Abstract
Leaching of metals (calcium (Ca), chromium (Cr), copper, (Cu), iron (Fe), and zinc (Zn)) of recycled concrete aggregates (RCAs) were investigated with four different leachate extraction methods (batch water leach tests (WLTs), toxicity leaching procedure test (TCLP), synthetic precipitation leaching procedure test (SPLP), and pH-dependent leach tests). WLTs were also used to perform a parametric study to evaluate factors including (i) effects of reaction time, (ii) atmosphere, (iii) liquid-to-solid (L/S) ratio, and (iv) particle size of RCA. The results from WLTs showed that reaction time and exposure to atmosphere had impact on leaching behavior of metals. An increase in L/S ratio decreased the effluent pH and all metal concentrations. Particle size of the RCA had impact on some metals but not all. Comparison of the leached concentrations of metals from select RCA samples with WLT method to leached concentrations from TCLP and SPLP methods revealed significant differences. For the same RCA samples, the highest metal concentrations were obtained with TCLP method, followed by WLT and SPLP methods. However, in all tests, the concentrations of all four (Cr, Cu, Fe, and Zn) metals were below the regulatory limits determined by EPA MCLs in all tests with few exceptions. pH-dependent batch water leach tests revealed that leaching pattern for Ca is more cationic whereas for other metals showed more amphoteric. The results obtained from the pH-dependent tests were evaluated with geochemical modeling (MINTEQA2) to estimate the governing leaching mechanisms for different metals. The results indicated that the releases of the elements were solubility-controlled except Cr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coarse aggregates are mainly used in the construction of highway base/subbase layers to develop a pavement layer that can not only freely drain but also provide load distribution to the underlying soil, reducing cracking in asphalt, rutting, and deformation in the base/subbase layer of highways (Tutumluer and Pan 2008). The use of recycle materials in pavement systems in lieu of natural aggregates has been an interest for some time (Tanyu et al. 2005; Li et al. 2009; Cetin et al. 2010). Crushed waste concrete, termed recycled concrete aggregate (RCA), is also considered as an alternative material to be used in roadway construction (Chen et al. 2013). Using recycled materials increases the sustainability of the project because the environmental and economic impacts associated from mining and processing of natural aggregates are minimized and/or eliminated. This also diverts concrete waste streams from landfills, saving valuable space. It ultimately creates an alternative aggregate that, in most cases, costs significantly less than the natural aggregate. Furthermore, RCAs are produced from the concrete pavement that has been laid over the pavement system during rehabilitation of the highways. Thus, the construction cost of the new pavement construction is reduced significantly due to the elimination of the transportation cost of conventional geomaterials typically used for base and subbase materials.
Recycled concrete aggregate use is widespread in many countries but it is shown that RCA materials have potential to leach metals (Engelsen et al. 2009, 2010; Chen et al. 2013). The cement paste in RCA is believed to be the primary source of leached constituents (Engelsen et al. 2010), and certain cement additives such as fly ash (mostly class C but also to a lesser extent class F) and steel slag (produced from both basic oxygen furnace (BOF) and electric arc furnace (EAF)) may intensify this issue as appreciable amounts of metals have been shown to leach from these industrial by products (Li et al. 2006; Cetin et al. 2012a, b, 2014; Daniels and Das 2014).
Engelsen et al. (2009) showed the pH of RCA tended to be highly alkaline. However, it should be noted that the pH of RCAs can change drastically based on the age and makeup of the material. It is very well known that certain metals tend to be released into the aqueous solutions at high pH values. These include metals such as chromium (Cr), zinc (Zn), and copper (Cu) (Engelsen et al. 2010; Chen et al. 2012). Leached Cr and Cu concentrations of RCAs tested in Engelsen et al. (2010) were higher than the US Environmental Protection Agency (EPA) drinking water maximum contaminant levels (MCLs) or EPA Secondary MCL (SMCL). Chen et al. (2012) found similar findings, where the leached Cr concentrations at pH range of 11 to 12.5 did not satisfy the EPA MCL.
The findings of the previous literature indicate that RCA may have to be evaluated for the leached concentrations of metals. Even though past studies investigated the impacts of changes in liquid-to-solid (L/S) ratio and particle size on the leaching of elements from RCA materials, there was no consistency within the results of these studies, which indicated the need for more research on these subjects. Moreover, the effects of exposure of freshly produced RCA to the atmosphere and reaction time that may affect the leached metal concentrations from RCA when RCA is exposed to these conditions were not studied in the literature before. Furthermore, it is known that pH is one of the most important parameters that control the leaching of both inorganic and organic constituents from solid phase into solution (Fruchter et al. 1990; Mudd et al. 2004). There are two main leaching controlling mechanisms: solubility and sorption (Fruchter et al. 1990; van der Sloot and Dijkstra 2004). Solubility control occurs when the solution in contact with the solid is saturated with regard to the constituent species of interest (Kosson et al. 1996) and is generally associated with the dissolution of metal oxides present in the solid, such as aluminum oxide, iron oxide, and zinc oxide (Kosson et al. 1996; Iyer et al. 1999). On the other hand, sorption processes control the release of elements that exhibit sorptive affinity to the active sites on the solid surface. Trace metals are likely controlled by sorption processes on sorption site surfaces, such as oxides, oxyhydroxides, organic matter, and clay (McBride 1994). Positively charged ions (such as Cu2+) that are not controlled by the dissolution of minerals are often controlled by the adsorption onto the sorption sites available on the solid surface, such as metal oxides and organic matter (van der sloot and Dijkstra 2004). Thus, it is necessary to understand the leaching behavior of metals and their leaching controlling mechanisms from roadways built with RCA which could provide information about the mobility of metals.
The objective of this study was to understand the impact of atmospheric exposure time, reaction time, L/S ratio, particle size, and pH on the leaching behavior of the selected metals (Ca, Cr, Cu, Fe, and Zn) and to identify the leaching controlling mechanisms of metals, and also to compare the concentration levels obtained from the batch water leach tests (WLT) against the concentration levels obtained from synthetic precipitation leaching procedure tests (SPLPs), and toxicity leaching procedure tests (TCLP), which were selected to simulate the exposure of RCA to potentially more acidic water environment.
Materials
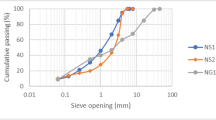
Five RCA samples from various locations across South Dakota were collected. Two of the RCA samples were collected from existing stockpiles of concrete obtained from mixture of undesignated sources (such as old concrete buildings, curb, gutters, and rigid pavement surfaces). These two samples are referred to as Eastern South Dakota (ESD) and Western South Dakota (WSD) RCA materials. ESD was collected from the aggregate plant on the east side of South Dakota (Sioux Falls, SD) while WSD was collected from the stockpile on the west side of South Dakota (WSD). Three of the RCA samples were from concrete recycled particularly from interstate highway pavement surfaces (samples referred as DOT1, DOT2, and DOT3). The DOT1 and DOT2 samples were crushed on site and used as a base layer on highway I-90 in Philip and Sioux Falls, SD, respectively. DOT3 was obtained from site, which consisted of freshly excavated concrete pavement layer. These samples were crushed later on with jaw crushers in the laboratory of South Dakota School of Mines and Technology. DOT3 samples were particularly created to test the effects of the atmospheric exposure on freshly produced RCA. All materials used in this study were composed primarily of sands and gravel size particles (Fig. 1).
