Abstract
In general, people may come in contact with mixtures of insecticides through domestic use, consumption of contaminated food or drinks, and/or living close to treated areas. We analyzed the toxic effects of diazinon on histological structure of liver and hematological parameters in male rats. DNA-damaging potential of diazinon was also investigated using the comet assay in blood cells and the micronucleus test in bone marrow. Two groups of six male rats orally received different amounts of diazinon: 1/50 and 1/25 LD 50 for 4 weeks (5 day/week). The present study showed that diazinon caused hypertrophy of sinusoids, central vein, and portal triad, in addition to the formation of oedema, vacuoles, hemorrhage, necrosis, and lymphoid infiltration in rats’ liver. A significant decrease in red blood cells, hemoglobin, hematocrite levels, and platelet counts was observed in the treated groups. However, the white blood cell count increased. Micronucleus test results revealed aneugenic effects of diazinon. Furthermore, we noticed an increase in comet tail length in treated groups. So, the comet assay confirmed the genotoxic potential of diazinon in vivo. On the assumption that all alterations observed in rats could be observed in human, it is necessary to raise the awareness about the health risk posed by this insecticide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insecticides are wildly used in agriculture as well as in health care facilities. In Tunisia, between the 1960s and the 1970s, an intensive control program was conducted principally against Anopheles (vectors of malaria). This program used mainly DDT (2,2bis (p-chlorophenyl)-1,1,1-trichloroethane), and malaria has not been reported in Tunisia since 1980 (Ben Cheikh et al. 1998). After stopping the use of organochlorine insecticides, the organophosphate insecticides (OPs) have become important in mosquito control strategies. Many studies have reported that OPs insect resistance in Tunisia is increasing (Ben Cheikh et al. 1998, 2008, 2009).
Exposure to insecticides affects most of the population, not only those occupationally exposed. In general, people may come into contact with mixtures of insecticides through domestic use, consumption of contaminated food or drinks, living close to treated areas. OPs compounds are known to inhibit acetylcholinesterase enzyme (AChE), which is responsible for the rapid hydrolysis of the neurotransmitter, acetylcholine, into choline and acetate during neurotransmission at cholinergic synapses (Fulton and Key 2001; Kwong 2002; Oruc and Usta 2007). This causes the accumulation of acetylcholine at cholinergic synapses leading to increased activation of nicotinic and muscarinic receptors. A range of late toxicities may also occur (Kalender et al. 2006).
Diazinon (O,O-diethyl-O-(2-isopropyl-4-methyl-6-pyrimidinyl)-phosphoro-thionate) is a commonly used OPs insecticide in agriculture and pest control. It has been classified by the World Health Organization (WHO) as class II toxic and moderately hazardous compound.
Toxic effects of diazinon are due to the inhibition of acetylcholinesterase, an enzyme needed for nervous system function. Diazinon itself is not a potent cholinesterase inhibitor. However, it can be become to more toxic diazoxon due to the enzymatic reaction in birds, fish, insects, and mammals (Zhang and Pehkonen 1999). It is activated by the cytochrome P450 mediated reactions in liver to oxons which are potent acetylcholinesterase inhibitors (Kappers et al. 2001). Detoxification occurs through hydrolysis of the oxon by calcium-dependent arylesterases (Aesterases; paraoxonase) or by binding of the oxon to, or hydrolysis of the oxon by carboxylesterases (B-esterases) (Furlong et al. 2000; Li et al. 1993).
Diazinon causes changes in liver enzymes and biochemical indices and swelling of mitochondria in hepatocytes (Kalender et al. 2005; Abdel-Daim et al. 2015). It also causes toxic effects on blood cells, spleen, thymus, and lymph nodes of rats (Handy et al. 2002). In addition, the diazinon’s mutagenicity studies, its ability to cause genetic damage, showed that diazinon can in fact damage DNA in human blood cells, in cells from laboratory animals, and in bacteria (Grover et al. 2003). Besides, other studies proved that the rate of micronucleus has been elevated in the blood cells of diazinon-treated group of rats compared with that in the control group (Hariri et al. 2011).
