynamics conditions.
-
Development of the effect of concentration gradient of acid gas and kinetics of sequestration process.
-
Development of an understandable transformation theory for insoluble alkalinity of bauxite residue based on mineralogy.
-
Establishment of a comprehensive data set in relation to the dissolution behavior of alumina extraction processes, specifically solids (in particular hydrogarnet, tri-calcium aluminate and sodalite).
-
Development of a combined reaction transport and hydrological model for solution flow and reaction processes within bauxite residue profiles.
-
Development of a clear and economic process for the reduction of alkalinity and soda in bauxite residue, e.g., direct carbonation technology of residue by stack gas carbon dioxide.
-
Achievement of the synergistic action of waste and bauxite residue and development of a critical technology applicable to the hazardous wastes.
-
Development of a thorough understanding of the stresses and tolerance levels of halophytes, glycophytes, and microbes to the alkalinity of bauxite residue.
Much of the current and traditional research approaches have focused on removal and separation of alkaline substances. The conventional ideas of alkalinity transformation may require change, and some novel research directions are worth further exploration.
-
Stabilization and/or immobilization of alkaline substances in bauxite residue.
-
Soil formation process of in situ bioremediation in the amended bauxite residue disposal areas.
-
Remediation of bauxite residue disposal area through natural weathering processes.
-
Alkalinity transformation of bauxite residue by biomass based on microbiology.
The management of bauxite residue still remains a challenge for the alumina industry and innovative technologies for the development of emission reductions from bauxite residue may be developed further both in China and in the international community.
References
Alekseev AI (1985) Calcium hydroaluminates and hydrogarnets: synthesis, properties, and application. LGU, Leningrad
Alekseev AI, Barinova LD, Rogacheva NP, Kulinich OV (1984) Thermodynamic and experimental analysis of equilibriums in the sodium oxide calcium oxide carbon dioxide water system. Zh Prikl Khim 57:1256–1261
Banning NC, Phillips IR, Jones DL, Murphy DV (2011) Development of microbial diversity and functional potential in bauxite residue sand under rehabilitation. Restor Ecol 19(101):78–87
Banning NC, Sawada Y, Phillips IR, Murphy DV (2014) Amendment of bauxite residue sand can alleviate constraints to plant establishment and nutrient cycling capacity in a water-limited environment. Ecol Eng 62:179–187
Bi SW (2006) Technics of alumina production. Chemical industry press, Beijing (in Chinese)
Blenkinsop RD, Currell BR, Midgley HG, Parsonage JR (1985) The carbonation of high alumina cement, Part I. Cem Concr Res 15:276–284
Borra CR, Pontikes Y, Binnemans K, Gerven TV (2015) Leaching of rare earths from bauxite residue (red mud). Miner Eng. doi:10.1016/j.mineng.2015.01.005
Chen YM, Nie JX (2007) Adorsption of SO2 from flue gas with wastewater in red mud. Nonferrous Metals 59(4):153–155 (in Chinese)
Clark MW, Johnston M, Reichelt-Brushett AJ (2015) Comparison of several different neutralisations to a bauxite refinery residue: potential effectiveness environmental ameliorants. Appl Geochem 56:1–10
Cooling DJ, Hay PS, Guifoyle L (2002) Carbonation of bauxite residue. In: Proceedings of the 6th International Alumina Quality Workshop, Brisbane
Couperthwaite SJ, Johnstone DW, Mullett ME, Taylor KJ, Millar GJ (2014) Minimization of bauxite residue neutralization products using nanofiltered seawater. Ind Eng Chem Res 53(10):3787–3794
Courtney R, Harrington T, Byrne KA (2013) Indicators of soil formation in restored bauxite residues. Ecol Eng 58:63–68
Courtney R, Harris JA, Pawlett M (2014) Microbial community composition in a rehabilitated bauxite residue disposal area: a case study for improving microbial community composition. Restor Ecol 22(6):798–805