Physical and chemical properties of the RCA materials used in this study are summarized in Table 1. All samples classified as soils were suitable for supporting roadway pavements in accordance with the AASHTO soil classification. The compaction characteristics of RCAs were determined using the Modified Proctor Compaction Test (ASTM D1557) per roadway design specifications of South Dakota Department of Transportation (SDDOT 2004). The pH values of materials were determined by following EPA method 9045D and ranged between 10.5 and 12.52. The chemical compositions and total metal concentrations of RCA were determined by X-ray diffraction (XRD) and X-ray fluorescence (XRF) spectrometry analyses (US Environmental Protection Agency (EPA) method 6200). RCA materials were crushed and ground to a particle size smaller than 0.0045 mm. The powders were dried before they were scanned by an X-ray diffractometer. Mineral phases were determined at 40 kV/20 mA; Cu radiation; the scan range was between 5 and 80°; the step size was 0.020°; and scan speed was 10°/min. RCA materials possess high CaO content that ranged from 39 % (ESD) to 56 % (DOT1) by weight (Table 1). Table 1 also presents the total metal concentrations of RCAs in (%) for Ca and Fe and in mg/kg for Cr, Cu, and Zn.
Selection of target metals for the study
This study investigated the leaching behavior of five metals: calcium (Ca), chromium (Cr), copper (Cu), iron (Fe), and zinc (Zn). Cr, Cu, Fe, and Zn were selected because when leached to the soil and/or groundwater, at higher concentrations, they may pose environmental concerns. Particularly, Cr, Cu, and Zn are listed on the priority contaminant list by US EPA. Fe is listed on the secondary priority list by US EPA but is included in this study because its association with other metals could alter the leaching mechanisms of Cr, Cu, and Zn. Other potentially toxic metals including cadmium (Cd), lead (Pb), and silver (Ag) were not studied in the current study since the previous studies indicated that these metals did not exhibit leaching potential at alkaline pH conditions (pH > 10) (Engelsen et al. 2009, 2010; Chen et al. 2013). In addition to the metal concentrations, pH of effluent solutions obtained from the samples were also investigated because pH is shown to be one of the most important parameters significantly affecting the leaching behavior of metals (Engelsen et al. 2012). The pH of the effluent solutions is directly related to Ca concentrations leached to the aqueous solutions (Cetin et al. 2012a). Therefore, it was important to investigate the relationship between leached Ca concentrations and effluent pH.
Methods
Sample preparation for leaching tests
Overall there were four different methods used in this study to extract leachate. Preparation of the samples to be used in all leaching tests was the same. Samples were oven-dried overnight at 110 °C ± 5°, crushed and sieved through a US No. 10 sieve (2 mm). Each sample was mixed with deionized water (ASTM Type II) at optimum moisture content (wopt) of the RCA materials in a sample container (Table 1). Separate sample containers were used for each RCA sample and containers were sealed with plastic wrap to minimize contact with the atmosphere. The sample containers were transferred to a moisture-controlled humidity chamber (19 °C ± 2 and 95 % ± 5 relative humidity) and stored for 7 days (unless otherwise noted) before any leachate was extracted.
Leachate extraction tests
WLT method (ASTM D4793)
RCA materials were crushed and sieved through a US No. 10 sieve (2 mm) and were allowed to react with water for 7 days. After reaction ends, the samples were prepared at an L/S ratio of 10 to simulate a representative field sample (Kosson et al. 2002). The influent solutions were prepared with 0.02 M NaCl solution to provide stable reaction conditions for the release of metals (Cetin and Aydilek 2013). The tubes were rotated end-over-end at 28 rpm at approximately 22 °C for 72 h to reach equilibrium conditions. The samples were allowed to settle for 5 min before measuring pH values. The samples were filtered through a 0.2-μm pore size membrane disk filter using a 25-mm Easy Pressure syringe filter holder and a 60-mL plastic syringe into an acid-cleaned 50-mL centrifuge tube. The samples were acidified with 10 % trace metal grade nitric acid (HNO3) to a pH less than 2, and then stored in a refrigerator between 0 and 4 °C.
SPLP method
The SPLP method was performed to simulate leaching due to acid rain (Kosson et al. 2002; Pandey et al. 2012). Following EPA method 1312, extraction fluid was a 60/40 % by weight of the mixture of reagent grade sulfuric acid (H2SO4) and reagent grade HNO3, which was diluted with deionized water to a pH of 4.20 ± 0.05. All samples were prepared at an L/S ratio of 20:1 (twice the L/S ratio used in WLT method). Samples were rotated for 18 ± 2 h at 28 rpm and were subsequently vacuum filtered using 0.7-μm borosilicate glass fiber filters. The filters were acid-washed prior to use with 10 % trace metal grade HNO3 then rinsed with deionized water. Filtered samples were preserved with pH less than 2 with 10 % trace metal grade HNO3 and refrigerated at less than 4 °C.
TCLP method
The TCLP method is another common method that is used to simulate leaching of contaminants in an acidic environment (Kosson et al. 2002; Pandey et al. 2012). The leachate extraction based on TCLP was performed in accordance with EPA method 1311. The leachant used was prepared with 500 mL deionized water, 5.7 mL reagent grade glacial acetic acid (CH3COOH), and 64.3 mL 1 N sodium hydroxide (NaOH). This mixture was then diluted with deionized water to a final volume of 1 L, resulting in a pH of 2.88 ± 0.05. A 2.5-g sample was mixed with 50 mL of the TCLP leachant at L/S ratio of 20:1 (same L/S ratio as in the SPLP method). The same rotation, filtration, and leachate preservation procedures were followed in the SPLP tests.
pH-dependent leaching test
The effect of pH on leaching was studied following EPA method 1313. The reaction vessel was downsized from the recommended 500 to 50 mL polypropylene centrifuge tubes to accommodate available laboratory equipment. An L/S ratio of 10:1 was used with 3.5 g of sample. A rotation of 28 ± 2 rpm was used as specified by the method. Nine target pH values of 2, 4, 5.5, 7, 8, 9, 10.5, 12, and 13 (±0.05) were achieved through the addition of either 2 N trace metal grade HNO3 or 1 N potassium hydroxide (KOH) to deionized water. The required volume of liquid needed for each target pH was calculated using LeachXS™ Lite Data Template—Version 1.2 spreadsheet. The calculated volumes take the wopt of the material into consideration. Samples were pressure filtered through clean 0.2-μm pore size membranes using a 25-mm Easy Pressure syringe filter holder and a 60-mL plastic syringe into a fresh acid-cleaned 50-mL centrifuge tube. Samples were preserved with 10 % trace metal grade HNO3 to a pH less than 2 and refrigerated at less than 4 °C.
Influence of factor on leached metals in WLTs
WLTs (the most commonly used leaching method in environmental studies in USA) were used to investigate the influence of four factors on leachate, generated from five different RCA materials. Brief discussions on why such factors were considered for this investigation are presented below.
Changes in metal concentrations with time after adding water (reaction time)
Cement in concrete is allowed to react so that hydration of the cement occurs, forming calcium-silicate-hydrate (C–S–H) gel, which gives concrete its strength (Bullard et al. 2011). RCA may also still contain cement minerals such as dicalcium silicate (C2S), which is never completely hydrated during service life of the concrete (Poon et al. 2006). The rehydration of these cement grains and deteriorated cement paste may influence leaching potential of metals. This phenomenon was investigated by adding water to RCA and allowing 1, 7, and 28 days for the samples to react with water (referred to as reaction time) in the moisture-controlled humidity chamber. The number of days for reaction time were chosen based on typical time frames associated with reacting of chemical reagents (such as cement, lime, and fly ash) when mixed with water (Mamlouk and Zaniewski 2011).
