Abstract
This study investigates the effect of a mixed surfactant system on the desorption of polycyclic aromatic hydrocarbons (PAHs) from soil model systems. The interaction of a non-ionic surfactant, Tween 80, and an anionic one, sodium laurate, forming mixed micelles, produces several beneficial effects, including reduction of adsorption onto solid of the non-ionic surfactant, decrease in the precipitation of the fatty acid salt, and synergism to solubilize PAHs from solids compared with individual surfactants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of soils and sediments by polycyclic aromatic hydrocarbons (PAHs) is an environmental concern (Mc. Entee and Ogneva-Himmelberger 2008; Wang et al. 2008) due to the carcinogenic, mutagenic, and teratogenic properties of PAHs. They are largely formed by natural and anthropogenic pyrolysis of organic matter during forest fires, fossil fuel use, and chemical manufacture (Peters et al. 2008; Tobiszewski and Namiesnik 2012). These compounds are also resistant to natural degradation by their high hydrophobicity.
PAH concentrations in soils vary according to land use and soil type. There are differences in the content of PAHs between agricultural, forest, and urban soils (Wilcke 1996; Wilcke 2007). The last ones are found to be notoriously more contaminated than the previous ones, due mainly to the contribution of larger population, traffic density, and industrial areas (Lau et al. 2014).
Several attempts, involving physical, chemical, biological, and their combined technologies, have been made to remedy PAH-contaminated soils and groundwater. Surfactant-enhanced remediation (SER) has been suggested as a promising technology for the removal of hydrophobic pollutants from soil and groundwater (Bernardez and Ghoshal 2004; Zhu et al. 2004). Surfactant molecules above their critical micelle concentration (CMC) form aggregates in water, which are called micelles. These aggregates have a hydrophobic core and a hydrophilic outer surface. Micelles are capable of dissolving hydrophobic PAHs in their hydrophobic core, which results in an increased apparent aqueous solubility of the compounds (Lakraa et al. 2014; Wei et al. 2011).
In a soil-water-surfactant system, molecules of hydrophobic organic compounds (PAHs for instance) exist mainly in the following forms: solubilized in surfactant micelles, dissolved in the surrounding solution, sorbed directly on the soil particles, or sorbed in association with sorbed surfactants (Edwards et al. 1994). It was found that the efficiency of surfactants in aiding PAH desorption was strongly dependent on soil composition, surfactant structure, and PAH properties (Gan et al. 2009; Rodriguez-Escales et al. 2012; Crampon et al. 2014).
Cationic surfactants are not usually used in washing and desorption hydrophobic compounds from soils due to their strong adsorption on the soils that are mainly negatively charged (Barvinchenko et al. 2013). Anionic surfactants are more suitable; however, too hard subsurface water may be detrimental to the effectiveness of an anionic surfactant because these amphiphilic molecules may precipitate by the formation of insoluble salts with calcium and magnesium cations (Jafvert and Heath 1991). Non-ionic surfactants, instead, may stay adsorbed on soils (Chong et al. 2014) and hydrophobic compounds are associated to them, thus the global affinity of the contaminants by the soil is increased (Edwards et al. 1994; Zhang and Zhu 2012).
Mixtures of surfactants have received considerable attention in the last years because of their efficient solubilization, suspension, dispersion, and transportation capabilities (Yoshimura et al. 2004). In view of this, bi- and ternary combinations of ionic and non-ionic surfactants have been studied in relation to their mixed micelle formation (Torres et al. 2010; Liu et al. 2014, Wei et al. 2015) and adsorption at the air-water interface (Alargova et al. 2001; Zhang et al. 2002; Bakshi et al. 2005). Several theories have been adopted to analyze experimental results as well as to understand synergism in mixed surfactant systems (Clint 1992).
Mixtures of surfactants can have better properties than individual surfactants due to the formation of mixed micelles (Panda and Din 2013), so some attempts have been made to improve the performance of SER of contaminated soils by employing mixed surfactant systems (Zhou and Zhu 2007; Nizri and Magdassi 2005).