Fu GF, Tian FQ, Quan K (2005) Study on digestion of Chinese middle/low grade bauxite in lime Bayer process. J Northeast Univ (Nat Sci) 26(11):1093–1095 (in Chinese)
Gao Y (2014) Study on two-stage acid leaching process of alumina and iron oxide from guangxi pingguo alumina corporation produced red mud. Dissertation, Taiyuan University of Science and Technology (in Chinese)
Gherardi MJ, Rengel Z (2003) Deep banding improves residual effectiveness of manganese fertiliser for bauxite residue revegetation. Soil Res 41(7):1273–1282
Gräfe M, Klauber C (2011) Bauxite residue issues: IV. Old obstacles and new pathways for in situ residue bioremediation. Hydrometallurgy 108(1–2):46–59
Gu MM (2014) Research on key technology of comprehensive utilization of the red mud. Light Met 4:10–16 (in Chinese)
Gu HN, Wang N, Liu SR, Tian YJ (2012) Study on material composition and particles characteristics of red mud from the sintering alumina process. Rock Mine Anal 2:312–317 (in Chinese)
Guilfoyle L, Hay P, Cooling D (2005) Use of flue gas for carbonation of bauxite residue. In: Proceedings of the 7th International Alumina Guality Workshop, Perth, Australia
Hamdy MK, Williams FS (2001) Bacterial amelioration of bauxite residue waste of industrial alumina plants. J Ind Microbiol Biotechnol 27(4):288–233
Hanahan C, Mcconchie D, Creelman R (2004) Chemistry of seawater neutralization of bauxite refinery residues (red mud). Environ Eng Sci 21(2):125–138
Ji ZJ, Li SQ, Zhang CC, Tang XH (2014) Experimental studies on sodium removal of red mud by CO2. J Taiyuan Univ Technol 45(1):42–46 (in Chinese)
Jiang WC (2009) Study on recovering iron and alumina from Bayer red mud with lime soda sinter. Dissertation, Huazhong Univ Sci Technol
Jiang YF, Liu Y, Lin CX (2007) Chemistry and mineralogy of red mud and its utilization in Zhengzhou Alumina Refinery. Light Met 10:18–21 (in Chinese)
Johnston M, Clark MW, McMahon P, Ward N (2010) Alkalinity conversion of bauxite refinery residues by neutralization. J Hazard Mater 182(1–3):710–715
Jones BEH, Haynes RJ, Phillips IR (2011) Influence of organic waste and residue mud additions on chemical, physical and microbial properties of bauxite residue sand. Environ Sci Pollut Res 18(2):199–211
Kirwan LJ, Hartshorn A, McMonagle JB, Fleming L, Funnell D (2013) Chemistry of bauxite residue neutralisation and aspects to implementation. Int J Miner Process 119:40–50
Klauber C, Gräfe M, Power G (2011) Bauxite residue issues: II. Options for residue utilization. Hydrometallurgy 108(1–2):11–32
Krishna P, Babu AG, Reddy MS (2014) Bacterial diversity of extremely alkaline bauxite residue site of alumina industrial plant using culturable bacteria and residue 16S rRNA gene clones. Extremophiles 18(4):665–676
Li B (2002) Quantitative phase analysis of red mud of Bayer process by rietveld full-pattern fitting method. J Instrum Anal 6:68–72 (in Chinese)
Li XB, Liu XM, Liu GH, Peng ZH, Liu YX (2004) Study and application of intensified sintering process for alumina production. Chin Nonferrous Met 14(6):1031–1036 (in Chinese)
Li JQ, Long Q, Xu BJ (2009) Research on alumina recovery from red mud by sintering process. Light Met 11:11–13 (in Chinese)
Li R, Shi F, Fukuda K (2010) Interactive effects of various salt and alkali stresses on growth, organic solutes, and cation accumulation in a halophyte Spartina alterniflora (Poaceae). Environ Exp Bot 68(1):66–74
Liao CZ, Zeng LM, Shih K (2015) Quantitative X-ray diffraction (QXRD) analysis for revealing thermal transformations of red mud. Chemosphere 131:171–177
Lin JF, Li YD, Han MF, Yi YL, Gao LS, Dai YZ (2013) Capture of carbon dioxide with red mud. Environ Protec Chem Ind 33(6):549–552 (in Chinese)
Liu C (2007) The feasibility investigation to the concrete made by the red mud for Zhongzhou aluminium factory. Dissertation, Henan Polytech Univ (in Chinese)
Liu ZB, Li HX (2015) Metallurgical process for valuable elements recovery from red mud-a review. Hydrometallurgy 155:29–43
