Abstract
In membrane bioreactors applied to wastewater treatment, fouling is typically a complex function of sludge characteristics. A pilot-scale tertiary submerged membrane bioreactor (tMBR) was continuously operated for over 200 days to assess the effect of biomass physiological state and environmental stress on process performance. Sludge characteristics were evaluated in terms of suspended solid concentration (MLSS and MLVSS), apparent viscosity, bioflocculation state, filterability, bioactivity, biopolymeric clusters (BPCs) and soluble microbial products. During the initial period of the tMBR start-up, when MLSS was below 3000 mg/L, the biomass was found to be very sensitive to environmental stress by sudden oxygen increase or organic shock loading, resulting in temporary biomass deflocculation and BPC release, and consequently, severe induced membrane fouling. However, at higher MLSS values, low stable biomass growth (0.04 ± 0.002 kg MLVSS/kg COD) was measured, regardless of organic overloading shocks or feeding failures. This period was also characterised by low bioactivity, BPC content and membrane fouling. Statistical analysis showed that BPCs have an important role when compared with other sludge properties as indicators of its fouling potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last two decades, membrane bioreactor (MBR) technology has attained a significant market share in municipal wastewater treatment, increasing its value at a compound annual growth rate of 10–15 % (Marketsandmarkets 2014). This technology combines suspended biomass, similar to the conventional activated sludge process, with microfiltration or ultrafiltration membranes that replace gravity sedimentation and clarify the wastewater effluent. Among the commercially available process configurations and membrane modules of MBRs, the submerged hollow-fibre type is the most widely used in municipal wastewater treatment owing to lower operation costs (Judd 2010). The rapid market penetration of MBRs has principally been driven by superior effluent quality, suitable for unrestricted irrigation and other industrial applications. Such quality permits a significant reduction of total plant footprint compared to conventional activated sludge plant (Cote et al. 2012). However, despite considerable progress over the past two decades, membrane fouling is still the main factor limiting widespread application of this technology. This drawback leads to increased operating expenditure (OPEX), due to the cost of membrane aeration, cleaning requirements and membrane replacement (Fenu et al. 2010).
Fouling can be defined as the alteration in the membrane caused by specific physical and/or chemical interactions between the membrane and components of the microbial suspension, leading to membrane permeability loss. Several mechanisms have been proposed to explain this phenomenon, including pore clogging, adsorption of foulants, gel or cake formation, cake layer consolidation and osmotic pressure effects (Lin et al. 2014). Conventional filtration strategies to reduce fouling largely involve air scouring to induce favourable hydrodynamics in the vicinity of the membrane surface and physical cleaning through backwashing (i.e. permeate is used to flush the membrane backwards) or relaxation (when no filtration takes place), supplemented with periodic chemical cleaning in place (CIP) (Judd 2010). The rate and extent of fouling is typically a complex function of membrane properties, hydrodynamic and operating conditions, feedwater nature and microbial suspension characteristics (Le-Clech et al. 2006). Therefore, for a given system (i.e. feedwater and membrane) and favourable hydrodynamics, an appraisal of the impact operating parameters have on microbial suspension characteristics is necessary, in order to select the best operating conditions. Several authors have tried to identify the characteristics of the suspensions that have the greatest influence on fouling, among which are concentration of suspended solids in the mixed liquor (MLSS), viscosity, particle size distribution (PSD), biomass activity and biopolymer content (Le-Clech et al. 2006; Meng et al. 2009). Biopolymers are organic substances of microbial origin, consisting mainly of polysaccharides and proteins, which can be in the microbial flocs (extracellular polymeric substances—EPSs), or in the liquid phase as soluble microbial products (SMPs) and colloidal biopolymer clusters (BPCs). Based on the concept of utilisation and biomass associated products (Aquino and Stuckey 2008), biopolymer content is related to various microbial processes including substrate degradation, biomass decay, EPS hydrolysis and soluble biopolymer degradation.