Among the techniques used to detect genetic and genotoxic effects, the micronucleus test and comet assay are often used since they are appropriately and easily applied. For that reason, in the present study, the sub-acute toxicity of diazinon on genotoxicity markers was investigated using the comet assay for detecting the blood cells DNA damage and the micronucleus test of bone marrow cells under laboratory conditions. As well, some hematological indices and liver histological changes were evaluated.
Materials and methods
Animals
Eighteen male Wistar rats weighing 120–160 g, obtained from SIPHAT, (Pharmaceutical Industrial Society of Tunisia, Ben Arous Tunisia) were used in this study. Animals were acclimatized during 1 week before the experiments. They were randomly divided into three groups of six rats each one control group and two diazinon administered groups. Diets and water were given ad libitum to rats. Animals were housed at 24 ± 3 °C with 12 h light/dark cycle, 55 % humidity, and maintained during the experimental period in accordance with the guidelines for animal care of the Faculty of Medicine of Monastir, Tunisia.
Chemicals
We used a commercial formulation of diazinon, (Dazide®600 g/l). This product was purchased from a local market of agricultural products (Monastir, Tunisia).
Treatment
Two diazinon-treated groups (group 1, group 2) received an amount of 5 ml/Kg bw/day of corn oil containing respectively 26.8 mg/Kg bw and 53.6 mg/Kg bw for 4 weeks (5 days a week). Control group received an equal amount of corn oil. Diazinon was administered orally by gavage. Twenty-four hours after the last dose, animals were sacrificed under ether anesthesia and a blood sample was taken from each rat by heart tapping.
Follow-up of body and liver weight
Animal body weight was measured every week, whereas liver was weighed immediately after sacrifice as following:
Histological study
After blood sampling, rats were decapitated, and livers were removed, washed in ice-cold physiological saline, post-fixed in alcohol bouin, processed in a series of graded ethanol, and embedded in paraffin. Five micrometer-thick sections were cut and stained with hematoxylin and eosin (H & E) for light microscopy analysis. Hepatic changes were graded based on severity from normal to severe. Normal (0) represented the least detectable lesion; mild (+) represented an easily discernible lesion; moderate (++); represented a change affecting a large area of the tissue represented on the slide; and severe (+++) represented the maximal lesion observed (Araoud et al. 2014).
Hematological study
Freshly collected blood samples were analyzed for hematological analysis using an automatic hematological assay analyzer (Beckman Coulter, USA). Different hematological parameters were tested as follows: red blood corpuscles (RBC), white blood corpuscles (WBC), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet counts (PLT), hemoglobin (Hb) and hematocrite (HCT) levels, mean platelet volume (MPV).
Micronucleus test
Bone marrow slides were prepared by using the modified method of Schmid (1975). We used bovine serum albumin (BSA) prepared in phosphate buffered saline (PBS) as suspending medium instead of fetal calf serum (Seetharama Rao et al. 1983).
The bone marrow of both femur and tibia was flushed out using 2 ml of 5 % BSA centrifuged at 1000 rpm for 10 min. The pellet was collected and resuspended in required quantity of BSA (5 %), mixed thoroughly and a drop of the suspension was smeared on clean slides which were fixed in methanol and stained with Giemsa (5 %).
The slides were scored for the presence of MN in polychromatic erythrocytes (PCE) and normochromatic erythrocytes (NCE). About 1500 PCE were scanned from each animal to assess the percentage of MN-PCE and 500 PCE and NCE to estimate P/N ratio.
Comet assay/alkaline single-cell gel electrophoresis
The alkaline version of the comet assay was performed as described by Singh et al. (1988) with minor modifications. Cleaned microscopic frosted slides were dipped into 1 % normal melting agarose (NMA). Then, the slides were gently wiped to remove extra agarose, and then stored at room temperature. A mixture of whole blood (60 μl) and low melting point agarose (LMA) (60 μl) (prepared in 0.6 % PBS (pH 7.4)) were dropped onto the precoated slide with normal melting agarose.
Slides were placed on ice for 10 min and allowed to solidify. Cover slips were then removed and slides were immersed for 3 h at 4 °C, in the dark, in freshly prepared lysing solution (2.5 M NaCl, 100 Mm EDTA, 0.25 M NaOH, Triton X100 (1 %), DMSO (10 %), pH 10).