Atmosphere exposure
The effect of atmospheric exposure was evaluated to test the hypotheses that exposure of RCA to the atmosphere may cause carbonation which could form calcite precipitation in RCA materials. The carbonation process occurs when dissolved carbon dioxide reacts with dissolved portlandite (Ca(OH)2) and C–S–H gel in the concrete to form calcite (CaCO3) (Garrabrants et al. 2004; Gervais et al. 2004). Carbonation removes alkalinity and the hydroxide content of RCA, decreasing the pH of the leachate. It also degrades cement hydrates such as C–S–H and ettringite, which can have oxyanionic elements such as chromate (CrO4 2−) substituted in the structure (Baur et al. 2004; Mulugeta et al. 2011) leading to a greater release of certain metals. It should be noted that the carbonation process was not measured in this study (noted as a limitation of this study), but was used to describe the effects of atmospheric exposure based on effluent pH and leached metal concentrations.
The effect of exposing freshly produced RCA to atmosphere was investigated using samples from the DOT3 location where samples were obtained from an active construction site. All collected samples were freshly crushed in the laboratory at their arrival and were evaluated in three different stages: (i) samples allocated as DOT3 were evaluated right away and considered to represent freshly exposed RCA to atmosphere (simulating field conditions as closely as possible), (ii) samples allocated as EXP1.5 were exposed to atmosphere for 43 days (1.5 months) before testing, and (iii) samples allocated as EXP2 were exposed to atmosphere for 62 days (2 months) before testing. In this study, RCA samples were spread out on the floor in the laboratory to enable the contact of all RCA particles with the atmosphere at regular laboratory settings (room temperature and room relative humidity). Before running the leaching test, RCA materials were remixed and the procedure used for regular sample preparation for leaching tests was followed. After exposure, all samples were reacted with water for 1, 7, and 28 days in humidity room prior leaching tests.
Liquid-to-solid ratio
The effect of liquid-to-solid (L/S) ratio was investigated to better understand the effect of increase in water amount on RCA to leached metal concentrations. This information was intended to provide insight on different potential saturation conditions in the environment where the RCA may be exposed to in the field. All samples were prepared with 5:1, 10:1, 15:1, and 20:1 L/S ratios. Higher L/S ratios indicate higher water content and in according to Kosson et al. (2002), 10:1 L/S ratio represents typical field conditions. All samples were allowed to react with water for 7 days in humidity room prior to leaching tests.
Particle size
Important chemical and physical properties of RCA affecting leaching are related to particle size of the material. Fine particles may contain more cement paste, which largely controls the leaching of RCA (Engelsen et al. 2009). However, Chen et al. (2012) determined fine particles to have a tendency of possessing lower pH than coarse particles. Chen et al. (2012) claimed that carbonation may have caused this difference between coarse, sand, and fine particles of RCAs. Chen et al. (2012) and Edil (2012) speculated that fine RCA particles had greater surface area than those coarse RCA particles, which provided higher surface area for carbonation reaction to occur. It was claimed that cement content will decrease due to carbonation and it would ultimately decrease the pH of fine RCA particles. The particle sizes and shape of RCAs are dependent on the crusher used in the plant or onsite, which causes different gradation of RCA when these materials are used in highway construction. Therefore, it is important to determine the impacts of particle size of RCA on the leaching of metals. The particle size fractions tested in the current study were 9.5–4.75 mm, 4.75–2.36 mm, 2.36–1.19 mm, 1.19–0.6 mm, 0.6–0.3 mm, 0.3–0.15 mm, 0.15–0.105 mm, 0.105–0.075 mm, and less than 0.075 mm.
pH
The pH of the material and the environment are key factors affecting the release of many constituents for various kinds of materials. pH has a strong influence because dissolution and sorption processes are pH dependent (van der Sloot and Dijkstra 2004). The change in pH affects the release of metals from recycled materials by (1) increasing of dissolution and/or desorption of metal-bearing mineral phases under acidic conditions or (2) diminishing leaching due to precipitation and/or increasing of partitioning into a solid phase at higher pH or under alkaline conditions. To evaluate a specific threshold, different pH conditions were simulated as described above.
Metal analyses
The preserved leachate samples were analyzed using a PerkinElmer AAnalyst 100 flame atomic absorption spectrometer (AA). The AA was calibrated using known concentrations of diluted commercial multielement standard solutions. Analyses of metal concentrations within the linear range involved a single calibration standard solution. This was the case for all metals except Ca. For analysis at concentrations beyond the linear range, two or more calibration standards were used. Calibration standards were verified every five samples and at the end of each sampling session. These readings were to be within 5 % of the expected concentration or recalibration and resampling of the previous sample set was performed.
Minimum detection limits (MDLs) for AA were determined for each element. The MDLs for Ca, Cr, Cu, Fe, and Zn were determined as 2.5 mg/L, 2, 3, 4, and 10 μg/L, respectively.
Geochemical modeling
MINTEQA2, a numerical model developed by US EPA and aims to simulate equilibria and speciation of inorganic solutes in aqueous solutions, was used to determine the predominant oxidation states and leachate controlling mechanisms of the leached metals. Leached metal concentrations from WLTs and recorded pH of the leachate were used as an input in the MINTEQA2 geochemical modeling program. Table 2 presents the input data used in the geochemical modeling analyses in the current study. The log activity values of leached metal concentrations from RCAs at pH 0–14 were calculated along with the saturation indices of the leachates with respect to solids or minerals. Detailed information regarding the MINTEQA2 geochemical modeling analyses method could be found at Komonweeraket et al. (2015a).
Results and discussions
Duplicate WLTs were conducted to study the effect of reaction time, atmosphere exposure, L/S ratio, particle size, and pH on leaching behavior of metals in RCA. The averages of the results are presented in this study.
Influence of factors on leached metals from WLTs
Effects of reaction time
Effluent pH and leached metal concentrations of RCAs after 1, 7, and 28 days of reaction time are shown in Figs. 2 and 3. In all cases, the pH values of the leachates were highly alkaline (pH > 10) (Fig. 2). The rehydration and precipitation of Ca minerals in the RCA aggregate matrix occurring during the reaction process most likely caused the decrease in the effluent pH. Similar conclusions were reported (Sanchez et al. 2009), claiming that the precipitation of Ca as CaCO3 may be the reason for the decrease in effluent pHs.
There were not any clear correlations between the initial total metal content of RCA materials and leached metal concentrations. For instance, DOT1 had the lowest total Cu content among all RCA materials (Table 1) but showed the highest Cu leaching (Fig. 3). The results of this study indicate that leaching of metals from RCA is not significantly dependent on the total metal contents. Leached Zn concentrations from all RCAs were below the detection limit. This finding is consistent with the previous studies, which also found that at alkaline conditions, leaching potential of Zn decreases significantly (Engelsen et al. 2010; Chen et al. 2012). However, it was important to confirm this finding in this study as RCA is a recycled material and the properties may vary from one source to another.
Figures 2 and 3 show that an increase in reaction time leads to decrease in pH, Ca, and Cu concentrations. Cr and Fe concentrations generally showed an initial increase then slightly stabilized. Leached Fe concentrations from DOT3 RCA showed the opposite trend, decreasing in concentrations with reaction time. Moreover, a significant drop in the leached Ca concentrations (1.4 to 4.3 times) was observed in DOT3 with an increase in reaction time, while the leached Ca concentrations from other RCAs stabilized after 7 days of reaction time. DOT3 was freshly crushed RCA material and its immediate exposure to open atmosphere may cause significant release of Ca and Fe metals as a first flush.