Liu YJ, Naidu R (2014) Hidden values in bauxite residue (red mud): recovery of metals. Waste Manag 34(14):2662–2673
Liu PW, Zhang LH, Zhang XF, Pei Y, Li GK (2000) Theoretical basis of new technology in predesilicification and bauxite dressing-Bayer process and industrial technique of new technology. J Chem Ind Eng (China) 51(6):734–739 (in Chinese)
Liu LR, Aye L, Lu ZW, Zhang PH (2006) Analysis of the overall energy intensity of alumina refinery process using unit process energy intensity and product ratio method. Energy 31(8):1167–1176
Liu WC, Sun SY, Zhang L, Jahanshahi S, Yang JK (2012) Experimental and simulative study on phase transformation in Bayer red mud soda-lime roasting system and recovery of Al, Na and Fe. Miner Eng 39:213–218
Liu FX, An YY, Tang SG (2014a) Submerging test for dry stockpiling and consolidating red mud at the field. Sci Tech Eng 27:264–267
Liu W, Chen X, Li W, Yu Y, Yan K (2014b) Environmental assessment, management and utilization of red mud in China. J Clean Prod 84:606–610
Lockwood CL, Stewart DI, Mortimer RJG, Mayes WM, Jarvis AP, Gruiz K, Burke LT (2015) Leaching of copper and nickel in soil-water systems contaminated by bauxite residue (red mud) from Ajka, Hungary: the importance of soil organic matter. Environ Sci Pollut Res 22(14):10800–10810
Lu GL, Chi SJ, Bi SW (2010) Leaching of alumina and iron oxide from red mud. J Mater Metal (1):31–34, 67. (in Chinese)
MEC (Minerals Education Coalition) (2013) Alumina. Minerals education coalition. http://www.mineralseducationcoalition.org/minerals/aluminum. Accessed 10 May 2015
Mei XG, Sun ZY, Zuo WL (1997) Development on removal of Na from bauxite residue in foreign countries. Light Met 7:21–25 (in Chinese)
Mendez MO, Maier RM (2008) Phytoremediation of mine tailings in temperate and arid environments. Rev Environ Sci Bio Technol 7:47–59
Menzies NW, Fulton IM, Morrell WJ (2004) Seawater neutralization of alkaline bauxite residue and implications for revegetation. J Environ Qual 33(5):1877–1884
Menzies NW, Fulton IM, Kopittke RA, Kopittke PM (2009) Fresh water leaching of alkaline bauxite residue after sea water neutralization. J Environ Qual 38(5):2050–2057
Nan XL, Zhang TG, Liu Y, Dou ZH, Zhao QY, Jiang XL (2009) The main categories of red mud and its influence on the environment in China. Chin J Process Eng S1:459–464 (in Chinese)
Nan XL, Zhang TG, Wu YQ, Dou ZH (2010) A study on absorption of low-concentration SO2 by Bayer red mud. J Northeast Univ (Nat Sci) 31(7):986–989
NBSC (National Bureau of Statistics of the People’s Republic of China) (2013) Alumina production of China in 2013. http://data.stats.gov.cn/search/keywordlist. Accessed 12 May 2015
NBSC (National Bureau of Statistics of the People’s Republic of China) (2015) Alumina production of China in 2014. http://data.stats.gov.cn/search/keywordlist. Accessed 12 May 2015
Palmer SJ, Frost RL, Smith MK (2011) Minimising reversion, using seawater and magnesium chloride, caused by the dissolution of tricalcium aluminate hexahydrate. J Colloid Interf Sci 353(2):398–405
Palmer SJ, Smith M, Frost RL (2012) Implication of calcium hydroxide in the seawater neutralisation of bauxite refinery liquors. In: In 9th International Alumina Quality Workshop, Perth, WA
Pan XL, Yu HY, Tu GF (2015) Reduction of alkalinity in bauxite residue during Bayer digestion in high-ferrite diasporic bauxite. Hydrometallurgy 151:98–106
Pang YG, Wu L, Shen P, Li SQ (2012) Dynamics factors’ effects to the sodium removal of bayer mud by CO2. J Sci Univ (Eng Sci Edn) 44(Supp.1):235–239
Paradis M, Duchesne J, Lamontagne A, Isabel D (2007) Long-term neutralisation potential of red mud bauxite with brine amendment for the neutralisation of acidic mine tailings. Appl Geochem 22(11):2326–2333
Pontikes Y, Angelopoulos GN (2013) Bauxite residue in cement and cementitious applications: current status and a possible way forward. Resour Conserv Recy 73:53–63