Since MBR use began, MLSS has been considered one of the main foulant parameters. Nevertheless, most studies indicate that the MLSS concentration, considered alone, is a poor indicator of the fouling tendency of suspensions (Meng et al. 2009, Drews 2010; Van den Broeck et al. 2011). In general, the relationship between MLSS concentration and fouling appears to depend on the MLSS concentration range; below 6 g/L, an inverse relationship is often reported while a direct relationship is generally found above 15 g/L (Le-Clech et al. 2006). A greater MLSS effect may have an influence on sludge viscosity, so affecting the hydrodynamics of the filtration process (Trussell et al. 2007). In addition to MLSS, other factors such as sludge morphology (i.e. particle-size distribution) and BPCs are expected to cause membrane fouling (Wang and Li 2008). Since sludge flocs and free bacteria are considerably larger than membrane pores, particle size distribution (PSD) has an influence on the explanations of fouling focussed on cake-layer and consolidation mechanisms. Many authors report that cake layers formed by small flocs were denser than those by large ones, probably due to a higher specific cake resistance according to the Carman–Kozeny equation (Lin et al. 2011). In addition, small flocs have a greater tendency to settle on the membrane due to their lower back-transport velocity (Zeman and Zydney 1996). Meanwhile, it is widely reported that biopolymer matter strongly affected membrane fouling in an MBR. Soluble/colloidal biopolymers could enter into the membrane pores causing pore blocking; they form a gel layer, enhance floc attachment to the membrane, affect cake structure and induce osmotic effect (Lin et al. 2014).
Despite extensive research efforts to understand the effect of operating conditions on biomass characteristics and membrane fouling propensity, the variety of installations and feedwater characteristics sometimes lead to contradictory results, as in the case of sludge retention time (SRT) (Sabia et al. 2013; Villain and Marrot 2013). Moreover, determination of the actual membrane fouling rate is significantly affected by the fouling history of the membrane (Drews 2010). For this reason, several ex situ filterability tests such as the time-to-filter (TTF) or Delft filtration characterisation method (DFCm) have been introduced. Nevertheless, these tests may not accurately reflect the real operating conditions in MBRs and the correlation between the test methods and membrane fouling rates must be checked case by case (Drews 2010). In addition, the interconnection between sludge characteristics adds a particular complication in ascertaining which component of the suspension is the primary cause of membrane fouling (Van den Broeck et al. 2011). Therefore, pilot tests are generally required to characterise the suspensions with greatest influence on fouling under particular MBR operating conditions.
Recent studies have shown the capability of tertiary submerged membrane bioreactors (tMBRs) for treatment of secondary effluents from old plants with poor performance by secondary clarifiers (e.g. Vera et al. 2014). These clarifier malfunctions can compromise the economic feasibility of other tertiary treatments. In fact, growing interest in this technology has been seen recently in the Aquapolo project (Sao Paulo, Brazil), the largest wastewater reuse project in the southern hemisphere, which includes a tMBR in the treatment scheme (Koch 2014). One of the most attractive points of tMBR is its capability to operate under complete solid retention (without a waste sludge purge) due to extremely low sludge production in the substrate-limited conditions imposed. This advantage significantly reduces operating costs (Vera et al. 2014). There are several experiments with MBRs treating raw municipal wastewater that have demonstrated their capability to operate under complete sludge retention time limitations without drawbacks in biodegradation activity (e.g., Rosenberger et al. 2002). In these experiences, high biomass concentrations were required (12 to 24 g/L) in order to operate under substrate-limited conditions, which had a negative impact on oxygen transfer efficiency and hence on operating costs. In addition, Pollice et al. (2008) found that sludge filterability deteriorated under complete sludge retention, probably due to excessive increments in MLSS and sludge viscosity. In contrast, tMBRs offer the capability to operate under complete sludge retention time and moderate MLSS concentration, due to low organic content in the feedwater. However, the consequences of complete sludge retention for morphology, bioactivity, soluble biopolymer content and filterability of the microbial suspension must be studied in detail in order to optimise process performance.
The aim of this study is to assess biomass development in a tMBR and its relationship with membrane fouling. For this purpose, a pilot tMBR operated with complete sludge retention was continuously fed with a secondary effluent for 195 days. Biomass development was assessed by following the evolution of MLSS, MLVSS, viscosity, particle size distribution, TTF, bioactivity (heterotrophic and nitrifying activity) and colloidal and soluble biopolymer content (BPCs and SMPs). The relationships between membrane fouling rate and each property of the sludge were further analysed statistically to assess the role and significance of each.
