Abstract
Knowledge of the interaction between graphene-based materials and low-molecular-weight organic acids (LOAs) is essential to understand fate and effects of graphene-based materials in the aquatic environment, but this interaction remains poorly elucidated. In this study, the effects of LOAs on the physicochemical properties of graphene nanoplatelets (GNPs) in an aqueous medium and on the GNP toxicity to algae were studied. The unicellular green alga Scenedesmus obliquus was exposed to GNP suspensions in the presence of benzoic acid or gallic acid at various concentrations. The GNPs had smaller hydrodynamic sizes and the GNP suspensions were more stable and had higher or lower surface zeta potentials in the presence of LOAs than when LOAs were not present. The toxic effects in S. obliquus cultures incubated with GNP suspensions containing LOAs were related to the LOA concentration, and the presence of LOAs caused three effects: stimulation, alleviation, and synergistic inhibition. The intensities of the effects mainly correlated with the LOA concentration, the extent of agglomeration, and particle-induced oxidative stress. The results indicate that the environmental fates and toxicities of GNPs are strongly affected by the binding of GNPs to LOAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Graphene is one of the most fascinating nanostructures. Graphene has extraordinary physicochemical, electrical, and mechanical properties, and it has a wide range of uses in electronic devices, biomedicine, and environmental protection (Akhavan and Ghaderi 2010; Lakshmi and Vasudevan 2013; Mu et al. 2012). Increasing amounts of graphene-based materials are being used, and it is likely that graphene-based materials will be mass-produced in the future. This will probably mean that graphene-based materials will be released into the environment and that biota will be exposed to them (Hu and Zhou 2013; Seabra et al. 2014; Zhao et al. 2014). It is therefore essential that the fate and effects of graphene in the environment are understood (Radic et al. 2013; Zhao et al. 2014).
Graphene present in the environment is likely to pose risks to ecosystems (Hu and Zhou 2013). Algae, as key primary producers, play important roles in maintaining balance in ecosystems (Toussaint et al. 1995). Algae are small, reproduce quickly, and are sensitive to toxicants, so they are some of the most vulnerable aquatic organisms to engineered nanoparticles and can be considered to be indicator organisms (Schwab et al. 2011; Van Hoecke et al. 2009; Wang et al. 2012). Monolayer graphene and graphene nanopowder have been found to have 72-h median effect concentrations of 1.14 and 2.25 mg/L, respectively, for algae (Pretti et al. 2014). However, to the best of our knowledge, little information is available on the behavior of graphene in aquatic ecotoxicity tests and the toxic effects graphene has on algae. In particular, the toxicity of graphene to algae and the mechanisms through which graphene affects algae under different environmental conditions are poorly understood. Graphene has some properties that are similar or identical to the properties of carbon nanotubes (Akhavan and Ghaderi 2010). It has previously been shown that carbon nanotubes inhibit the growth of green algae (Long et al. 2012; Zhao and Liu 2012). It is widely accepted that oxidative stress is one of the mechanisms involved in the toxic effects caused by carbonaceous nanomaterials (Liu et al. 2013; Long et al. 2012). The effects of graphene on algae therefore urgently need to be assessed.
The amounts of effort focused on defining the natures of the interactions between dissolved organic matter and nanoparticles are currently increasing because of the concern about the fates and effects of engineered nanoparticles released into the aquatic environment (Lowry et al. 2012; Pan and Xing 2008; Wang et al. 2015). Low-molecular-weight organic acids (LOAs) are produced in considerable amounts during the decomposition of plant and animal matter (Strobel 2001). LOAs contribute a considerable proportion of naturally occurring dissolved organic matter. However, synthetic LOAs are typically used in manufactured products because synthetic LOAs are less expensive than LOAs derived from natural sources and because higher LOA yields can be achieved using synthetic processes than natural processes. It is noteworthy that LOAs have strong capacities to combine with carbon-based nanoparticles, so LOAs present in natural water directly affect the fates and stabilities of carbon-based nanoparticles in the water (Chang and Vikesland 2009; Wang et al. 2011). A fundamental understanding of the interactions between graphene and LOAs would improve our ability to evaluate the behavior and effects of graphene in the environment, particularly the aquatic environment.