Pulford ID, Hargreaves JSJ, Ďurišová J, Kramulova B, Girard C, Balakrishnan M, Batra VS, Rico JL (2012) Carbonised red mud—a new water treatment product made from a waste material. J Environ Manag 100:59–64
Rao PP (2010) Analysis on basic characteristics of Bayer’s dry red mud and the operation feature of the yard. J Eng Geo 18(3):340–344
Samal S, Ray AK, Bandopadhyay A (2013) Proposal for resources, utilization and processes of red mud in India—a review. Int J Miner Process 118:43–55
Santini TC, Hinz C, Rate AW, Carter CM, Gilkes RJ (2011) In situ neutralisation of uncarbonated bauxite residue mud by cross layer leaching with carbonated bauxite residue mud. J Hazard Mater 194:119–127
Santini TC, Kerr JL, Warren LA (2015) Microbially-driven strategies for bioremediation of bauxite residue. J Hazard Mater 293:131–157
Schmalenberger A, O’Sullivan O, Gahan J, Cotter PD, Courtney R (2013) Bacterial communities established in bauxite residues with different restoration histories. Environ Sci Technol 47(13):7110–7119
Si CH, Ma YQ, Lin CX (2013) Red mud as a carbon sink: variability, affecting factors and environmental significance. J Hazard Mater 244–245:54–59
Smith P (2009) The processing of high silica bauxites—review of existing and potential processes. Hydrometallurgy 98(1–2):162–176
Sun YD (2009) Research and implementation on storage process of “half-drying mixed red mud”. Energy Saving Nonferrous Met 3:20–25 (in Chinese)
Suryavanshi AK, Scantlebury JD, Lyon SB (1999) Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem Concr Res 26(5):717–727
Sushil S, Batra VS (2012) Modification of red mud by acid treatment and its application for CO2 removal. J Hazard Mater 203–204:264–273
Thornber MR, Binet D (1999) Caustic soda adsorption on Bayer residues. In: 5th International Alumina Quality Workshop, Bunbury, AQW Inc
Tuazon D, Corder GD (2008) Life cycle assessment of seawater neutralised red mud for treatment of acid mine drainage. Resour Conserv Recy 52(11):1307–1314
USGS (United States Geological Survey) (2015) Mineral commodity summaries: Bauxite and alumina statics information. United States Government Printing Office, Washington. http://minerals.usgs.gov/minerals/pubs/commodity/bauxite/mcs-2015-bauxi.pdf. Accessed 13 June 2015
Wang PS (2005) Characteristics and rapid hardening mechinsm of red mud from alumina production with sintering process. Nonferrous Metals 3:115–119 (in Chinese)
Wang KQ (2012) Physic-chemical properties of red mud in Shanxi. Light Met 4:25–28 (in Chinese)
Wang KQ, Li SH (2007) Study on aluminum recovery from red mud with hydrochloric acid. Nonferrous Metals 7:16–19 (in Chinese)
Wang L, Xiu PS (2011) Red mud stacking and environmental protection in the alumina refinery. Liaoning Chem Ind 10:1056–1059 (in Chinese)
Wang Q, Li J, Zhao Y, Luan ZK (2009) Study on the dealkalization of red mud by suspension and carbonation. Chin J Environ Eng 3(12):2275–2280
Wang LY, Li XL, Huo E (2010a) Research on dealkalization of bauxite residue. Sci Tech Info 7:367–368
Wang YS, Yang G, Zhang JP (2010b) A process for sodium elimination from red mud of alumina production. China Patent No. CN102190322B (in Chinese)
Wang YS, Yang G, Zhang JP (2010c) Novel process for sodium elimination from red mud of alumina production. Nonferrous Met 62(3):61–64 (in Chinese)
Wang Z, Han MF, Zhang YH, Zhou FS (2013) Study on the dealkalization technics of Bayer process red mud with CO2 by carbonation. Bull Chin Ceramic Soc 32(9):1851–1855 (in Chinese)
Whittington BI (1996) The chemistry of CaO and Ca(OH)2 relating to the Bayer process. Hydrometallurgy 43(1):13–35
Whittington BI, Cardile CM (1996) The chemistry of tricalcium aluminate hexahydrate relating to the Bayer industry. Int J Miner Process 48(1):21–38
Whittington BI, Fallows TM, Willing MJ (1997) Tricalcium aluminate hexahydrate (TCA) filter aid in the Bayer industry: factors affecting TCA preparation and morphology. Int J Miner Process 49(1):1–29
Whittington BI, Fletcher BL, Talbot C (1998) The effect of reaction conditions on the composition of desilication product (DSP) formed under simulated Bayer conditions. Hydrometallurgy 49(1):1–22