Materials and methods
Feedwater
The tMBR was fed with secondary effluent from a conventional activated sludge wastewater treatment plant, designed only for carbon removal (Table 1). This effluent exhibited a high variable chemical oxygen demand (COD = 559 mg/L, on average), mainly as particulate matter (CODs/COD = 0.14 and COD/SST = 1.35), with a low readily biodegradable fraction (5-day biochemical oxygen demand BOD5 to COD ratio was approximately 0.22). This high particulate content was attributed to the short SRT (<4 days) and oxygen deficiency in the activated sludge process, which frequently resulted in episodes of sludge deflocculation. Nitrogen compounds were mainly in the form of ammonium (43 mg/L, on average). In terms of the total COD, this feedwater is comparable to a moderate loaded wastewater but mainly composed of suspended slowly biodegradable organic matter.
MBR
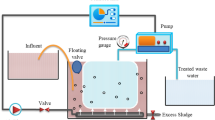
A cylindrical 220-L deposit was equipped with ZeeWeed® ZW-10 (GE Water & Process Technologies) hollow-fibre membranes with a 0.04-μm-rated pore diameter, 1.9-mm external diameter and 0.9 m2 of filtering surface area, assembled vertically (see Vera et al. 2014). ZeeWeed® consists of a woven reinforcing braid on which a PVDF membrane is cast. The effluent (permeate) was extracted from the top header of the module under slight vacuum. Throughout the experimental time, ultrafiltration was carried out at constant permeate flux (J = 35 L/h m2), registering transmembrane pressure as a function of time. Furthermore, backwashing was applied each 7 min at constant conditions (J B = 60 L/h m2 and t B = 30 s) for fouling removal. Although air sparging was not supplied during the filtration phase, constant air scouring at 3.1 Nm3/h m2 was applied during backwashing, to improve its efficiency. The process tank was stirred by air bubbling from the bottom, at a flow rate of 1.2 Nm3/h. A compressor also supplied additional air (0.3 Nm3/h) for the biological processes. The bioreactor was run at a hydraulic retention time of 7.6 h without sludge removal except for sampling. During the experimental time, the biological suspension was routinely characterised to analyse the influence of microbial suspension characterisation on membrane fouling. Membrane chemical cleaning was carried out by soaking with NaOCl (1000 ppm) for 12 h.
Analytical methods
Total and soluble chemical oxygen demand (COD and CODs, respectively), 5-day biochemical oxygen demand (BOD5), total suspended solids (TSS), mixed liquor suspended solids (MLSS), mixed liquor volatile suspended solids (MLVSS), time to filter (TTF) and turbidity were determined in conformity with the standard methods (2005). The analyses of ammonium–nitrogen (N–NH3) were carried out by the Nessler method using a DR-5000 Hach spectrophotometer. Dissolved organic carbon (DOC) concentration was measured with a TOC-meter (TOC-5000A, Shimadzu). The difference in DOC concentration between the filtrated suspension through a 0.45-μm nitrocellulose membrane filter (HA, Millipore) and the MBR permeate was assigned as biopolymer clusters (BPC). Nitrate–nitrogen (N–NO3 −) and nitrite–nitrogen (N–NO2 −) were analysed through ion chromatography using a Compact IC plus 882 device supplied by Metrohm. Microbial floc size distribution was measured using a Malvern Mastersizer 2000 instrument with a detection range of 0.02–2000 μm. To quantify supernatant turbidity, the biomass samples were centrifuged at 1300 g for 2 min. The biological activities were evaluated by respirometric measurement described in Ginestet et al. (1998) using allylthiourea (86 μM) and azide (24 μM) as selective inhibitors of ammonia and nitrite oxidation, respectively. The sludge viscosity was determined by using the concentric cylinder rotational viscosimeter Visco Star plus (FungiLab, Spain), with a rotational speed of 100 rpm.
Results and discussion
Evolution of sludge characteristics and process performance
The pilot plant was operated continuously for 195 days under similar operating conditions (HRT = 7.2 h and complete retention time) to study biomass development. To facilitate analysis, the experimental period has been divided into five phases according to process stability: initial biomass growth (phase I, 1st–21st day), unsteady operation (phase II, 22nd–60th day) and steady biomass growth (phase III, 61st–84th day; phase IV, 85th–157th day; phase V, 158th–195th day).