Allowing the RCA samples to react for a longer period of time may increase the rehydration rate of cement particles in RCA, which may eventually cause encapsulation of particles. Thus, this may also contribute on immobilization of metals that are attached to surface of RCA particles. It is speculated in this study that rehydration of cement in RCA matrix may have also immobilized the leached Ca (except in DOT3 sample) and Cu in the aqueous solutions.
Immobilization of metals with cement, including Cr and Fe, has been observed through the following mechanisms as cited by Gougar et al. (1996): CrO4 − and Fe3+ can substitute for SO4 2− and Al3+, respectively, in ettringite. However, it is believed that the self-cementing process may not have fully completed after 1 day of adding water in the current study, as evidenced by the initial increases in Cr and Fe concentrations. After the 7 and 28 days, the results showed Cr and Fe concentrations almost stabilized (Fig. 3). These results suggest that significant hydration of cement particles in RCA occurs after almost a week of mixing with water. The kinetics of hydration is dependent on several factors including the type of cement minerals present in the RCA matrix. For instance, C2S (2CaOSiO2), a primary mineral in portland cement, is very slow to hydrate compared to other cement minerals such as C3S (3CaOSiO2) and C3A (3CaOAl2O3) (Mamlouk and Zaniewski 2011). The C2S present in RCA but the amount depends on the age, particle size, and amount of cementitious materials used in the original concrete mix (Poon et al. 2006). Although the amount was not measured in this study, the presence of C2S in RCA may have caused self-cementation to occur and let to the stabilization of leached metal concentrations from RCA with longer reaction time (i.e., 7 to 28 days). In addition, carbonation of remaining cement particles on the surface of RCA particles may have caused the precipitation of calcite and encapsulate the surface of these particles. This may have decreased the leaching rate of Cr and Fe (Van Gerven et al. 2007). Lange et al. (1996) similarly found that carbonation of cement had been shown to reduce the leached concentrations of Zn which was a metal that tended to have similar leaching patterns from recycled materials (Komonweeraket et al. 2015a, b).
Effects of atmosphere exposure
Leaching results of the DOT3 samples, which were freshly crushed, exposed to the atmosphere for 1.5 (Exp1.5) and 2 (Exp2) months were compared to the original freshly crushed DOT3 leaching data (Fig. 4). The pH of freshly crushed DOT3 sample that has not been exposed to atmosphere for a period of time is slightly higher than the samples exposed for 1.5 and 2 months because it was the “freshest” sample and expected to experience the least amount of carbonation. It is well known that carbonation process tends to lower the pH in aqueous solutions (Stumm and Morgan 1996) which may have lead the slight decrease in the pH of the DOT3 effluent solutions in 1.5 and 2 months of atmosphere exposure.
Leached Ca concentrations were much higher in freshly crushed DOT3 samples. Leachate pH was slightly higher in 1.5-month samples compared to the 2-month samples indicating that effects of atmosphere exposure continued to impact the leaching process. These results may also be interpreted as that the changes in Ca concentration occur significantly when the samples are exposed to atmosphere initially but then does not change much over time (Fig. 4).
The similar trend was also observed for the leaching of Cu metal (Fig. 4). Leached Cu concentrations decreased with an increase in exposure time while at the atmospheric conditions, leaching of Cr and Fe concentrations increased (Fig. 4). These results showed that exposing RCAs to the atmosphere may greatly influence the leaching behavior of metals in different ways. Even though it is not measured in the current study, it is speculated that carbonation process is most likely to occur on the surface of the RCA particles when they are left open to the atmosphere and may impact the leaching of different metals in dissimilar ways.
The increases in Cr and Fe concentrations demonstrate the increased solubility of these materials, which is believed to occur due to carbonation process. Enhanced solubility of Cr has been shown in carbonated materials (Mulugeta et al. 2011; Pandey et al. 2012). As recycled concrete becomes carbonated, cement hydrates are degraded releasing Cr as well as Fe, which was also observed in the current study (Fig. 4). On the contrary, carbonation may cause the decrease in Cu concentrations. Carbonation process may be the phenomenon that slightly lowers the material pH and decreases the solubility of Cu minerals resulting in a copper carbonate precipitate (Garrabrants et al. 2004) such as Cu2(OH)2CO3(s) or Cu3(OH)2(CO3)2(s) (Stumm and Morgan 1996).
Effects of liquid-to-solid ratio
Figure 5 illustrate the pH and the leaching behavior of Ca, Cr, Cu, and Fe with changes in L/S ratio. It was observed that an increase in the L/S ratio caused a decrease in pH and metal concentrations. For all RCA materials, both pH and Ca concentrations decreased with increasing the L/S ratio. The effluent pHs of the RCA materials, in decreasing order, were DOT3, ESD, WSD, DOT1, and DOT2. Leached Ca concentrations from RCA materials followed the same order indicating that there is direct correlation between effluent pHs and leached Ca concentrations from RCAs. Garrabrants et al. (2004) claimed that leached Ca concentrations in carbonated Portland cement mortar decreased with an increase in L/S ratio due to formation and precipitation of calcite. Carbonation of the remaining cement mortar on RCA particles could be the reason to observe a decrease in the leached Ca concentrations from RCA materials which may have also impacted the effluent pH values.
Increasing the liquid content of the leaching test diluted the leachate as indicated by the decreasing dissolved metal concentrations. Thus, dissolution of the samples did not increase, indicating the RCA materials to be very insoluble. These findings are consistent with the results of previous studies conducted on other waste materials (Kosson et al. 2002; Kim and Hesbach 2009; Ward et al. 2009). The fresh DOT3 is the RCA which were the least exposed to atmosphere among all RCAs (the least potential for carbonation occurrence) had the highest Ca and Cu concentrations.
Leached Cr concentrations decreased with L/S ratio was most probably due to its sorption onto insoluble ettringite and/or hydrous ferric oxide (HFO). The effluent pH influences this sorption process of Cr process such that lower pH improves adsorption of oxyanionic species (CrO4 −) to amorphous hydroxides such as iron oxides (Cornelis et al. 2008; Mulugeta et al. 2011).
Fe concentrations decreased with an increase in L/S ratios, which can be attributed to the presence of insoluble Fe precipitates in the RCA materials. Aqueous Fe released from RCA likely formed Fe(OH)3 and/or FeCO3 precipitates, which are very insoluble at high pH (pH > 10) (Gitari et al. 2008).
Effect of particle sizes
Leaching from different particle sizes in RCA was studied for nine different fractions. pH was relatively stable in each particle size fraction indicating the pH of the leachate is more dependent on the RCA material itself rather than particle size. Figure 6 shows the concentrations of Cu and Cr metals generally decrease with particle size while no consistent relationship was found between the particle sizes and the leaching of Ca and Fe.
The average pH change was 2 % with most samples having increased pH in the larger size fractions. Ca concentrations were variable among each sample ranging between 38 mg/L (DOT1—9.5–4.75 mm) to 2040 mg/L (ESD—9.5–4.75 mm). The leaching Ca decreased in the larger size fractions except for ESD. Cr decreased with increasing particle size for all RCAs. Leached Cu concentration decreased significantly with increasing particle sizes with an average decrease of 83 %. Leached Cu concentrations were between 0.0016 mg/L (DOT2 fraction 9.5–4.75 mm) and 0.271 mg/L (WSD fraction <0.075 mm). Leached Fe concentrations fluctuated. Overall concentrations ranged from 0.043 (DOT2 fraction 9.5–4.75 mm) to 1.28 (DOT1 fraction 0.6–0.3 mm). The average percent difference between the smallest and largest fractions was 94 %.