The slides were then removed from lysing solution and placed in the electrophoresis tank. The tank was filled with freshly made alkaline electrophoresis solution (20 mM EDTA, 10 N NaOH, pH 10) to cover the slides, and then they were left for 20 min in the dark to allow DNA unwinding and alkali-labile site expression. Electrophoresis was carried out for 20 min at 37 V and 300 mA. The slides were then washed for three times with 1 ml of neutralizing solution (0.4 M TrisBase, pH 7.5) for 5 min each.
After neutralization, gels were stained with 50 μl of ethidium bromide solution (20 μg /ml) and covered with cover slips for 20 min. The slides were scored without knowledge of the dose group. Image of 100 randomly selected comets (50 cells from each of two replicate slides) were taken at ×400 magnification. The tail length (TL) was measured on screen using the Comet Score computer-based image analysis system (TriTek Corp., Sumerduck, VA, USA). TL indicates the extent of migration of genetic material in the direction of the anode (Singh et al. 1988) and is expected to be proportional to the level of single-strand breaks and alkali-labile sites.
Statistical analysis
The results are presented as mean ± SD. Statistical analysis has been carried out using SPSS (Statistical Packages for Social Science, version 20.0, IBM Corporation, New York, USA). The statistical analyses were conducted using one-way analysis of variance (ANOVA) followed by the post hoc Tukey’s test, used for comparison. A p value ≤0.05 was considered statistically significant.
Results
Body weight gain (%) and relative liver weight (%)
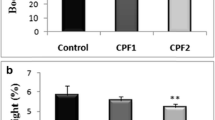
In the current study, no significant change of body weight gain was observed in the treated groups in comparison with the control group (Fig. 1a) after 4 weeks of diazinon treatment. However, we noted a decrease in the relative liver weight of treated rats in comparison with the controls (Fig. 1b). That decrease was only significant (p ≤ 0.01) in groups treated with the highest dose.
Histological findings
Light microscopic observation of the liver of the control rats exhibited a normal histological architecture with adjacent sinusoids radiating from the central veins (CV) towards the periphery of the liver lobules and normal portal triad (Fig. 2a, b). The hepatocytes were of normal shape with dispersed chromatin.
Light micrographs of rat liver tissue stained by hematoxylin-eosin (HE) in control and treated groups after 4 weeks of oral treatment. The control reveal a normal liver parenchyma and a normal portal triad (a, b) (×400). Rats treated with the lowest dose (26.8 mg/Kg bw/day) of diazinon show a moderate changes in the liver parenchyma (d, g) (×400, ×1000, respectively). Rats treated with the highest dose (53.6 mg/Kg bw/day) reveal a severe alteration in the liver parenchyma and hyperthrophy of portal triad (c, e, f, h, and i) (×400, ×400, ×400, ×1000, ×1000, respectively). CV central vein, S sinusoids, LI lymphoid infiltrate, V vacuoles, Nc necrotic cell, He hemorrhage, DS dilated sinusoid, DCV dilated central vein, Dg degenerating cell, O oedema, and portal triad (PVB portal vein branch, HAB hepatic artery branch, BD bile duct)
The administration of the lowest dose of diazinon to mature rats induced lymphoid infiltration (Fig. 2d), dilated sinusoids, cytoplasm vacuolization, degenerating cells, and moderate hepatocellular necrosis (Fig. 2g).
The highest dose of diazinon induced the formation of oedema and dilated central vein (Fig. 2c), hemorrhage, and severe hepatocellular necrosis randomly distributed throughout the parenchyma (Fig. 2h), as well as an increase in inflammatory cell infiltration, cytoplasmic vacuolation, and dilated sinusoids (Fig. 2e, h, and i) was observed. In addition, a dilated portal triad was observed (Fig. 2f). Our findings showed that histological changes of the liver were proportional to the dose (Table 1).
Hematological findings
The hematological parameter data are presented in Table 2. A non-significant difference between treated groups and control group was observed in MCV, MPV, MCH, and MCHC levels. However, we noted a decrease in RBC, HCT, and HGB levels in treated groups in comparison with the control. That decrease was significant (p ≤ 0.05) only in the group treated with the higher dose. Also, we observed a decrease in PLT count in treated groups in comparison with the control. The administration of the lower dose of diazinon induced a significant decrease (p ≤ 0.01) in PLT count. That decrease is more significant in groups treated with the higher dose.