The plant started without any sludge inoculum, so the biomass developed from the microorganisms entered with feedwater (i.e. secondary effluent). As shown in Fig. 1, MLSS and MLVSS rapidly increased during phase I due to microorganism retention and growth, resulting from the high (F/M)C imposed (11.5–5.0 kg CODs/kg MLVSS day). Nevertheless, at the end of phase I, to maintain complete nitrification with a proper dissolved oxygen concentration, airflow rate to the bioreactor was increased to 1.5 Nm3/h, producing a shock to the microorganisms, which started to produce BPCs and generate white foam (Fig. 4b). This relationship between intense biopolymer production and foam is commonly observed in MBRs and attributed to proteins that have properties as surface-active agents (Di Bella et al. 2011). This foam disappeared after the 40th day. In addition, from the 44th to 57th day, the tMBR suffered sporadic events of organic overloading shocks, and feedwater COD increased from 670 to 2230 mg COD/L due to temporary malfunction of the WWTP. This significantly increased suspended solid concentration in the tMBR. The unstable period was identified as phase II. After that, biomass started to grow continuously during a 135-day period (phases III–V), with the exception of another shock load (123rd–127th day) and a period of feeding failure (146th–157th day). During this period of stable growth, microorganisms were progressively subjected to severe substrate limitation (from 0.26 to 0.10 kg COD/kg MLVSS day), which may justify the low sludge-yield coefficient (Y obs ) of 0.04 ± 0.002 kg MLVSS/kg COD. Due to the starvation conditions, it was expected that available substrate was mainly utilised by microorganisms for non-growth activities rather than biosynthesis, which reduced net sludge production (Rosenberger et al. 2002). In addition, previous studies in similar conditions have demonstrated the existence of predator species in the tMBR, especially sessile ciliates and free-swimming ciliates, which are known to be responsible for a reduction in the population of smaller bacteria (Wang et al. 2013). The sludge yield was within the typical range of those reported for secondary MBRs operated with complete sludge retention (0.03–0.12 kg MLVSS/kg COD (e.g., Pollice et al. 2004)). It was also consistent with predicted values (0.05–0.1 kg MLVSS/kg COD) of ASM-based models considering the concepts of endogenous respiration and slow hydrolysis at long SRTs (>100 days) (Amanatidou et al. 2015). At the same time, the MLVSS/MLSS ratio decreased from 0.85 of day 60 to 0.82 of day 195 (phases III–V), which indicated a slight inert solid accumulation in the sludge. This is in accordance with previous studies, suggesting that MBRs operated for long SRTs develop slow-growing bacteria that may degrade inert substances and avoid significant accumulation in the sludge (van Loosdrecht and Henze 1999).
Table 2 shows the evolution of sludge bioflocculation, viscosity and filterability. During phases I–II, results revealed an increase in bioflocculation, detected in supernatant turbidity and mean particle size behaviour, due to biomass development. It should also be noted that unsteady conditions (phase II) greatly affected sludge characteristics, as seen from the variability of turbidity measurements. Once the steady biomass growth period was achieved (phases III–V), average turbidity slightly increased from 16 to 27 NTU. As expected, the small floc content (i.e. d 10, 10 % of the volume distribution being below this value) decreased from 44 to 29 μm during the same period. These results indicated some degree of sludge deterioration after long operation times. Probably, the main reason for the observed behaviour is the progressive substrate limitation imposed, which decreased biomass activity. Wilén et al. (2000) studied the effect of microbial activity on floc stability and concluded that the bacterial metabolism was important in maintaining the strength of the flocs. Nevertheless, this slight deflocculation did not have a significant impact on process performance since no significant decrease in the substrate consumption rate nor appreciable release of SMPs was observed. Regarding rheological properties, apparent viscosity seems to be related to MLSS, as previously reported (Trussell et al. 2007). Based on this approach, a critical MLSS value can be identified (~3000–3500 mg/L); over it, apparent viscosity significantly increased (phases IV–V, from 2.0 to 4.0 mPa s). Finally, the results presented in Table 2 also underline the link between bioflocculation and sludge filterability, measured as TTF. Similar results have been reported by Van den Broeck et al. (2011), reflecting the key role of small floc size in filterability.