Recently, we had study the mixture formed by Tween 80 with sodium fatty acid salts as sodium laurate (SL) or miristate to modify the apparent solubility of some PAHs in water (Sales et al. 2011). The mentioned surfactants formed mixed micelles that were characterized, showing synergistic effect to increase the solubilization in water of naphthalene but not of phenanthrene. These differences could be attributed to different solubilization sites of the substrates in the mixed micelles.
SER is based on two processes: (a) enhanced solubilization of pollutants by micelles; (b) desorption of the contaminants from the soil to the aqueous solution. Hence, it is important to know whether the studied surfactant mixtures could be efficient to desorb naphthalene or other PAHs form soils and whether we could find synergism in this analysis.
Experiments involving soils require large amounts of solid with an extensive previous treatment: it is necessary to separate particle size and to characterize the content of salts, organic material, pH, surface area, etc. Silica gel possesses similar characteristics to those of the inorganic portion of the soils; it is granular and porous, and built of repetitive sequences of orthosilicate groups. The union sites of pollutants in the inorganic moiety of soils should be similar to the union sites of the contaminants in silica, thus this solid can mimic the portion of soil interacting with pollutants (Paria 2008). In addition, silica is a reproducible matrix where it is possible to study only the effect of changing different variables in the system, without any modification in the solid. For that reason, we began to work with silica gel as a soil model, trying different work conditions in search of synergism. The final idea was to apply the best washing conditions found in silica gel, working with sediments obtained from San Roque Lake, Córdoba, Argentina.
The objectives of this study were the following: (i) to investigate whether the presence of an anionic surfactant could diminish the sorption of the non-ionic surfactant onto solids; (ii) to analyze whether the presence of non-ionic surfactant could diminish the precipitation of the anionic one; and (iii) to evaluate whether a mixed surfactant system, efficient to increase the apparent solubility of the PAHs in water, was equally efficient to desorb the pollutants from a solid matrix. Naphthalene and phenanthrene were chosen as representative PAHs due to their different physical properties, mostly in molecular weight which discriminates the two PAHs in terms of hydrophobicity, tendency for bioaccumulation, resistance to degradation, and overall environment persistence. The experimental results could be used to understand the performance of this kind of mixtures in the remediation of polluted soils and to provide valuable information in the design of surfactant remediation technologies. It is important to mention that the chosen surfactants are environmentally safe compounds.
Experimental section
Materials
Aqueous solutions were made up of water purified in a Millipore apparatus. Methanol HPLC grade (Sintorgan) was used without purification.
The PAHs were obtained from Sigma-Aldrich and their purity was greater than 98 %.
The surfactants, Tween 80 and sodium laurate, were obtained from Sigma-Aldrich and used as received.
Silica gel 60 (Merck) has a particle diameter between 63 and 200 μm, pH = 7, and a surface area of 500 m2/g.
The experiments with soils were conducted with a sample of sediment of San Roque Lake, Córdoba, Argentina, previously characterized as consisting of 6.4 % of sand, 69.7 % of silt, 23.9 % of clay, 12.61 % of organic material, pH = 6.10, and a specific surface area of 250 m2/g (Borgnino et al. 2010). Previously to be used, the sample of soil was washed several times with methanol and buffer solution to eliminate possible interferences in the following determinations.
All the solutions in this work were prepared in buffer pH 9.155, (Na2B4O7 0.01 M, NaCl 0.02 M).
Preparation of PAH-contaminated solid matrices
Stock solutions of naphthalene (Naph) or phenanthrene (Phen) were prepared in ethylic ether with adequate concentrations. Fifty milliliters of these stock solutions were added to 20 g of dried silica gel or sediment. The mixtures were stirred and mixed thoroughly. The solvent was left to evaporate in a fume hood for 3 days and was afterwards completely eliminated by a vacuum pump to get a constant weight. The quantification of the PAH content was done by UV-visible spectrophotometry (Multispec 1501) at 267 nm for Naph and 251 nm for Phen from silica gel. The procedure consisted of vigorously shaking for 24 h a measured amount of contaminated silica gel with methanol. After that, the mixture was filtered or centrifuged and the amount of PAH was quantified. This procedure was done in triplicate.