The aim of this study was to investigate the effects LOAs have on the physicochemical behaviors and effects of graphene nanoplatelets (GNPs) in aquatic ecotoxicity tests. Two naturally occurring aromatic LOAs, benzoic acid (BA) and gallic acid (GA), were used as model compounds. The sizes and zeta potentials of GNPs in algal cultures were determined in the absence and presence of LOAs. The toxic effects caused by GNPs in Scenedesmus obliquus (a green alga) at the population level were measured. Changes in the antioxidant capacities of the algae caused by GNPs were measured using biochemical assays.
Materials and methods
Test materials and test medium
GNPs were purchased as powders with thickness of 1–4 nm and particle size of 2 μm from PlasmaChem GmbH (Berlin, Germany). Detailed information provided by the manufacturers is given in Table S1 of the Supplementary material. BA (CAS RN 65-85-0) with a purity of 99 % and GA (CAS RN 149-91-7) with a purity of ≥99.5 % were purchased from Shanghai Lingfeng Chemical Reagent CO., LTD (Shanghai, China) and Sinopharm Chemical Reagent Co., LTD (Shanghai, China), respectively. The algae growth and test medium were prepared at pH 7.8 ± 0.2 according to the OECD guideline (OECD 2006).
Treatments and concentrations tested
A stock 5 g/L GNP suspension in ultra-pure water was prepared and stirred for 24 h in the dark at room temperature and then stored at 4 °C until use. The stock solution was sonicated for 30 s in a temperature-controlled sonication bath (150 W, 40 Hz, 25 °C) before an aliquot was added to an experimental tank. A test GNP suspension was prepared by adding, dropwise, an aliquot of a stock GNP suspension to the algae culture medium. A stock 10 g/L LOA solution in analytical-grade dimethylsulfoxide (DMSO) was prepared. The actual LOA concentrations that were used in the tests (1, 10, and 40 mg/L) were determined using an ultraviolet–visible spectrophotometer (UV1102; Shanghai Tian Mei Scientific Instrument Co., Shanghai, China). A suspension of a GNP and LOA complex was prepared by mixing an aliquot of the GNP stock suspension with a dilute LOA solution.
Physicochemical analysis
The morphology of GNPs was characterized by using transmission electron microspectroscopy (TEM, FEI, Tecnai G2F20, US). Each test sample was immediately analyzed after being prepared. The average hydrodynamic sizes of the GNPs in 1 and 5 mg/L GNP suspensions were measured using the dynamic light scattering method, and the zeta potentials of the GNPs in 1 and 5 mg/L GNP suspensions were measured using the phase analysis light scattering method. Both methods were performed using a Nano S90 ZetaSizer (Malvern Instruments, Malvern, UK).
Suspensions of GNPs in the absence and presence of LOAs were allowed to settle for 96 h under the same conditions as were used in the toxicity tests. These sedimentation experiments were performed as previously described by Quik et al. (2010). The hydrodynamic sizes and zeta potentials of GNPs in 1 and 5 mg/L GNP suspensions (a toxicologically relevant concentration range, including the highest concentration used in the toxicity tests) were determined using the ZetaSizer mentioned above when 96 h had passed.
A 20-mL aliquot of a 1 mg/L GNP suspension was added to each of a series of amber vials. Half of the aliquots also contained BA and the other half also contained GA, and the initial BA and GA concentrations ranged from 1 to 60 mg/L. The suspensions were adjusted to pH 7.8 ± 0.2 by adding 1 M NaOH or 1 M HCl, and then, the contents of the vials were stirred at 100 rpm for 24 h at 20 °C, to allow equilibrium to be reached. The test samples were then centrifuged at 15,000 rpm for 30 min at 20 °C (using a D3024 high speed micro-centrifuge; Scilogex, Rocky Hill, CT, USA), and then, each sample was passed through a 0.22-μm Durapore membrane filter (EMD Millipore Corp., Billerica, MA, USA). The equilibrium LOA concentrations in the samples were determined using the ultraviolet–visible spectrophotometer described above. The Freundlich model (Eq. (1)) was fitted to the sorption isotherm for the tests performed using each LOA.
where q e is the equilibrium adsorption capacity (mg/g), K F is the Freundlich adsorption coefficient ((mg/g)(mg/L)−(1/n)), C e is the equilibrated concentration (mg/L), and 1/n is the Freundlich intensity parameter.