As expected, high COD removal and complete nitrification were obtained after the first days of operation, corresponding with the rapid biomass growth observed in phase I (Fig. 2). However, there was considerable variability in COD removal efficiencies during the whole experimental period, between 79 and 97 %. This range may be attributable to influent COD fluctuations (110–960 mg/L) since permeate COD values decreased from 81 mg/L (on average) in the initial phase to less than 35 mg/L during phases III–V. Nitrifying biomass followed the typical trend, always achieving ammonium removal rates higher than 98 % after the first 20 days, with stable behaviour after increasing airflow rate (17th day). In phases III–V, average concentrations for nitrogen compounds in the permeate were 0.53, 50.3 and 0.03 mg N/L for NH4 +, NO3 − and NO2 −, respectively.
Biomass activity and colloidal/soluble biopolymer content
Respiration tests with various substrates were periodically carried out to elucidate the physiological state and activity of the microbial community. Endogenous specific uptake rate (SOUR endogenous) is defined as oxygen consumption of the microbial population in the absence of external substrates. It mainly consists of the maintenance energy requirement. In addition, heterotrophic, ammonium-oxidising (AOB) and nitrite-oxidising (NOB) bacteria were examined by adding potassium acetate, ammonium chloride and sodium nitrite, respectively, and measuring respiration consumption after dosing these substrates in excess. Figure 3 shows that endogenous SOUR continuously decreased with operation time. Similar trends have been reported by many authors, revealing the influence of MLSS (i.e. (F/M)C ratio) on microbial activity (Pollice et al. 2004). During the stable biomass growth period (phases III–V), low average SOUR values were observed (from 4.9 to 2.8 mg O2/g MLVSS h), similar to those usually reported for MBRs operated in endogenous conditions (Pollice et al. 2004). This is consistent with the low biomass growth observed. In addition, SOUROHO behaved the same, decreasing with operation time. It is notable that the endogenous uptake rate was approximately 60 % of that with a carbon substrate, confirming the substantial organic carbon limitation imposed on the microorganisms in the tMBR.
Autotrophic activity is here expressed as specific oxygen uptake rate of ammonia oxidation bacteria, SOURAOB, and nitrite oxidation bacteria, SOURNOB. Both rates tended to decrease with operation time, a trend similar to the ammonium feed to microorganism ratio ((F/M)N) in the tMBR. Assuming a theoretical factor for oxygen consumption of 4.57 (Metcalf and Eddy, Inc. 2003) and subtracting the endogenous consumption for recalculation of SOURAOB, ammonia uptake rates declined from 5.1 ± 1.5 to 1.4 ± 0.7 mg N/g MLVSS h, corresponding to a (F/M)N drop from 6.0 to 1.2 mg N/g MLVSS h. Similarly, based on a factor of 1.1 for SOURNOB, nitrite uptake rates reached 3.2 ± 0.8 mg N/g MLVSS h at the end of phase I, increased to 6.0 ± 1.2 mg N/g MLVSS h during phase II and then decreased to 1.7 ± 0.9 mg N/g MLVSS h (phase V). Consistent with this, complete nitrification was continuously observed after 20 days of operation.
Many researchers have suggested that colloidal and soluble biopolymers (BPCs and SMPs) play an important role in membrane fouling (Meng et al. 2009; Lin et al. 2014). For this reason, biopolymer content was routinely analysed (Fig. 4b). BPC in the tMBR was estimated from the difference between DOC concentrations in the suspension supernatant and the permeate. SMP concentration was assumed to be the DOC concentration in the permeate. Secondary effluents are mainly composed of organic matter (EfOM) of different origins, where biopolymers represent an important part. Therefore, feedwater DOC concentration was also measured in order to assess biopolymer production and degradation by the microorganisms in the tMBR. During the experimental period, EfOM ranged between 10.9 and 37.7 mg DOC/L, with an average of 21.3 ± 5.1 mg DOC/L (Fig. 4a). As shown in Fig. 4b, BPCs tended to accumulate in tMBR, following the same trend as EfOM during the first 17 days of phase I. In addition, corresponding with the increasing airflow rate, BPCs rapidly increased while white foam appeared. Net BPC release is a common response of microorganisms to environmental stresses, which cause release of proteins and polysaccharides due to sludge deflocculation (Lin et al. 2014). This deflocculation process is consistent with the high supernatant turbidity (Table 2) and the presence of white foam, attributed to some proteins with surface-active properties (Di Bella et al. 2011). From day 24, BPCs started to decrease until day 40, when concentration reached 7.5 mg/L. At this point, the sum of BPC and SMP concentrations was similar to EfOM concentration; it was thus assumed that deflocculation had ceased. Nevertheless, an organic shock load in the feedwater again led to a significant rise in BPCs. After that, during the biomass stable-growth period (phases III–V), BPCs were progressively biodegraded/adsorbed by the biomass until a very low stable concentration was reached (1.85 mg/L, on average). Furthermore, given the low microbial activity reached in this period, lower biopolymer production was expected because of the low substrate utilisation.