While, an increase in WBC count was observed in treated groups compared to the control group. These findings showed that WBC count changes were proportional to the dose. This parameter increased significantly (p ≤ 0.05) after the administration of the lower dose of diazinon (26.8 mg/Kg). The administration of the higher dose (53.6 mg/Kg) induced an even greater increase in WBC counts. This increase was significant (p ≤ 0.01).
Micronucleus test
The results of micronucleus assay carried out on rat bone marrow cells to assess the potential of diazinon to induce cytogenetic damage are listed in Table 3. The cytotoxic effects of diazinon were evaluated by the determination of PCEs in total erythrocytes (PCEs and NCEs). An insignificant difference between the control group and the treated groups was observed in P/N ratio. So, for treated groups, there was no sign of increased bone marrow cytotoxicity.
The micronucleus induction in the bone marrow of rats exposed to diazinon was also observed. There was a significant (p ≤ 0.01) increase of MN-PCE percentage in the treated groups compared with the control group. Thus, all dose levels can be considered mutagenic.
Comet assay
Figure 3 displays the results of DNA damages in rat blood cells according to comet analysis. All doses of diazinon could induce DNA damage at different levels. A statistically significant (p ≤ 0.01) increase of comet tail length was observed in the group treated with the higher dose (53.6 mg/kg) compared with control group. Figure 3 also demonstrates that DNA damage increased with the increase of the diazinon dose.
Discussion
Insecticides are designed to interfere with living species and are necessarily toxic. Diazinon is not only used to protect fruits and vegetables from harmful insects but it is also used as an anti-parasitic for sheep and cattle. It has a toxic effect when it is orally or dermally administered (Garfitt et al. 2002).
In the present study, decreases in body weight gain and relative liver weights were observed. Also, Kalender et al. (2006) and Ogutcu et al. (2006) suggested that diazinon did not affect body weight gain in rats. The minor drop in body weight gain may be due to a food intake disturbance. However, the relative liver weight decrease observed in treated groups is an indicator of hepatic toxicity. It may be due to enhanced catabolic processes such as glycogenolysis, lipolysis, or proteolysis (Belaid-Nouira et al. 2013).
Our results of histological studies demonstrated that diazinon caused many structural changes in the liver tissue of Wistar rats. It was induced an intense cytoplasmic vacuolation, degeneration of hepatocytes, and appearance of moderate necrotic cell’s foci. Necrosis is produced as a result of cell degeneration accompanied by organelles swelling and amorphous cytoplasm followed by shrinking and dissolution of nuclei (Campos-Pereira et al. 2012). Cytoplasmic vacuolation is known to be caused by an excess of lipids accumulation in the cytoplasmic vesicles but it was also suggested that vacuolar formation is a cellular defense mechanism against toxic substances. The segregation of these substances in vacuoles could be a way to prevent them from interfering with cellular metabolism (Mollendorf 1973). All liver sections of the treated rats have shown an abundance of dilated sinusoids, dilated central veins, hypertrophy of portal triad, and mild hemorrhaging in both treated groups as well, while oedema was only observed in the group treated with a higher dose. However, variable intensities of these changes were noticed depending upon the dosage of treatment.
Another study of Al-Attar (2015) demonstrated that oral administration of 50 mg/Kg bw/day of diazinon along 3 weeks induce severe histopathological alterations including vacuole formations in hepatocytes, dilated sinusoids, appearance of necrotic cell’s foci, and damage of liver structure.
These findings support earlier reports on hepatic alterations induced by organophosphate insecticides (Yehia et al. 2007; Tripathi and Srivastav 2010; Mansour and Mossa 2010).
In addition, the lymphoid infiltration detected in liver sections of treated groups with diazinon is in perfect agreement with WBC count results that prove the presence of inflammation. These observations are in concordance with those reported by Mossa et al. (2012) and Elzoghby et al. (2014) which have shown that treatment with organophosphate insecticides induces lymphocyte infiltration. However, an earlier study of Lari et al. (2015) demonstrated that oral administration of 15 mg/Kg bw/day of diazinon during 28 days did not show any major histopathological changes in liver.