Density functional theory quantum chemical calculations were performed using Dmol3 code. Calculations were performed for isolated molecules and compounds using the local density approximation method and the local density approximation with empirical dispersion corrections “DFT-D” method. The conductor-like screening model “COSMO” was used to simulate the solvent (water) environment (Klamt 1995). A dielectric constant of 78.54 was selected for water. The adsorption energy (E a) was calculated using the equation below.
where E complex, E GNP, and E anionic-LOA refer to the energies of the anionic LOA-GNP complexes, the isolated GNP, and anionic LOA, respectively.
Algal growth assays
A culture of the unicellular green alga S. obliquus was obtained from the Chinese Academy of Sciences Institute of Hydrobiology (Wuhan, China). The algal cell culture medium recommended by the OECD was used in the algal growth assays, which were conducted following OECD guideline 201 “Freshwater Alga and Cyanobacteria, Growth Inhibition Test” (OECD 2006). Algae that were growing exponentially were resuspended in fresh medium to give a final cellular density of 3 × 105 cells/mL. Control experiments were included to ensure that the observed effects were associated with exposure to the test materials. Flasks containing various concentrations of the test materials (GNP at 0.5, 1, and 5 mg/L and BA or GA at 0, 1, 10, and 40 mg/L) were inoculated with algae and incubated for 96 h. Every flask was shaken by hand three times each day. The algal cell density was determined after 96 h to allow the specific growth rate to be calculated. Growth inhibition (%) was calculated by dividing the specific growth rate for a treatment by the mean specific growth rate for the controls. The DMSO concentration was 0.4 % v/v DMSO in the test and control media. The control suspensions were adjusted to pH 6.5, which was the lowest pH value found in the test suspensions.
Antioxidant capacity assays
The ferric-reducing antioxidant power was measured using a commercial total antioxidative capacity (T-AOC) assay kit, obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The T-AOC assay was used because it is simple and gives reproducible results. Test algae were cultured for 96 h and then harvested by centrifuging the medium. The harvested cells were resuspended in 1 mL phosphate buffer and then analyzed using the T-AOC assay following the method described by Chen et al. (2014) with the slight modifications described below. The absorbance at 520 nm and the stability of the color of the Fe2+-o-phenanthroline complex produced were measured using a spectrophotometer. The modified T-AOC end point was estimated using the equation T-AOC = ([ODsample (520 nm) − ODblank (520 nm)] / 0.01) / ([30 × dilution of the system] / algal cell ODsample (680 nm), where OD is the optical density.
Statistical analysis
All data are expressed as means ± standard deviation (SD). Based on normality and homogeneity of variance, statistically significant differences between the test treatments were determined by t test at a significance level of p < 0.05.
Results and discussion
Impacts of LOAs on physicochemical properties of GNPs
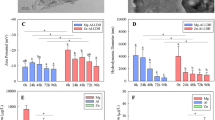
The TEM image of GNPs is presented in Fig. S1. The TEM image reveals that the GNPs used in this study aggregated intensely. The hydrodynamic sizes of GNPs in the absence and presence of LOAs are shown in Fig. 1. The mean initial GNP sizes in the 1 and 5 mg/L suspensions were 2217 ± 666 nm and 981 ± 69 nm, respectively. The initial GNP size was lower when an LOA was present than when no LOA was present, and the initial GNP size decreased as the LOA concentration increased (Fig. 1a, b), implying that the effect of the LOA on the GNP size depended on the LOA concentration. After 96 h, the GNP size in the 1 mg/L suspension (in the absence of LOAs) had decreased to 1030 ± 99 nm and the GNP size in the 5 mg/L suspension had increased to 2179 ± 907 nm. This indicated that suspensions of GNPs at higher concentrations are more likely to deteriorate than are suspensions of GNPs at lower concentrations. At different times after the suspension was prepared, the GNP size was lower in the presence of an LOA than in the absence of an LOA, and the size decreased as the LOA concentration increased (Fig. 1c, d). After 96 h, however, the GNPs were markedly larger in the 1 mg/L suspension containing 40 mg/L BA than in the 1 mg/L suspension without BA present (p < 0.01) (Fig. 1c). The hydrodynamic sizes of the GNPs were lower in the presence of GA than in the presence of BA, implying that GA improved the stability of the GNP suspension in the culture medium more effectively than did BA.