One of the mechanisms affecting leaching by particle size is the cement composition within each fraction. Engelsen et al. (2010) claimed that the leaching of trace metals is mostly due to the cement paste content and it is speculated that there may be more cement paste in certain fractions than others, which may yield the results obtained from the current study. However, the cement paste content in RCA materials was not measured in the current study to confirm this relationship.
pH-dependent WLTs
pH-dependent leaching tests were conducted to investigate the leaching behaviors and leaching controlling mechanisms of RCA materials. pH of the RCA leachate was adjusted from 2 to 13. The acid neutralizing capacity (ANC) and pH-dependent leaching of Ca, Cr, Cu, Fe, and Zn are shown in Fig. 7. The leaching of Ca showed a cationic pattern whereas the other metals followed an amphoteric pattern (Fig. 7). These patterns are consistent with past studies of RCA and Portland cement as presented by Chen et al. (2012), Engelsen et al. (2009, 2010), Garrabrants et al. (2004), and Sanchez et al. (2009).
EPA drinking water standards were exceeded in the acidic leaching range for Cr, Cu, and Fe. Cr concentrations exceeded the EPA MCL (100 μg/L) for all RCA materials after a pH of 5. Cu exceeded the SMCL (1 μg/L) at pH < 2 and Fe exceeded the EPA SMCL (300 μg/L) at pH < 7. A normal field environment would have neutral pH conditions, so it is unlikely that the leaching of Cr and Cu would cause health issues. Fe concentrations may exceed the SMCL in the environment, but this optional federal standard is in place for improved taste in drinking water (EPA 2009), and Fe leaching from RCA would likely not be detrimental to the environment.
Recycled concrete aggregates were shown to have limited acid neutralizing capacity. At a neutral to slightly acidic pH, the majority of the samples displayed some buffering capacity. Outside of this range, the RCA materials showed rapid pH changes upon acid addition. This plateau could be due to buffering from the dissolution of calcite in the carbonated materials depending on the degree of carbonation (Chen et al. 2012; Garrabrants et al. 2004). For the specimens that experienced limited carbonation, Ca(OH)2 precipitate could be the main buffering solid phase.
The materials listed in order of increasing ANC are as follows: ESD, DOT1, WSD, DOT3, and DOT2. The RCA materials from concrete recyclers (ESD) had the lowest ANC. This may be due to these materials being more carbonated or by having different sources of concrete; the original concrete may have come from building demolitions and not just pavement. Table 1 shows ESD to be the only materials with a greater percentage of SiO2 than CaO, which may be due to the different sources of concrete in these materials. Moreover, it should be noted that the remaining cement content has greater impact on ANC of the RCA materials than SiO2/CaO ratios. However, the remaining cement content of RCA materials used in this study was not measured and such comparison could not be made. It is strongly recommended that in the future studies, the cement content of RCA materials should be evaluated to understand not only ANC of these materials more in detail but also its correlation to the other parameters and processes such as effluent pH and leaching behavior elements.
Leaching of major elements from wastes, under equilibrium conditions, is dominated by solubility processes (Kosson et al. 2002). Thus, the major elements studied, Ca and Fe, were likely solubility-controlled. For Ca, increasing the solution pH likely caused Ca2+ to precipitate as a carbonate, and decreasing the pH caused dissolution of this solid (Garrabrants et al. 2004; Komonweeraket et al. 2015c). Ca showed a cationic leaching pattern, which steadily continued to decrease as effluent pH of the RCA materials increased. Ca concentrations soared with increasing acidity due to dissolution of calcite and cement hydrates. More carbonated samples again would have less Ca released because Ca(OH)2 is more soluble than CaCO3 (Engelsen et al. 2009; Garrabrants et al. 2004). The order of Ca leaching from lowest to highest was MD1, ESD, MD2, DOT2, WSD/DOT1, and DOT3. These results were similar to the ANC study demonstrating a strong relationship between pH and Ca leaching.
Cr, Cu, Fe, and Zn had amphoteric leaching forming a U-shaped curve with increased concentrations at extreme acidic and alkaline pH values and minimum concentrations between these limits (Fig. 7c–f). At a pH near 10.5, the leached concentrations of these metals were the lowest. Acidic conditions caused the greatest release of Cr, Cu, Fe, and Zn as shown in Fig. 7 due to greater dissolution of metal-bearing minerals. Geochemical modeling of the RCA materials used in the current study would provide additional knowledge into the processes controlling leaching of these metals and allow to determine whether they were solubility controlled or sorption controlled from metal-bearing minerals.
Geochemical modeling results
MINTEQA2, a numerical model, was used to determine the predominant oxidation states and leachate-controlling mechanisms of the leached constituents from RCA materials. Metal concentrations from pH-dependent leaching tests and specific pH of the leachate collected in leaching tests were used as an input in the MINTEQA2 geochemical modeling program.
The two main equilibrium mechanisms that control the leaching of elements from industrial waste byproducts such as RCA, fly ash, steel slag, etc. are solubility (dissolution-precipitation) and sorption (Fruchter et al. 1990; Mudd et al. 2004). In the case that dissolution-precipitation reactions control the leaching of elements, geochemical equilibria models based on thermodynamic data have been shown to successfully predict aqueous concentrations, assuming equilibrium between the leachate and the solubility-controlling solids (Allison et al. 1991). A more complex model that incorporates sorption of kinetic algorithms is required to predict solute concentrations if sorption reactions or dissolution kinetics control the leaching of metals (Fruchter et al. 1990).
Speciation analyses resulted in that the dominant oxidation states of Ca, Cr, Cu, Fe, and Zn were Ca2+, Cr(VI) as CrO4 2−, Cu2+, Fe3+, and Zn2+, respectively. Log activity diagrams were created by plotting MINTEQA2-based log activities of the elements of interest against laboratory-measured leachate pH to discern whether the concentrations of these elements were controlled by mineral solubility (Fig. 8). If release of an element is controlled by mineral solubility, calculated activities of elements of interest should consistently fall in close proximity to the stability/solubility line of the mineral or solid (Garavaglia and Caramuscio 1994).
Figure 8a shows that the leaching mechanism of Ca is controlled by the dissolution and precipitation of different Ca-bearing minerals depending on the effluent pH. It should be noted that carbonate concentrations were not measured in this study and therefore, they could not be put as input into the geochemical modeling (Table 2). In the future studies, these measurements should be done and used in the geochemical modeling analyses. Based on the results obtained from geochemical modeling analyses in this study for Ca, it was observed that anhydrite (CaSO4) mineral was controlling the leaching of Ca until a pH of ∼8–9. Around a pH of 8 to 9, dissolution/precipitation of calcite appears to potentially also contribute to the leaching of Ca. The leached concentrations of Ca appear to become oversaturated over the solid phase line of calcite after a pH of 9. Oversaturation with respect to calcite could be due to the presence of gypsum from the remaining cement paste on the RCA particles, which may cause the release of more Ca than dissolution of calcite (Langmuir 1997). Figure 8a shows that the Ca(OH)2 starts controlling the leaching behavior of Ca at pH > 10. These results showed that calcite may not seem to have a significant role in controlling the leaching mechanisms of Ca, although previous findings from Stumm and Morgan (1996) indicated that calcite had a great influence on the leaching of Ca at alkaline conditions. Based on these results, although it appears that carbonation may not have great influence in this case, more evaluation is needed to confirm this.