Determination of adsorption of Tween 80 and precipitation of SL in the presence of solid matrices
We studied the adsorption of Tween 80 and the precipitation of SL in contact with the solid matrices with each surfactant alone or in mixtures. A weighted amount of solid matrix was placed into a capped borosilicate glass tube with a measured volume of solution of one or both surfactants in buffer pH 9.155. These solutions were shacked for 48 h at 25 °C. Afterwards, the supernatant was separated by centrifugation or filtration and the amount of Tween 80 and SL was quantified.
Tween 80 adsorbed on silica gel was quantified by UV-visible spectrophotometry at 272 nm. The presence of SL did not interfere with this quantification.
Tween 80 and SL adsorbed onto soil were measured by difference with the supernatant by H1-NMR (Bruker Avance II 400 MHz) using benzoic acid as an internal standard. We used the signal at 3.73 ppm corresponding to the oxyethylene groups in the molecule of Tween 80 for quantification. For SL, the methylene signal was used at 2.18 ppm corresponding to the methylene group nearest the carbonyl group. For benzoic acid we used the signal at 7.9 ppm corresponding to protons 2 and 6 in the aromatic ring. The extract of the soil did not show signals of interference.
Determination of recovery percentage of PAH with surfactants from contaminated matrices
Batch tests were performed in capped borosilicate glass tubes for solubilization of Naph and Phen in single and binary surfactant solutions with different total concentration from different solid matrices. In these mixtures, the molar ratio of nonionic surfactant in relation to the ionic one was varied. In addition, different solid matrices with different amounts of PAH were assayed. Each solid-surfactant-PAH system involved at least three batch tests in order to work out an average. A weighted amount of contaminated matrix was added to each tube in an amount considerably higher that the required to saturate the solution with the PAH; the corresponding surfactant solution was then added. The mixtures were treated for 1 min in a vortex (TTS 3 digital). Afterwards, the samples were left shaking for a period of 48 h in a thermostated shaker bath maintained at 25 ° C. Finally, the samples were centrifuged to separate the solid phase. An adequate aliquot of the supernatant was withdrawn to the necessary concentration adding 50 % of methanol to carry out the quantification of the sample by UV-visible absorption in a Shimadzu Multispec 1501 spectrophotometer when silica gel was the solid. To avoid Tween 80 interference, we performed the quantification of PAHs by derivative absorbance measurements. Naph was determined in the second derivative spectrum at 291.8 nm and Phen was determined in the first derivative spectrum at 295.5 nm. Tween 80 and SL did not present signals at these wavelengths.
When the solid matrix consisted of sediments from San Roque Lake, the quantification was done using HPLC (Varian 5500). The conditions of quantification were as follows: Column C18, solvent methanol:water/80:20, flow 1 mL/min, λ = 254 nm. In those conditions, the retention time for naphthalene was 8 min.
Results and discussion
Effect of sodium laurate on the adsorption of Tween 80 on silica gel
The adsorption of nonionic surfactants onto a hydrophilic surface is a known effect (Zheng et al. 2012), dependent in part on surfactant concentration (Zhou and Zhu 2007). At low surfactant concentration, the nonionic surfactants are sorbed as monomers and lie parallel to the solid surface through surface interactions with both types of surfactant moieties. With the increase in surfactant concentration, adsorption increases dramatically as the surface micelle (admicelle) (Lopata et al. 2010) or bilayers form on the adsorbent through association or hydrophobic interaction between the hydrocarbon chains of the surfactants; a plateau is reached corresponding to a maximum sorption amount with the surfactant concentration around CMC. The effectiveness of surfactants decreases when a significant amount of them is adsorbed by the soil, since the amount of surfactant available for solubilizing the contaminant decreases, thus its mobility through the medium to which it is applied is reduced. Additionally, adsorption of surfactant increases the hydrophobicity of the soil. As a result, removed solubilized organic compounds will be re-adsorbed on the soil surface (Ahn et al. 2008).