The GNP zeta potential in the culture medium changed from −9.3 to −15.9 mV as the pH increased from 4.1 to 10.0 (Fig. S2). The GNP zeta potentials were <0, suggesting that each GNP could have been stabilized by a surface layer of electrolyte anions, causing the GNP to have a negative surface charge. The primary GNPs contain <7 at.% oxygen (Table S1), so the zeta potentials could have been also negative because of oxygen-containing functional groups at the GNP surfaces being ionized. The hydrodynamic sizes and zeta potentials of the GNPs were not significantly different when the culture medium contained and did not contain DMSO (p > 0.05) (Fig. S3), implying that the GNPs and DMSO did not interact under the conditions we used. The surface charges of GNPs in the absence and presence of LOAs are shown in Fig. 2. The initial GNP zeta potentials in 1 and 5 mg/L suspensions were −19.0 ± 0.8 nm (as shown in Fig. 2a) and −17.9 ± 0.5 nm (as shown in Fig. 2b), respectively. The GNP zeta potentials at 0 h in the absence and presence of LOAs were not significantly different. However, the GNP zeta potential in the 1 mg/L suspension was significantly lower when the BA concentration was 1 mg/L than when no BA was present (p < 0.05) and was higher when the BA concentration was 40 mg/L than when no BA was present (Fig. 2a). After 96 h, the GNP zeta potentials in the 1 and 5 mg/L suspensions were −18.8 ± 0.5 nm (as shown in Fig. 2c) and −17.3 ± 0.3 nm (as shown in Fig. 2d), respectively. The GNP zeta potentials at 0 and 96 h were not significantly different. During the 96-h exposure tests, the GNP zeta potential increased as the BA concentration increased (Fig. 2c) but decreased as the GA concentration increased (Fig. 2d).
The pK a values for the LOA –COOH and –OH groups are shown Table S2. All of the –COOH groups will be ionized but the –OH groups will not be ionized at the pH values of most natural waters (pH 6.5–8.5). LOA anions will therefore have strongly affected the physicochemical properties of the GNPs under the conditions used in our study. As mentioned above, the surface charges of the GNPs became more positive when BA was present but more negative when GA was present. After 96 h, the 1 mg/L GNP suspension pH was 6.9 and the 40 mg/L GA suspension pH was 7.1. This suggests that the GA anions adsorbed onto the GNP surfaces induced more negative surface charge than did the H+ ions that were adsorbed. However, it appears that H+ donors may have contributed to the surface charge being more positive when BA was present than when no BA was present. The surface was much less charged in the GA system than in the BA system, implying that GA anions interacted more strongly with the GNP surfaces than did BA anions. This was supported by adsorption equilibrium isotherms (Table 1). The Freundlich model fitted the isotherms for the adsorption of the LOAs on the GNPs well (determined from the goodness-of-fit (R 2) of the model to the data). The GNP K F values were higher for GA than for BA, indicating that the GNPs had higher adsorption capacities for GA anions than for BA anions. Hyung and Kim (2008) found that higher molecular weight fractions of dissolved organic matter are more favorably adsorbed than lower molecular weight fractions of dissolved organic matter onto multiwalled carbon nanotubes.
Quantum chemical calculations at the molecular level were performed to investigate in a theoretical way the differences between the affects BA and GA had on the physicochemical properties of the GNPs (Table 1). The absolute E a values, calculated using the local density approximation method and the local density approximation with DFT-D method, were lower for the anionic BA–GNP complex than for the anionic GA–GNP complex. This implies that GA will interact much more strongly than BA with GNP surfaces. Therefore, GA will be more effective than BA in increasing the dispersion stability of a GNP suspension.