Figure 8b indicates that the leaching of Cr(VI) in the effluent solution is not controlled by chromium (hydr)oxides. Engelsen et al. (2010) also found that the leaching process of Cr(VI) at alkaline pHs could not be described with dissolution or precipitation of metal hydroxides. It has been claimed that CaCrO4 and Cr(VI)-ettringite minerals may control the leaching of Cr(VI) metal species at highly alkaline conditions (pH > 10) (Johnson et al. 1999; Astrup et al. 2006; Karamalidis and Vouidrias 2008). Figure 8c shows the variation of log activity of Ca2+ values corresponding to log activity of CrO4 2− values. According to the solid line that represents the CaCrO4 (s), this line is approximately 3 to 10 orders of magnitude above the log activity of Ca2+ and log activity of CrO4 2− values. This indicates that the solubility of CrO4 2− may be controlled by CaCrO4(s). In general, the solubility of Cr(VI) at high pH values is controlled by Cr(VI)-ettringite minerals (Astrup et al. 2006; Karamalidis and Vouidrias 2008; Engelsen et al. 2010). At pH values greater than 10, the Cr(VI) replaces SO4 2− in ettringite minerals. This substitution of SO4 2− anion is dependent on the amount of Cr(VI) concentrations in the effluent solutions (Engelsen et al. 2010). However, this claim could not be tested in this study since SO4 2− measurements were not taken to be put in the geochemical modeling.
Based on Fig. 8d, the crystalline phase of CuO mineral Tenorite(c) is controlling the solubility of Cu(II) metal species in the aqueous solutions collected from RCAs. Previous studies also claimed that at pH > 9, tenorite or precipitation of Cu(OH)2(s) controls the leaching of Cu(II) (Engelsen et al. 2010; Garavaglia and Caramuscio 1994; Fruchter et al. 1990). Cu(OH)2(s) is also known as a solid phase that controls the leaching of Cu metals especially under alkaline conditions (Apul et al. 2005).
In the geochemical modeling analysis, it was found that Fe3+ is the dominant oxidation state of Fe metals in the aqueous solutions of the RCAs. Apul et al. (2005) and Komonweeraket et al. (2015a) also claimed that the predominant Fe species in similar waste materials were Fe3+. It is well known that Fe solubility is controlled by hydroxide minerals (Fruchter et al. 1990; Gitari et al. 2009). Figure 8e indicates that solubility of Fe is more likely controlled by hematite (Fe2O3) minerals rather than Fe(OH)3-amorphous. These results are consistent with Black et al. (1992), which claimed that the solubility of Fe metals was controlled by Fe2O3 and Fe3O4. Fruchter et al. (1990) and Mudd et al. (2004) do not support the findings in this current study about the solubility controlling the phase of Fe; however, these previous studies did not include high basic conditions, i.e., pH > 12 (Fig. 8e). At such pH levels, it is possible for Fe 3+ to be controlled by hematite instead of ferrihydrite (Fe(OH)3). In addition, X-ray diffraction analysis indicated that hematite is the primary mineral phase of Fe in the RCAs used in this study.
Solubility of Zn metals is mainly controlled by precipitation and dissolution reactions in the soil matrix (Murarka et al. 1992). The results of geochemical analysis indicated that zincite (ZnO) and Zn(OH)2 were determined as solid phases that controlled the leaching of Zn at pH > 7 (Fig. 8f). This finding is also consistent with the findings of Garavaglia and Caramuscio (1994), which also reported that zincite and Zn(OH)2 were the two main (hydr)oxides controlling the leaching of Zn.
Dijkstra et al. (2002) suggested that including surface precipitation of Zn on the soil particles in the speciation analyses would provide more detailed information about the leaching behavior of Zn. This, however, was not the scope of this study; therefore, it was not included in the MINTEQA2 analysis of the Zn. The adsorption of Zn onto Fe and Al (oxy)hydroxide minerals tends to occur often at neutral pHs (Dijkstra et al. 2004). Since, the pHs of the effluent solutions of the RCA materials are very high and the ZnO solid line closely matches the Zn2+ concentrations (Fig. 8f). The sorption of Zn onto Fe and Al (oxy)hydroxide minerals was not observed in the current study.
Comparisons of the results from WLT, TCLP, and SPLP test methods
All three leach tests conducted in this study were very similar to each other in terms of sample preparation, but they differ in terms of test duration and L/S ratio. The L/S ratio used in the preparation of WLTs samples was 10:1 while it was 20:1 for the samples prepared for SPLP and TCLP tests. WLT samples were rotated for 72 h while TCLP and SPLP samples were rotated only for 18 ± 2 h.
Figures 9 and 10 illustrate the results from SPLP and TCLP tests compared to the WLT results. Overall, the TCLP test results showed the most conservative leaching. The extraction fluid used in the TCLP is the most acidic (pH 2.88) of the three tests and resulted in greater leaching due to the increased degradation of the cement paste and greater solubility of metals at the lower pH (Engelsen et al. 2010). Similar trends were observed within pH-dependent WLTs results. Leached metal concentrations were highest at acidic pH conditions (pH < 7).
The leached pH from the WLT and SPLP tests were similar, yet the WLT yielded greater concentrations of Ca, Cr, and Cu than the SPLP. Figure 9 shows that the concentrations of Ca, Cr, and Cu from WLTs are up to 2 and 3 times higher than the concentrations obtained from SPLP tests, respectively. The pH of the SPLP extraction fluid was 4.20 and the pH of the WLT influent solution was 7.50. In addition, the influent solution used in WLTs was 0.02 M NaCl, indicating that there was significant amount of dissolved Cl concentrations in the aqueous solutions. More leaching would be expected from the SPLP due to its lower pH. However, these results indicate the chemical composition of the extract/influent solutions affected leaching more than the effluent pH of SPLP samples (pH ∼4.2). The WLT influent solution is thought to have caused increase in the leaching of Ca, Cr, and Cu due to its chloride (Cl) content. Increased Cl concentrations may increase Ca, Cu, and Cr leaching by forming mobile Cl metal complexes (Engelsen et al. 2012). However, Fe concentrations were lower in the WLT than in the SPLP. Fe-(hydr)oxide starts dissolving dramatically at pH < 6 (Cetin et al. 2012a). The pH values of the leachates collected from SPLP tests were buffered around pH = 4.2 while the effluent pH values measured from the WLTs samples were very alkaline (pH > 11). This indicates pH was the dominant factor on controlling the leaching of Fe from RCA materials than dissolved Cl concentrations present in the WLT aqueous solutions.
Figure 10 shows that the concentrations of Ca, Cr, Cu, and Fe in TCLP tests are up to 10, 2, 2, and 10 times higher respectively than the concentrations of these elements obtained from the WLTs. The pHs of the effluent solutions of the samples in TCLP tests (pH ∼2.9) were more acidic than those in WLTs and SPLP, which was most probably the reason for the higher leached element concentrations of the samples collected from the TCLP tests.
Conclusions
Laboratory batch leach tests of RCA collected from five different locations (two of which from existing stockpiles developed from a mix of concrete sources and the three of which from concrete recycled from interstate highway pavement surfaces) were performed to investigate the effect of several factors that RCA may encounter during storage or in service that may affect the effluent pH and leached metal concentration. The following conclusions were derived from this study:
-
1.
Concentrations of all four (Cr, Cu, Fe, and Zn) metals were below the regulatory limits determined by EPA MCLs in all tests with few exceptions (WLTs, SPLPs, TCLPs) even though the samples were collected from different sources and were tested with different leachate extraction methods. No consistent relationship was found between the total metal content of RCA materials and leached metal concentrations.
-
2.
pH of the leachate and leached Ca and Cu concentrations decreased with an increase in time after the water is added to the sample (reaction time). On the other hand, leached Cr and Fe concentrations increased throughout the 7 days of reaction time and stabilized thereafter. This indicated that allowing the samples to react with water for 7 days may have increased the rehydration rate of cement particles in RCA matrix, which could enhance the immobilization of the metals.