Table 1 shows the effect of SL on the adsorption of Tween 80. In the absence of SL and in the presence of [TW80] = 5 × 10−3 M, the final [TW80] at the equilibrium in the presence of silica gel is 0.42 × 10−3 M. The adsorption of the surfactant on silica is high; yet in the presence of increasing quantities of SL, it is notably reduced. It was previously described that TW80 and SL form mixed micelles with attractive interactions between both surfactants (Sales et al. 2011). The formation of these mixed micelles could be the main reason for the decrease observed in the adsorption of TW80 that, at XTW80 = 0.2, is ten times lower than in the initial situation, without the addition of SL. The remaining concentration of Tween 80 in the solution is still about 400 times higher than the CMC of the surfactant.
The adsorption of the non-ionic surfactant onto silica gel is also higher than that onto the soil. This is clear from the adsorption density data and from the higher surface area of the silica (500 m2/g) as compared to that of the soil (250 m2/g).
Effect of Tween 80 on the precipitation of sodium laurate in the presence of soil
The solutions of SL in contact with silica gel or soil turn turbid due to the precipitation of the surfactant in contact with the solid. However, the addition of Tween 80 to these solutions produces a marked decrease in turbidity, reducing the loss of SL. A quantitative measurement of this reduction in the presence of soil is shown in Table 2. A similar tendency is observed in the presence of silica gel.
The addition of a small amount of Tween 80 to SL ([TW80] = 3 × 10−3 M, XTW80 = 0.13), reduces in a 43 % the precipitation of SL; likewise, the addition of SL diminishes the adsorption of Tween 80. The formation of mixed micelles could explain these effects. As was previously determined (Sales et al. 2011), this system presents strong attractive interactions between both surfactants conferring high stability to the mixed micelles. This behavior can be explained considering that the headgroups of ionic surfactants will cause electrostatic self-repulsion and that the bulky groups in the non-ionic surfactants will cause steric self-repulsion in the micelles of the pure compounds. In mixed systems, these two effects are weakened by dilution effects after mixing, and the electrostatic self-repulsion of the anionic surfactants is replaced by the attractive ion-dipole interaction between the hydrophilic groups of the both surfactants. That is the reason why the formation of mixed micelles is favored in this kind of mixtures (Zhou and Rosen 2003).
The observed results indicate that the mixtures of anionic/non-ionic surfactants could have advantages as media to extract contaminants from solids, due to the increased surfactant concentrations that it is possible to maintain in solution in the mixed micelles, as compared to single surfactant solutions.
PAH desorption from silica gel studies
The ability of the mixtures Tween 80/LS to extract PAHs from solids was evaluated by varying different experimental conditions, including several concentrations and ratios of surfactants and different amounts of contaminants in the solids.
Table 3 summarizes the experiments carried out. Rexp means the percentage of recovery of PAH from solids with respect to the original amount of pollutant, defined by Eq. 1
where n w are the mole in aqueous solution and n s the mole in the solid.
Tween 80 enhances the solubilization of PAHs compared with buffer, even though part of the surfactant is adsorbed into the silica gel. SL, instead, is not a good system alone, resulting almost as effective as buffer, probably due to the enormous precipitation of the surfactant in contact with the solid matrix, which reduces its concentration in solution at values lower than the CMC.
However, when Tween 80 and SL are mixed, they exhibit synergic effect. This is evidenced by parameter E (Table 3) being higher than 1, defined in Eq. 2 corresponding to the ratio between the experimental recovery percentage (R exp) and the theoretical percentage (R theor, Eq. 3). This last percentage was calculated assuming that there is no interaction between surfactants, so each surfactant desorbed the same amount as if it was alone.
where R Tween80, R SL, and R buffer are the values to the recovery of the PAHs by Tween 80, SL, and buffer, respectively.