Impacts of LOAs on 96-h growth inhibition toxicity of GNPs
The effects of GNPs in the absence and presence of LOAs on growth inhibition effects on algal cells after 96 h are shown in Fig. 3. The 0.5, 1, and 5 mg/L GNP treatments in the absence of LOAs caused 17 ± 7 %, 22 ± 2 %, and 34 ± 1 % growth inhibition, respectively. The GNP suspensions therefore appeared to induce significant toxic effects in the algae. The 5 mg/L GNP suspension caused more inhibition than did the 0.5 and 1 mg/L GNP suspensions, implying that the toxicity of the GNPs depended on the GNP concentration. No toxic effects were found to be caused by 4 % v/v DMSO or by the suspensions being at pH 6.5 (Fig. S4).
Growth inhibition rates of Scenedesmus obliquus exposed to 0.5, 1, and 5 mg/L GNPs in the absence and presence of three different concentrations (1, 10, and 40 mg/L) of benzoic acid (BA) (a) and gallic acid (GA) (b), respectively. Values expressed as mean ± standard deviation (n = 3). Data within all columns in each figure with a letter in common are not significantly different at p < 0.05 level
The roles the LOAs played in the toxicity of GNPs to the algae were explored by performing a set of experiments with different LOAs and different concentrations (Fig. 3). As is shown in Fig. 3a, the presence of 1 or 10 mg/L BA caused the growth inhibition rate to become negative, implying that BA mitigated the toxicity of the GNPs to S. obliquus. A hormesis effect occurred when the BA concentration was low to moderate. Hormesis is a dose–response relationship that is characterized by stimulation at low doses and inhibition at high doses (Calabrese 2008). The toxicities of 0.5 and 5 mg/L GNPs to the algae were not significantly different when BA was absent and when the BA concentration was 40 mg/L (p > 0.05). 1 mg/L GNPs was significantly more toxic in the presence of 40 mg/L BA than when only 1 mg/L GNPs was present. The growth inhibition rate was not significantly different when only 40 mg/L BA was present and when 1 mg/L GNPs and 40 mg/L BA were present, implying that BA alone may contribute to the increase in the toxicity. The growth inhibition rates at GA concentrations of 1 and 10 mg/L were lower than or similar to the growth inhibition rates in the absence of GA (Fig. 3b), suggesting that low and moderate GA concentrations mitigated the toxicity of the GNPs to S. obliquus. However, the GNPs were significantly more toxic in the presence of 40 mg/L GA than when no LOA was present (p < 0.05). The growth inhibition rates were not significantly different when only 40 mg/L GA was present and when 0.5 or 1 mg/L GNPs and 40 mg/L GA were present, implying that GA alone may have been responsible for increasing the toxicity. The growth inhibition rate was significantly higher when 5 mg/L GNPs and 40 mg/L GA were present than when only 5 mg/L GNPs was present or when only 40 mg/L GA was present. This implies that synergistic effects between GNPs and GA occurred at the higher GNP concentrations.
Hydrodynamic size and surface charge might play an important role in governing the behavior and effect of engineered nanoparticles (Li et al. 2013; Song et al. 2012). Based on the present study’s results, there is no direct correlation between the physicochemical properties and the algal toxicity (Fig. S5). Furthermore, the agglomeration of carbon-based nanomaterials has been found to affect the growth of photoautotrophs in aquatic systems because of shading (i.e., an indirect effect) in previous studies (Hu et al. 2015; Long et al. 2012; Pretti et al. 2014; Schwab et al. 2011). As mentioned above, the presence of LOAs decreased the hydrodynamic sizes of the GNPs in the culture medium. This behavior was particularly apparent at high LOA concentrations. It is therefore reasonable to conclude that LOAs can promote shading by increasing the stability of a GNP suspension and that this may increase the toxicity of the GNPs to the algae.