-
3.
Effluent pH and leached Ca and Cu concentrations from DOT3 samples decreased while Cr and Fe concentrations were increased when they were exposed to atmosphere for a longer period of time. These results indicate that exposing RCAs to the atmosphere may greatly influence the leaching behavior of metals.
-
4.
Increasing the liquid-to-solid ratio (L/S) decreased the leached concentrations of Ca, Cr, Cu, and Fe from the RCA due to the dilution of leachate in RCA materials. This indicates that saturation level of the RCA constructed in the field may alter the leaching potential of metals.
-
5.
Leached Cr and Cu concentrations from the larger particles were lower than the ones leached from finer particles of RCA materials. However, no consistent trend was observed between the particle sizes and leaching of Ca and Fe metals.
-
6.
pH-dependent leaching tests showed a cationic leaching pattern for Ca with concentrations increasing with decreasing pH. Amphoteric leaching patterns were observed in Cr, Cu, Fe, and Zn such that the concentrations were at minimum level at neutral pH and increased significantly at acidic and basic conditions. DOT2 and WSD RCAs showed a higher ANC than the other RCAs. This is likely due to the higher CaO contents of DOT2 and WSD RCAs than the CaO contents of the other RCA materials.
-
7.
Geochemical modeling analyses indicated that leaching of Fe, Cu and Zn were controlled by the dissolution-precipitation of CuO, Fe2O3-Fe(OH)3, and ZnO-Zn(OH)2 metal-bearing minerals, respectively. These minerals/solids are either originally present in RCA materials or form in the aqueous solutions.
-
8.
Geochemical modeling analyses in this study indicate that potentially multiple solid phases may have control over leaching of Ca depending on the pH conditions. At pH < 9, leaching of Ca appears to be controlled by anhydrite, around pH of 8∼9 by calcite (CaCO3), and at pH > 9, by the dissolution of calcium hydroxide Ca(OH)2 may have control over leaching of Ca as the solution becomes oversaturated over the solid phase line of calcite.
-
9.
Leaching of Cr from RCA, on the other hand, appears not to be controlled by Cr-bearing (hydr)oxides but by the Ca(CrO4)(s) in accordance with the geochemical modeling. However, it should be noted that at high pH, CrO4 2− is likely to be controlled by ettringite mineral although presence of ettringite could not be observed in this study. This could potentially be due to the limitation of the study where SO4 2− measurements of the effluent solutions were not obtained.
-
10.
The SPLP and TCLP tests simulated leaching potential of metals under acidic conditions. Overall, the TCLP tests provided the highest leached element concentrations due to its very acidic influent solutions (pH ∼2.9). On the other hand, the concentrations of Ca, Cr, and Cu leached from SPLP were lower than the ones measured from the WLT due to the high Cl concentrations in the influent solutions. However, Fe concentrations were lower in the WLT than in the SPLP. SPLP leached 10 times higher Fe concentrations than the WLTs indicating that the lower pH of the SPLP (pH = 4.2), as compared the alkaline pH of WLTs (pH > 11), had a greater influence on the leaching behavior of Fe metals. Observations from these tests indicate that the pH of the water that RCA may come in contact with in the field may also alter the leaching potential of metals.
Limitations of this study
The results presented in this research are based on the five RCA samples collected from South Dakota and the concentration of the metals evaluated in this study. RCA is a byproduct and its composition may vary from one source to another. Other important parameters such as alkalinity and electrical conductivity measurements were not studied. These parameters are generally used to measure carbonation amount, which was not the focus of this study. Carbonate and sulfate concentrations were not measured in this study but previous studies were used to support the results and trends observed. Future studies will include the measurement of carbonate and sulfate concentrations in the leachate.
References
Allison JD, Brown DS, Novo-Gradac KJ (1991) MINTEQA2/PRODEFA2 a geochemical assessment model for environmental systems: version 3.0 user’s manual. U.S. Environmental Protection Agency, Washington, D. C, EPA/600/3-91/021
Apul DS, Gardner KH, Eighmy TT, Fallman A-M, Comans RNJ (2005) Simultaneous application of dissolution/precipitation and surface complexation/surface precipitation modeling to contaminant leaching. Environ Sci Technol 39(15):5736–5741
Astrup T, Dijkstra JJ, Comans RNJ, Van der Sloot HA, Christensen TH (2006) Geochemical modeling of leaching from MSWI air-pollution-control residues. Environ Sci Technol 40(11):3551–3557
Baur I, Keller P, Mavrocordatos D, Wehrli B, Johnson CA (2004) Dissolution-precipitation behaviour of ettringite, monosulfate, and calcium silicate hydrate. Cem Concr Res 34(2):341–348
Black CJ, Brockway D, Hodges S, Milner A et al (1992) Utilisation of Latrobe Valley brown coal fly ash. In: Barton CM (ed) Proc. energy, economics & environment-gipssland basin symp. AusIMM, Melbourne, pp 149–155
Bullard JW, Jennings HM, Livingston RA, Nonat A, Scherer GW, Schweitzer JS, Scrivener KL, Thomas JJ (2011) Mechanisms of cement hydration. Cem Concr Res 41(12):1208–1223
Cetin B, Aydilek AH (2013) pH and fly ash type effect on trace metal leaching from embankment soils. Resour Conserv Recycl 80:107–117
Cetin B, Aydilek AH, Guney Y (2010) Stabilization of recycled base materials with high carbon fly ash. Resour Conserv Recycl 54:879–892
Cetin B, Aydilek AH, Li L (2012a) Experimental and numerical analysis of metal leaching from fly ash-amended highway bases. Waste Manag 32(5):965–978
Cetin B, Aydilek AH, Guney Y (2012b) Leaching of trace metals from high carbon fly ash stabilized highway base layers. Resour Conserv Recycl 58:8–17
Cetin B, Aydilek AH, Li L (2014) Trace metal leaching from embankment soils amended with high-carbon fly ash. Journal of Geotechnical and Geoenvironmental Engineering-ASCE 140(1):1–13
Chen J, Bradshaw S, Benson CH, Tinjum JM, Edil TB (2012) pH-dependent leaching of trace elements from recycled concrete aggregate. In: Hryciw RD, Athanasopoulos-Zekkos A, Yesiller N (eds) Geocongress 2012. American Society of Civil Engineers, Oakland, pp 3729–3738
Chen J, Tinjum JM, Edil TB (2013) Leaching of alkaline substances and heavy metals from recycled concrete aggregate used as unbound base course. Transp Res Rec 2349:81–90
Cornelis G, Johnson CA, Gerven TV, Vandecasteele C (2008) Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: a review. Appl Geochem 23(5):955–976
Daniels JL, Das G (2014) Practical leachability and sorption considerations for ash management. Proceedings of Geocongress, Atlanta, GA, 14p
Dijkstra JJ, Van der Sloot HA, Comans RNJ (2002) Process identification and model development of contaminant transport in MSWI bottom ash. Waste Manag 22(5):531–1
Dijkstra JJ, Meeussen JCL, Comans RNJ (2004) Leaching of heavy metals from contaminated soils: an experimental and modeling study. Environ Sci Technol 38(16):4390–4395
Edil TB (2012) Recycled unbound materials. Minnesota Department of Transportation, St. Paul, Report No. 2012–35