The observed effect is also dependent on the amount of contaminant present in the silica gel. When the solid has a smaller amount of contaminant (i.e., a more realistic situation), the synergic effect is more important. SL solubilizes 20 times more when there is less Naph (Rexp = 11 % against 0.54 %, when Naph content in the solid diminishes 89 times). Tween 80, instead, increases 7.6 times the desorption of Naph at the same surfactant concentration in the least contaminated solid (Table 3). In addition, when the composition of the mixture is enriched in SL (X TW80 = 0.13), the results are better. This fact agrees with previous studies of solubilization of PAHs in buffer solution, where the synergic effect on the solubilization of Naph shows the maximum at molar ratio 0.13 (Sales et al. 2011). Figure 1 displays the enhanced desorption of both PAHs due to the mixture of surfactants in the better conditions.
The synergic effect may be attributed to the formation of mixed micelles (Sales et al. 2011) that, in this case, reduces the adsorption of Tween 80 into the solid, and reduces the precipitation of SL in contact with silica gel. Hence, there are more surfactants in solution which can produce the enhanced desorption of the pollutants. Additionally, the mixed micelle has a better solubilization power for Naph than the micelles of individual surfactants, as was demonstrated (Sales et al. 2011).
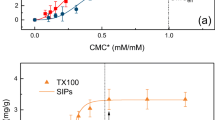
Similar results were previously described in desorption of phenanthrene from contaminated soil by mixtures of Triton X100 (TX100) with sodium dodecyl sulfate (SDS) (Zhou and Zhu 2007). The sorption amount of TX100 onto soil from the mixed solution was less than that of single TX100 solution, and decreased as the mole fraction of SDS in solution increased. In addition, TX100-SDS mixed solution was more effective for desorption of phenanthrene from the contaminated soil than the single TX100 solution, in a similar way to the results described in this work.
Naphthalene desorption from sediments
Figure 2 shows the enhanced desorption of Naph from sediment due to the mixture of surfactants. The synergistic effect can also be seen, probably ascribed to the decrease in the adsorption of Tween 80 onto the soil and to the decrease in the precipitation of SL in contact with sediment. Both effects result from the formation of mixed micelles between the two surfactants (Sales et al. 2011). Guo et al. found increased desorption of p-nitrochlorobenzene and reduction of the sorption of the surfactants by the soil in mixtures of sodium dodecylbenzenesulfonate and Tween 80, and they also attributed the observed results to the formation of mixed micelles between both surfactants (Guo et al. 2009).
Our results from the studies in sediment are in concordance with those obtained in silica gel, so this last solid matrix is a good model of soils, that allows studying interaction of surfactants, and pollutant desorption processes, in a more simple way.
Conclusions
The formation of mixed micelles produces a combination of beneficial effects to desorb pollutants from solid matrices: (i) in mixed micelles the sorption of Tween 80 onto solid is reduced; (ii) the interaction between Tween 80 with sodium laurate in the micelles diminishes the precipitation of the last surfactant in contact with solids; (iii) the concentrations of both surfactants in the solution are larger in that way; (iv) the capability of mixed micelles to solubilize PAHs is greater than that of individual surfactants. All these effects contribute to producing a synergic effect in desorption of PAHs from solids.
These results afford new insights into encouraging the use of mixtures of surfactants in soil remediation.