Impacts of LOAs on GNP-induced total antioxidant capacity
The degree of oxidative stress that occurred was determined by measuring the T-AOC induced by GNPs in the absence and presence of LOAs (Fig. 4). The T-AOC was significantly higher in the algae exposed to GNPs than in the algae in the controls. Furthermore, the T-AOC depended on the GNP concentration. These findings indicate that the GNPs may have caused oxidative damage in the algae by causing reactive oxygen species to be produced. Oxidative stress has previously been found to be an important toxicity pathway for materials in the graphene family and to occur when fresh algae are exposed to graphene oxide (Hu et al. 2015; Nogueira et al. 2015; Pretti et al. 2014).
Total antioxidant capacity (T-AOC) of Scenedesmus obliquus after 96 h of exposure to 0.5, 1, and 5 mg/L GNPs in the presence of three different concentrations (1, 10, and 40 mg/L) of benzoic acid (BA) (a) and gallic acid (GA) (b), respectively. Values expressed as mean ± standard deviation (n = 3). Data within all columns in each figure with a letter in common are not significantly different at p < 0.05 level
The T-AOCs in the treatments in which only the LOAs were present were negligible, suggesting that the LOAs did not cause much oxidative damage to the algae (Fig. 4). The presence of either BA or GA significantly decreased the T-AOC induced by the GNPs, implying that the adsorption of LOAs onto the GNPs may have inhibited the production of reactive oxygen species induced by the presence of the GNPs and, therefore, decreased the amount of oxidative stress that occurred. These results also indicate that a decrease in the T-AOC may have been responsible for the toxicity of GNPs to the algae being lower when the LOAs were present at low and moderate concentrations than when the LOAs were not present or were present at higher concentrations.
Conclusions
In summary, we have shown that the presence of LOAs increased the stability of a GNP suspension in algal culture medium. The Freundlich isotherm and simulated E a values indicated that GA interacted much more strongly than BA with the GNPs, which may have caused GA to affect the physicochemical properties of the GNPs in the aquatic medium more than did BA. The growth inhibition caused by the GNPs was mitigated by the presence of low and moderate concentrations of the LOAs. Specifically, hormesis occurred when the GNPs were present with low and moderate BA concentrations. The presence of LOAs decreased the T-AOC, and this may be why the GNPs were less toxic to the algae when the LOAs were present than when the LOAs were absent. The GNPs were more toxic to the algae when the LOA concentrations were high than when the LOA concentrations were lower or no LOAs were present, and this could have been because LOAs were directly toxic to the algae and because a large amount of disagglomeration occurred. Our results show that the actual risks posed by GNPs in aquatic systems may be affected by the presence of LOAs.
References
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4:5731–5736
Calabrese EJ (2008) Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 27:1451–1474
Chang X, Vikesland PJ (2009) Effects of carboxylic acids on nC60 aggregate formation. Environ Pollut 157:1072–1080
Chen Y, Miao Y, Huang L, Li J, Sun H, Zhao Y, Yang J, Zhou W (2014) Antioxidant activities of saponins extracted from Radix Trichosanthis: an in vivo and in vitro evaluation. BMC Complement Altern Med 14:86
Hu X, Zhou Q (2013) Health and ecosystem risks of graphene. Chem Rev 113:3815–3835
Hu C, Wang Q, Zhao H, Wang L, Guo S, Li X (2015) Ecotoxicological effects of graphene oxide on the protozoan Euglena gracilis. Chemosphere 128:184–190
Hyung H, Kim JH (2008) Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: effect of NOM characteristics and water quality parameters. Environ Sci Technol 42:4416–4421
Klamt A (1995) Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem 99:2224–2235
Lakshmi J, Vasudevan S (2013) Graphene-a promising material for removal of perchlorate (ClO4 −) from water. Environ Sci Pollut Res Int 20:5114–5124
Li R, Wang X, Ji Z, Sun B, Zhang H, Chang CH, Lin S, Meng H, Liao YP, Wang M, Li Z, Hwang AA, Song TB, Xu R, Yang Y, Zink JI, Nel AE, Xia T (2013) Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano 7:2352–2368