Engelsen CJ, van der Sloot HA, Wibetoe G, Petkovic G, Stoltenberg-Hansson E, Lund W (2009) Release of major elements from recycled concrete aggregates and geochemical modelling. Cem Concr Res 39(5):446–459
Engelsen CJ, van der Sloot HA, Wibetoe G, Justnes H, Lund W, Stoltenberg-Hansson E (2010) Leaching characterisation and geochemical modelling of minor and trace elements released from recycled concrete aggregates. Cem Concr Res 40(12):1639–1649
Engelsen CJ, Wibetoe G, van der Sloot HAH, Lund W, Petkovic G (2012) Field site leaching from recycled concrete aggregates applied as sub-base materials in road construction. Sci Total Environ 427–428:86–97
EPA (2009). Water Drinking Limits. < http://water.epa.gov/drink/contaminants/>. Accessed 14 March 2016
Fruchter JS, Ral D, Zachara JM (1990) Identification of solubility-controlling solid phases in a large fly ash field lysimeter. Environ Sci Technol 24(8):1173–1179
Garavaglia R, Caramuscio P (1994) Coal fly-ash leaching behaviour and solubility controlling solids. Stud Environ Sci 60:87–2
Garrabrants AC, Sanchez F, Kosson DS (2004) Changes in constituent equilibrium leaching and pore water characteristics of a Portland cement mortar as a result of carbonation. Waste Manag 24(1):19–36
Gervais C, Garrabrants AC, Sanchez F, Barna R, Moszkowicz P, Kosson DS (2004) The effects of carbonation and drying during intermittent leaching on the release of inorganic constituents from a cement-based matrix. Cem Concr Res 34(1):119–131
Gitari WM, Petrik LF, Etchebers O, Key DL, Okujeni C (2008) Utilization of fly ash for treatment of coal mines wastewater: solubility controls on major inorganic contaminants. Fuel 87(12):2450–2462
Gitari WM, Fatoba OO, Petrik LF, Vadapalli WRK (2009) Leaching characteristics of selected South African fly ashes: effect of pH on the release of major and trace species. J Environ Sci Health A 44:206
Gougar MLD, Scheetz BE, Roy DM (1996) Ettringite and C-S-H Portland cement phases for waste immobilization: a review. Waste Manag 16(4):295–303
Iyer RS, Stanmore BR, Pullammanappallil PC (1999) Solid–liquid mass transfer during leaching of calcium from dilute slurries of flyash. Chemical engineering research and design. Trans Inst Chem Eng Part A 77(8):764
Johnson CA, Kaeppeli M, Brandenberger S, Ulrich A, Bauman W (1999) Hydrological and geochemical factors affecting leachate composition in municipal solid waste incinerator bottom ash part II. The geochemistry of leachate from Landfill Lostorf, Switzerland. J Contam Hydrol 40:239–259
Karamalidis AK, Vouidrias EA (2008) Anion leaching from refinery oily sludge and ash from incineration of oily sludge stabilized/solidified with cement. Part II. Modeling. Environ Sci Technol 42:6124–6130
Kim AG, Hesbach P (2009) Comparison of fly ash leaching methods. Fuel 88:926–937
Komonweeraket K, Cetin B, Aydilek AH, Benson CH, Edil TB (2015a) Effects of pH on the leaching mechanisms of elements from fly ash mixed soils. Fuel 140:788–802
Komonweeraket K, Cetin B, Benson CH, Aydilek AH, Edil TB (2015b) Leaching characteristics of toxic constituents from coal fly ash mixed soils under the influence of pH. Waste Manag 38:174–184
Komonweeraket K, Cetin B, Aydilek AH, Benson CH, Edil TB (2015c) Geochemical analysis of leached elements from fly ash stabilized soil. J Geotech Geoenviron Eng ASCE. doi:10.1061/(ASCE)GT.1943-5606.0001288, 04015012
Kosson DS, van der Sloot HA, Eighmy TT (1996) An approach for estimation of contaminant release during utilization and disposal of municipal waste combustion residues. J Hazard Mater 47(1–3):43–45
Kosson DS, van der Sloot H, Sanchez F, Garrabrants AC (2002) An integrated framework for evaluating leaching in waste management and utilization of secondary materials. Environ Eng Sci 19(3):159–204
Lange LC, Hills CD, Poole AB (1996) The effect of accelerated carbonation on the properties of cement-solidified waste forms. Waste Manag 16(8):757–763
Langmuir D (1997) Aqueous environmental geochemistry. Prentice-Hall, Inc., New Jersey
Li L, Benson CH, Edil TB, Hatipoglu B (2006) Groundwater impact from coal ash in highways. Waste Resour Manag 159(4):151–162
Li L, Benson CH, Edil TB (2009) Mechanical performance of pavement geomaterials stabilized with fly ash in field applications. Coal Combust Gasification Prod 1:43–49
Mamlouk MS, Zaniewski JP (2011) Materials for civil and construction engineers. Prentice Hall, Upper Saddle River
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, New York
Mudd GM, Weaver TR, Kodikara J (2004) Environmental geochemistry of leachate from leached brown coal ash. J Environ Eng ASCE 130(12):1514–1516
Mulugeta M, Engelsen CJ, Wibetoe G, Lund W (2011) Charge-based fractionation of oxyanion-forming metals and metalloids leached from recycled concrete aggregates of different degrees of carbonation: a comparison of laboratory and field leaching tests. Waste Manag 31(2):253–258
Murarka IP, Rai D, Ainsworth CC (1992) Geochemical basis for predicting leaching of inorganic constituents from coal-combustion residues. Waste testing and quality assurance symposium, Washington, DC, pp 279–8
Pandey B, Kinrade SD, Catalan LJ (2012) Effects of carbonation on the leachability and compressive strength of cement-solidified and geopolymer-solidified synthetic metal wastes. J Environ Manag 101:59–67
Poon CS, Qiao XC, Chan D (2006) The cause and influence of self-cementing properties of fine recycled concrete aggregates on the properties of unbound sub-base. Waste Manag 26(10):1166–1172
Sanchez F, Langley White MK, Hoang A (2009) Leaching from granular cement-based materials during infiltration/wetting coupled with freezing and thawing. J Environ Manag 90(2):983–993
SDDOT (2004) 2004 standard specifications for highway granular bases and surfacing. < http://www.sddot.com/business/contractors/Specs/default.aspx>. (1 Jan 2016)
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, New York
Tanyu BF, Edil TB, Benson CH, Kim WH (2005) Development of methodology to include structural contribution of alternative working platforms in pavement structure. J Transp Res Rec 1936:70–77
Tutumluer E, Pan T (2008) Aggregate morphology affecting strength and permanent deformation behavior of unbound aggregate materials. J Mater Civ Eng 20(9):617–627
van der Sloot HA, Dijkstra JJ (2004) Development of horizontally standardized leaching tests for construction materials: a material based or release based approach? ECN-C--04-060, Dutch Ministry of Housing, Spatial Planning and the Environment
Van Gerven T, Cornelis G, Vandoren E, Vandecasteele C (2007) Effects of carbonation and leaching on porosity in cement-bound waste. Waste Manag 27(7):977–985
Ward CR, French D, Jankowski J, Dubikova M, Li Z, Riley KW (2009) Element mobility from fresh and long-stored acidic fly ashes associated with an Australian power station. Int J Coal Geol 80:224–236
Acknowledgments
Recycled concrete materials were provided by the South Dakota Department of Transportation (SDDOT). Endorsement by SDDOT is not implied and should not be assumed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bestgen, J.O., Cetin, B. & Tanyu, B.F. Effects of extraction methods and factors on leaching of metals from recycled concrete aggregates. Environ Sci Pollut Res 23, 12983–13002 (2016). https://doi.org/10.1007/s11356-016-6456-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6456-0