The study of hematological constituents of rats treated with diazinon showed a decrease in RBC, HGB and HCT which may be due to a decrease in the rate of erythropoiesis, hemosynthesis and/or an increase in the rate of erythrocyte destruction in hemopoietic organs. As we have shown in the results section, a significant reduction in the platelet count in diazinon treated rats was observed. This could be due to the effect of free radicals in bone marrow which leads to a decrease in platelet production. This is shown through the micronucleus test which found an increased MN formation in bone marrow cells. This increase is proof that diazinon induced bone marrow toxicity.
However, no significant difference was observed in MCV, MPV, MCH, and MCHC levels. MCH is a marker for hemoglobin in erythrocytes. In this study, RBC and hemoglobin counts decreased at the same rate in diazinon-treated rats. Because of this, no significant change was observed in MCH counts. These results are in concordance with those of Kalender et al. (2006), Hariri et al. (2011) and Yassa et al. (2011). In addition, the significant increase of WBC may be due to the activation of an animal’s defense mechanism that has been treated (Kalender et al. 2006).
In the present study, the micronucleus test proved that 26.8 and 53.6 mg/Kg of diazinon induced the formation of bone marrow micronuclei in a dose-dependent manner. That could be via an aneugenic mode of action (Hayashi et al. 1989). Hariri et al. (2011) found that the oral administration of 20 mg/Kg bw/day of diazinon for 4 weeks induced a significant increase of micronucleus frequency in bone marrow cells.
Also, Sarabia et al. (2009) demonstrated that intraperitoneal injection of diazinon increased the percentage of polychromatic erythrocytes with micronucleus and induced DNA fragmentation in cells of testicular tissues.
Several studies have shown the involvement of reactive oxygen species (ROS) and oxidative stress in insecticide toxicity, and it is generally accepted that ROS eventually cause DNA damage (Bertram and Hass 2008; Heikal et al. 2012). Although, MN test can detect clastogenic and aneugenic potential of chemicals and it is unable to detect single strand breaks (Vikram et al. 2008) which is detected by the comet assay. The comet assay is commonly used as a biomarker for DNA damage; it has been widely used in the field of genetic toxicology and environmental biomonitoring.
Figure 3 shows the amount of DNA damage (tail length) determined with the comet assay. As the amount of damage increases in a cell, more DNA migrates into the tail region. Tail length significantly increased at the concentration of 53.6 mg/Kg bw/day.
These findings support earlier reports on diazinon-related DNA effects (Hariri et al. 2011; Yassa et al. 2011) point to a main mechanism of diazinon action in vivo: genotoxicity, which was established by the comet assay.
Another study on human lymphocytes in cell culture (Muranli et al. 2015) reported that diazinon significantly increase the MN frequency and the comet tail length at different concentrations. Furthermore, a previous report (Tisch et al. 2002) showed, using the comet assay, some evidence of potential carcinogenicity of diazinon in human nasal mucosa cells in culture. The increase of comet tail length and of MN frequency demonstrated the DNA-damaging potential of diazinon.
Overall, the present findings indicate that diazinon could induce the MN of bone marrow cells, DNA damage of blood cells and the modification of blood parameters which could be a sign of hematopoietic organs’ alteration. Besides, an intense structural modification was observed in liver parenchyma of rats.
The increase of DNA damage in bone marrow and blood cells revealed in our study, in addition to the induction of DNA methylation alterations in response to diazinon exposure in vitro (Zhang et al. 2012) bring back into question the result of combined exposures of different insecticides and its potential to generate cancer.
On the assumption that all alterations observed in rats could be observed in human, it is necessary to raise the awareness about the health risk posed by this insecticide.