Liu Y, Zhao Y, Sun B, Chen C (2013) Understanding the toxicity of carbon nanotubes. Acc Chem Res 46:702–713
Long Z, Ji J, Yang K, Lin D, Wu F (2012) Systematic and quantitative investigation of the mechanism of carbon nanotubes’ toxicity toward algae. Environ Sci Technol 46:8458–8466
Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. Environ Sci Technol 46:6893–6899
Mu Q, Su G, Li L, Gilbertson BO, Yu LH, Zhang Q, Sun YP, Yan B (2012) Size-dependent cell uptake of protein-coated graphene oxide nanosheets. ACS Appl Mater Interfaces 4:2259–2266
Nogueira PF, Nakabayashi D, Zucolotto V (2015) The effects of graphene oxide on green algae Raphidocelis subcapitata. Aquat Toxicol 166:29–35
Organisation for Economic Co-operation and Development (OECD) (2006) Freshwater alga and cyanobacteria, growth inhibition test, Nr. 201. OECD guidelines for the testing of chemicals. OECD, Paris, http://www.oecd.org
Pan B, Xing B (2008) Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ Sci Technol 42:9005–9013
Pretti C, Oliva M, Di Pietro R, Monni G, Cevasco G, Chiellini F, Pomelli C, Chiappe C (2014) Ecotoxicity of pristine graphene to marine organisms. Ecotox Environ Safe 101:138–145
Quik JT, Lynch I, Van Hoecke K, Miermans CJ, De Schamphelaere KA, Janssen CR, Dawson KA, Stuart MA, Van De Meent D (2010) Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water. Chemosphere 81:711–715
Radic S, Geitner NK, Podila R, Käkinen A, Chen P, Ke PC, Ding F (2013) Competitive binding of natural amphiphiles with graphene derivatives. Sci Rep 3:2273
Schwab F, Bucheli TD, Lukhele LP, Magrez A, Nowack B, Sigg L, Knauer K (2011) Are carbon nanotube effects on green algae caused by shading and agglomeration? Environ Sci Technol 45:6136–6144
Seabra AB, Paula AJ, de Lima R, Alves OL, Durán N (2014) Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol 27:159–168
Song M, Yuan S, Yin J, Wang X, Meng Z, Wang H, Jiang G (2012) Size-dependent toxicity of nano-C60 aggregates: more sensitive indication by apoptosis-related Bax translocation in cultured human cells. Environ Sci Technol 46:3457–3464
Strobel BW (2001) Influence of vegetation on low-molecular-weight carboxylic acids in soil solution—a review. Geoderma 99:169–198
Toussaint MW, Shedd TR, Van der Schalie WH, Leather GR (1995) A comparison of standard acute toxicity tests with rapid-screening toxicity tests. Environ Toxicol Chem 14:907–915
Van Hoecke K, Quik JT, Mankiewicz-Boczek J et al (2009) Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests. Environ Sci Technol 43:4537–4546
Wang Z, Chen JW, Sun Q, Peijnenburg WJGM (2011) C60-DOM interactions and effects on C60 apparent solubility: a molecular mechanics and density functional theory study. Environ Int 37:1078–1082
Wang Z, Chen JW, Li X, Shao J, Peijnenburg WJGM (2012) Aquatic toxicity of nanosilver colloids to different trophic organisms: contributions of particles and free silver ion. Environ Toxicol Chem 31:2408–2413
Wang Z, Quik JT, Song L, Van Den Brandhof EJ, Wouterse M, Peijnenburg WJGM (2015) Humic substances alleviate the aquatic toxicity of polyvinylpyrrolidone-coated silver nanoparticles to organisms of different trophic levels. Environ Toxicol Chem 34:1239–1245
Zhao X, Liu R (2012) Recent progress and perspectives on the toxicity of carbon nanotubes at organism, organ, cell, and biomacromolecule levels. Environ Int 40:244–255
Zhao J, Wang Z, White JC, Xing B (2014) Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ Sci Technol 48:9995–10009
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 21407080) and the Foundation Research Project of Jiangsu Province (Grant Nos. BK20140987 and BK20150891). We also thank the anonymous reviewers for helping to improve the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 954 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Gao, Y., Wang, S. et al. Impacts of low-molecular-weight organic acids on aquatic behavior of graphene nanoplatelets and their induced algal toxicity and antioxidant capacity. Environ Sci Pollut Res 23, 10938–10945 (2016). https://doi.org/10.1007/s11356-016-6290-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6290-4