Wong JWC, Ho G (1994) Sewage sludge as organic ameliorant for revegetation of fine bauxite refining residue. Resour Conserv Recy 11(1):297–309
Wu C, Zou Q, Xue SG, Mo JY, Pan WS, Lou LQ, Wong MH (2015) Effects of silicon (Si) on arsenic (As) accumulation and speciation in rice (Oryza sativa L.) genotypes with different radial oxygen loss (ROL). Chemosphere 138:447–453
Xu GD, Ao H, She YG (2012) Current status and development trend of aluminum industry in world and strategy suggestions in China under background of sustainable development. Chin J Nonferrous Met 22(7):2040–2050 (in Chinese)
Xu L, Shi GD, Li YK, Zhong QW, Luo YZ, Yu P (2015) Study of scandium pre-enrichment from red mud leached by hydrochloric acid. Nonferr Met (Extract Metallurg) 1:54–56
Xue SG, Zhu F, Kong XF, Wu C, Huang L, Huang N, Hartley W (2016) A review of the characterization and revegetation of bauxite residues (Red mud). Environ Sci Pollut Res 23(2):1120–1132
Yang CM, Zhang ML, Liu J, Shi DC, Wang DL (2009) Effects of buffer capacity on growth, photosynthesis, and solute accumulation of a glycophyte (wheat) and a halophyte (Chloris virgata). Photosynthetica 47(1):55–60
Yang GJ, Yu HY, Li W, Li HH (2010) Pilot plant test of sulfur removal by red mud. Light Met 9:26–29 (in Chinese)
Yi YL, Han MF (2012) Characteristics and mechanism of sodium removal by the synergistic action of flue gas and waste solid. Environ Sci 33(7):2522–2527
Yu YB, Wang KQ, Wang H (2009) Study on properties of red mud of shanxi aluminium plant. J Taiyuan Univ Technol 1:63–66 (in Chinese)
Zhang TA (2011) A process for alumina production based on the transformation of calcification-carbonization. China Patent No. CN201110275013.6 (in Chinese)
Zhang GJ (2014a) Quick opening pressure filter in dry red mud stockpiling. Auto Appl 6:35–36
Zhang TA (2014b) Technology of aluminum metallurgy. Science Press, Beijing
Zhang KY, Hu HP, Zhang LJ, Chen QY (2008) Surface charge properties of red mud particles generated from Chinese diaspore bauxite. T Nonferr Met Soc 18(5):1285–1289
Zhao QJ, Chen QY, Yang QF (2004) The trends of Chinese alumina production with combined process. Int J Min Met Mater 5:127–130 (in Chinese)
Zheng XF, Hu J, Jiang M, Xue ZX (2010) Study on optimization of deaklaization process on adding lime to red mud produced by low temperature Bayer process. Light Met 4:23 (in Chinese)
Zhou QX, Song YF (2004) Contaminated soil remediation: principles and methods. Science Press, Beijing (in Chinese)
Zhu GH (2012) Study on recycling of titanium dioxide from red mud slag leached by sulfuric acid. Dissertation, Taiyuan Univ Sci Technol (in Chinese)
Zhu Q, Qi B (2009) Development and status of red mud comprehensive utilization technology in China. Lig'ht Met 8:7–10 (in Chinese)
Zhu XB, Li W, Guan XM (2015) An active dealkalization of red mud with roasting and water leaching. J Hazard Mater 286:85–91
Zhu F, Xue SG, Hartley W, Huang L, Wu C, Li XF (2016a) Novel predictors of soil genesis following natural weathering process of bauxite residue. Environ Sci Pollut Res 23(3):2856–2863
Zhu F, Li YB, Xue SG, Hartley W, Wu H (2016b) Effects of iron-aluminiumoxides and organic carbon on aggregate stability of bauxite residues. Environ Sci Pollut Res. doi:10.1007/s11356-016-6172-9
Acknowledgments
Financial supports from the Environmental Protection’s Special Scientific Research for Chinese Public Welfare Industry (No. 201509048), the National Natural Science Foundation of China (No. 41371475) and the Open-End Fund for the Valuable and Precision Instruments of Central South University (No. CSUZC201610) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Xue, S., Kong, X., Zhu, F. et al. Proposal for management and alkalinity transformation of bauxite residue in China. Environ Sci Pollut Res 23, 12822–12834 (2016). https://doi.org/10.1007/s11356-016-6478-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6478-7