References
Abdel-Daim MM, Taha R, Ghazy EW, El-Sayed YS (2015) Synergistic ameliorative effects of sesame oil and alpha-lipoic acid against subacute diazinon toxicity in rats: haematological, biochemical and antioxidant studies. Can J Physiol Pharmacol 150719144142003
Al-Attar AM (2015) Effect of grapeseed oil on diazinon-induced physiological and histopathological alterations in rats. Saudi J Biol Sci 22:284–292
Araoud M, Neffeti F, Douki W, Khaled L, Najjar MF, Kenani A, Houas Z (2014) Toxic effects of methamidophos on paraoxonase 1 activity and on rat kidney and liver and ameliorating effects of alpha-tocopherol. Environ Toxicol. doi:10.1002/tox.22095
Belaid-Nouira Y, Bakhta H, Haouas Z, Flehi-Slim I, Neffati F, Najjar MF, Ben Cheikh H (2013) Fenugreek seeds, a hepatoprotector forage crop against chronic AlCl3 toxicity. BMC Vet Res 9:22
Ben Cheikh H, Ben Ali-Haouas Z, Marquine M, Pasteur N (1998) Resistance to organophosphorus and pyrethroid insecticides in Culex pipiens (Diptera: Culicidae) from Tunisia. J Med Entomol 35:251–260
Ben Cheikh R, Berticat C, Berthomieu A, Pasteur N, Ben Cheikh H, Weill M (2008) Characterization of a novel high-activity esterase in Tunisian populations of the mosquito Culex pipiens. J Econ Entomol 101:484–491
Ben Cheikh R, Berticat C, Berthomieu A, Pasteur N, Ben Chiekh H, Weill M (2009) Genes conferring resistance to organophosphorus insecticides in Culex pipiens (Diptera: Culicidae) from Tunisia. J Med Entomol 46:523–530
Bertram C, Hass R (2008) Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol Chem 389:211–220
Campos-Pereira FD, Oliveira CA, Pigoso AA, Silva-Zacarin ECM, Barbieri R, Spatti EF, Marin-Morales MA, Severi-Aguiar GDC (2012) Early cytotoxic and genotoxic effects of atrazine on Wistar rat liver: a morphological, immunohistochemical, biochemical, and molecular study. Ecotoxicol Environ Saf 78:170–177
Elzoghby RR, Hamuoda AF, Abdel-Fatah A, Farouk M (2014) Protective role of vitamin C and green tea extract on malathion induced hepatotoxicity and nephrotoxicity in rats. Am J Pharmacol Toxicol 9:177–188
Fulton MH, Key PB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 20:37–45
Furlong CE, Li WF, Richter RJ, Shih DM, Lusis AJ, Alleva E, Costa LG (2000) Genetic and temporal determinants of pesticide sensitivity: role of paraoxonase (PON1). Neurotoxicology 21:91–100
Garfitt SJ, Jones K, Mason HJ, Cocker J (2002) Exposure human to the organophosphate diazinon: data from a volunteer study with oral and dermal doses. Toxicol Lett 134:105–113
Grover P, Danadevi K, Mahboob M, Rozati R, Banu BS, Rahman MF (2003) Evaluation of genetic damage in workers employed in pesticide production utilizing the Comet assay. Mutagenesis 18:201–205
Handy RD, Abd-El Samei HA, Bayomy MFF, Mahran AM, Abdeen AM, El-Elaimy EA (2002) Chronic diazinon exposure: pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology 172:13–34
Hariri AT, Moallem SA, Mahmoudi M, Hosseinzadeh H (2011) The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine 18:499–504
Hayashi M, Yoshimura I, Sofuni T, Ishidate MJ (1989) A procedure for data analysis of the rodent micronucleus test involving a historical control. Environ Mol Mutagen 13:347–356
Heikal TM, Mossa AH, Nawwar GA, MEl-sherbiny M, Ghanem HZ (2012) Protective effect of a synthetic antioxidant acetyl gallate derivative against dimethoate induced DNA damage and oxidant antioxidant status in male rats. Environ Anal Toxicol 2:7
Kalender S, Ogutcu A, Uzunhisarcili M, Acikgoz F, Durak D, Ulusoy Y, Kalender Y (2005) Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicology 211:197–206
Kalender Y, Uzunhisarcikli M, Ogutcu A, Acikgoz F, Kalender S (2006) Effects of diazinon on pseudocholinesterase activity and haematological indices in rats: the protective role of Vitamin E. Environ Toxicol Pharmacol 22:46–51