References
Ahn CK, Woo SH, Park JM (2008) Enhanced sorption of phenanthrene on activated carbon in surfactant solution. Carbon 46:1401–1410
Alargova RG, Kochijashky II, Sierra ML, Kwetkat K, Zana R (2001) Mixed micellization of dimeric (Gemini) surfactants and conventional surfactants. II. CMC and micelle aggregation numbers for various mixtures. J Colloid Interface Sci 235:119–129
Bakshi MS, Kaur G, Ahmad I (2005) Synergistic interactions in mixed micelles of alkyltriphenylphosphonium bromides and triblock polymers. Colloid Surf A Physicochem Eng Asp 253:1–8
Barvinchenko VN, Lipkovskaya NA, Fedyanina TV (2013) Adsorption of a cationic surfactant, miramistin, from aqueous solutions on the surface of highly dispersed silica. Colloid J 75:623–627
Bernardez LA, Ghoshal S (2004) Selective solubilization of polycyclic aromatic hydrocarbons from multicomponent nonaqueous-phase liquids into nonionic surfactant micelles. Environ Sci Technol 38:5878–5887
Borgnino L, Garcia MG, del Hidalgo MV, Avena M, De Pauli CP, Blesa MA, Depetris PJ (2010) Modeling the acid-base surface properties of aquatic sediments. Aquat Geochem 16:279–291
Clint JH (1992) Surfactant Aggregation. Blackie & Son Ltd, Glasgow, p 134
Chong Z-Y, Liao X-Y, Yan X-L, Sun L, Zhao D, Liang T (2014) Enhanced desorption of PAHs from manufactured gas plant soils using different types of surfactants. Pedosphere 24:209–219
Crampon M, Bureau F, Akpa-Vinceslas M, Bodilis J, Machour N, Le Derf F, Portet-Koltalo F (2014) Correlations between PAH bioavailability, degrading bacteria and soil characteristics during PAH biodegradation in five diffusely contaminated dissimilar soils. Environ Sci Pollut Res 21:8133–8145
Edwards DA, Zafar A, Luthy RG (1994) Distribution of nonionic surfactant and phenanthrene in a sediment/aqueous system. Environ Sci Technol 28:1550–1560
Gan S, Lau EV, Ng HK (2009) Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J Hazard Mater 172:532–549
Guo H, Liu Z, Yang S, Sun C (2009) The feasibility of enhanced soil washing of p-nitrochlorobenzene (pNCB) with SDBS/Tween80 mixed surfactants. J Hazard Mater 170:1236–1241
Jafvert C, Heath J (1991) Sediment- and saturated-soil-associated reactions involving an anionic surfactant (dodecylsulfate). 1. Precipitation and micelle formation. Environ Sci Technol 25:1031–1038
Lau EV, Gan S, Ng HK, Poh PE (2014) Extraction agents for the removal of polycyclic aromatic hydrocarbons (PAHs) from soil in soil washing technologies. Environ Pollut 184:640–649
Lakraa J, Tikariha D, Yadav T, Das S, Ghosh S, Satnami ML, Ghosh KK (2014) Mixed micellization of Gemini and cationic surfactants: physicochemical properties and solubilization of polycyclic aromatic hydrocarbons. Colloids Surf A Physicochem Eng Asp 451:56–65
Liu Z-Y, Li Z-Q, Song X-W, Zhang J-C, Zhang L, Zhao S (2014) Dynamic interfacial tensions of binary nonionic–anionic and nonionic surfactant mixtures at water–alkane interfaces. Fuel 135:91–98
Lopata JJ, Werts KM, Scamehorn JF, Harwell JH, Grady BP (2010) Thermodynamics of mixed anionic/nonionic surfactant adsorption on alumina. J Colloid Interface Sci 342:415–426
Mc. Entee JC, Ogneva-Himmelberger Y (2008) Diesel particulate matter, lung cancer, and asthma incidences along major traffic corridors in MA, USA: a GIS analysis. Health & Place 14:817–828
Nizri G, Magdassi S (2005) Solubilization of hydrophobic molecules in nanoparticles formed by polymer-surfactant interactions. J Colloid Interface Sci 291:169–174
Panda M, Din K (2013) Solubilization of polycyclic aromatic hydrocarbons by Gemini–conventional mixed surfactant systems. J Mol Liq 187:106–113