Kappers WA, Edwards RJ, Murray S, Boobis AR (2001) Diazinon is activated by CYP2C19 in human liver. Toxicol Appl Pharmacol 177:68–76
Kwong TC (2002) Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monit 24:144–149
Lari P, Abnous K, Imenshahidi M, Rashedinia M, Razavi M, Hosseinzadeh H (2015) Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health 31:367–376
Li WF, Costa LG, Furlong CE (1993) Serum paraoxonase status: a major factor in determining resistance to organophosphates. J Toxicol Environ Health 40:337–346
Mansour SA, Mossa AH (2010) Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic Biochem Physiol 96:14–23
Mollendorf A (1973) Cytology cell physiology. Academic, New York
Mossa AH, Heikal TM, Omara EAA (2012) Physiological and histopathological changes in the liver of male rats exposed to paracetamol and diazinon. Asian Pac J Trop Biomed 2:1683–1690
Muranli FDG, Kanev M, Ozdemir K (2015) Genotoxic effects of diazinon on human peripheral blood lymphocytes. Arh Hig Rada Toksikol 66:153–158
Ogutcu A, Uzunhisarcikli M, Kalender S, Durak D, Bayrakdar F, Kalender Y (2006) The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of vitamin E. Pestic Biochem Physiol 86:93–98
Oruc EO, Usta D (2007) Evaluation of oxidative stress responses and neurotoxicity potential of diazinon in different tissues of Cyprinus carpio. Environ Toxicol Pharmacol 23:48–55
Sarabia L, Maurer I, Bustos-Obregón E (2009) Melatonin prevents damage elicited by the organophosphorous pesticide diazinon on mouse sperm DNA. Ecotoxicol Environ Saf 72:663–668
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Seetharama Rao KP, Abdul Rahiman M, Koranne SP (1983) Bovine albumin as a substitute for fetal calf serum in the micronucleus test. Int Symp on Recent Trends in Med Genet 28
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Tisch M, Schmezer P, Faulde M, Groh A, Maier H (2002) Genotoxicity studies on permethrin, DEET and diazinon in primary human nasal mucosal cells. Eur Arch OtoRhinoLaryngol 259:150–153
Tripathi S, Srivastav AK (2010) Liver profile of rats after long-term ingestion of different doses of chlorpyrifos. Pestic Biochem Physiol 97:60–65
Vikram A, Tripathi DN, Pawar AA, Ramarao P, Jena GB (2008) Pre-bled-young-rats in genotoxicity testing: a model for peripheral blood micronucleus assay. Regul Toxicol Pharmacol 52:147–157
Yassa VF, Girgis SM, Abumourad IMK (2011) Potential protective effects of vitamin E on diazinon-induced DNA damage and some haematological and biochemical alterations in rats. J Medit Ecol 11:31–39
Yehia MAH, El-Banna SG, Okab AB (2007) Diazinon toxicity affects histophysiological and biochemical parameters in rabbits. Expl Toxicol Pathol 59:215–225
Zhang Q, Pehkonen SO (1999) Oxidation of diazinon by aqueous chlorine: kinetics, mechanisms, and product studies. J Agric Food Chem 47:1760–1766
Zhang X, Wallace AD, Du P, Lin SM, Baccarelli AA, Jiang HM, Jafari N, Zheng YN, Xie HH, Soares MB, Kibbe WA, Hou LF (2012) Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ Toxicol Pharmacol 34:959–968
Acknowledgments
This work was supported by funds allocated to the Research Unit of Histology and Genetic UR 12 ES 10 by the “Ministère Tunisien de l’Enseingement Supérieur et de la Recherché Scientifique”. The authors thank the personnel of the Laboratory of hematology, University Hospital of Monastir, in their help in the determination of hematological parameters. We also thank the reviewers for their helpful comments and Prof. Moncef Rassas for English proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that there is no competing of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Ezzi, L., Haouas, Z., Salah, I.B. et al. Toxicopathic changes and genotoxic effects in liver of rat following exposure to diazinon. Environ Sci Pollut Res 23, 11163–11170 (2016). https://doi.org/10.1007/s11356-016-6314-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6314-0