Paria S (2008) Surfactant-enhanced remediation of organic contaminated soil and water. Adv Colloid Interf Sci 138:24–58
Peters S, Talaska G, Jönsson BAG, Kromhout H, Vermeulen R (2008) Polycyclic aromatic hydrocarbon exposure, urinary mutagenicity and DNA adducts in rubber manufacturing workers. Cancer Epidemiol Biomed Prev 17:1452–1459
Rodriguez-Escales P, Sayara T, Vicent T, Folch A (2012) Influence of soil granulometry on pyrene desorption in groundwater using surfactants. Water Air Soil Pollut 223:125–133
Sales PS, de Rossi RH, Fernández MA (2011) Different behaviours in the solubilization of polycyclic aromatic hydrocarbons in water induced by mixed surfactant solutions. Chemosphere 84:1700–1707
Tobiszewski M, Namiesnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119
Torres MF, Sales PS, de Rossi RH, Fernández MA (2010) Aggregation behaviour of Brij-35/ perfluorononanoic acid mixtures. Langmuir 26:17858–17866
Wang X, Cheng H, Xu X, Zhuang G, Zhao C (2008) A wintertime study of polycyclic aromatic hydrocarbons in PM2.5 and PM2.5–10 in Beijing: assessment of energy structure conversion. J Hazard Mater 157:47–56
Wei J, Huang G, Yu H, An C (2011) Efficiency of single and mixed Gemini/conventional micelles on solubilisation of phenanthrene. Chem Eng J 168:201–207
Wei Y, Liang X, Tong L, Guo C, Dang Z (2015) Enhanced solubilization and desorption of pyrene from soils by saline anionic-nonionic surfactant systems. Colloid Surf A Physicochem Eng Asp 468:211–218
Wilcke W (1996) Polyciclic aromatic hydrocarbons (PAHs) in soil—a review. J Plant Nutr Soil Sci 163:229–248
Wilcke W (2007) Global patterns of polycyclic aromatic hydrocarbons (PAHs) in soil. Geoderma 141:157–166
Yoshimura T, Ohno A, Esumi K (2004) Mixed micellar properties of cationic trimeric-type quaternary ammonium salts and anionic sodium n-octyl sulfate surfactants. J Colloid Interface Sci 272:191–196
Zhang L, Luo L, Zhao S, Yu J (2002) Studies of synergism/antagonism for lowering dynamic interfacial tensions in surfactant/alkali/acidic oil systems, part 2: synergism/antagonism in binary surfactant mixtures. J Colloid Interface Sci 251:166–171
Zhang D, Zhu L (2012) Effects of Tween 80 on the removal, sorption and biodegradation of pyrene by Klebsiella oxytoca PYR-1. Environ Pollut 164:169–174
Zheng G, Selvam A, Wong JWC (2012) Enhanced solubilization and desorption of organochlorine pesticides (OCPs) from soil by oil-swollen micelles formed with a nonionic surfactant. Environ Pollut 46:12062–12068
Zhou Q, Rosen MJ (2003) Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: the regular solution approach. Langmuir 19:4555–4562
Zhou W, Zhu L (2007) Enhanced desorption of phenanthrene from contaminated soil using anionic/non-ionic mixed surfactant. Environ Pollut 147:350–357
Zhu L, Chen B, Tao S (2004) Sorption behavior of polycyclic aromatic hydrocarbons in soil-water system containing nonionic surfactant. Environ Eng Sci 21:263–272
Acknowledgments
This research was supported in part by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Técnica (ANPCyT), Ministerio de Ciencia y Tecnología de la Provincia de Córdoba (MINCyT) and Universidad Nacional de Córdoba (UNC). P. S. thanks ANPCyT for a research fellowship. We also thank Dr. Laura Borgnino for the gift of the sample of sediment used in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Sales, P.S., Fernández, M.A. Synergism in the desorption of polycyclic aromatic hydrocarbons from soil models by mixed surfactant solutions. Environ Sci Pollut Res 23, 10158–10164 (2016). https://doi.org/10.1007/s11356-016-6242-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6242-z