On the other hand, SMPs remained nearly constant throughout the experimental study, with an average of 11.1 ± 2.0 mg DOC/L (Fig. 4b). During stable biomass conditions (phases III–V), a mass balance between EfOM and SMPs revealed a net removal of 52 ± 7 %. Therefore, feedwater fluctuations and microbial processes appear to only affect BPC concentration, which in turn correlates with the biopolymer fraction retained by the membrane. Consequently, it is expected that BPC evolution affects membrane fouling, consistently with previous studies (Wang and Li 2008).
Correlation between different biomass characteristics
Table 3 shows the multicollinearity (expressed by Pearson’s coefficient r) of the sludge parameters previously discussed. As expected, MLSS was highly correlated with the apparent viscosity (r = 0.852). This correlation is frequently reported in many studies. For example, Wu and Huang (2009) obtained a similar correlation coefficient in different sludges from MBRs. MLSS also correlated (r = −0.826) with endogenous SOUR, confirming its influence on microbial activity, in agreement with previous studies (Pollice et al. 2004). The low coefficient (r = −0.557) obtained for nitrification activity (SOURAOB) should be attributed to the considerable variability associated with unstable conditions in phases I–II. It should be noted that the autotrophic population shows a lower growth rate and therefore requires more time to become accustomed to the new conditions. Results also highlighted a moderate correlation (r = −0.735) between MLSS and BPC. This may be related principally to the development of the slow-growing population after a long operation time, which are able to degrade the biopolymers, as proposed by other authors [e.g. Sabia et al. 2013].
Among the parameters affecting suspension filterability, expressed by TTF, only supernatant turbidity presented a moderate correlation (r = 0.775), revealing a considerable effect of bioflocculation on sludge filterability. In contrast, results indicated that MLSS did not directly correlate to sludge filterability.
Membrane performance
Submerged hollow-fibre MBRs are frequently operated under temporised filtration/backwashing cycles (Judd 2010). This operation mode was applied in the present work. During a filtration cycle, transmembrane pressure (TMP) linearly increased with elapsed time. This behaviour can be explained by the incompressible cake model (Vera et al. 2014), where TMP within a filtration cycle can be expressed in terms of the initial transmembrane pressure (TMP i ), fouling rate (r f ) and elapsed time (t) (Eq. [1]):
Based on this approach, r f can be used as a quantification parameter of reversible fouling and TMP i as another parameter associated with residual fouling phenomena after backwashing.
The evolution of membrane performance, expressed in terms of TMP i and TMP f (transmembrane pressure at the end of the filtration cycle), is shown in Fig. 5a. During phase I, TMP i tended to rapidly increase up to 28–36 kPa (higher than the technical operating values), despite two chemical cleanings performed on days 4 and 8. Identical TMP i trends were observed between chemical cleanings, where most of the fouling was observed on the first day of operation. A theoretical explanation for this behaviour has been proposed, considering a specific deposited mass remaining on the membrane surface after backwashing (Charfi et al. 2015). Therefore, residual fouling depends on backwashing effectiveness and the properties and quantity of the mass deposited after backwashing (i.e. r f and filtration time). For given backwashing conditions and a fixed filtration time (7 min), TMP i is expected to depend on r f . Consistently, r f followed a similar trend to TMP i , suddenly increasing to 35–65 Pa/s, which is 20–40 times higher than typically found in full-scale MBRs (Fig. 5b) (Drews 2010). After increasing airflow rate on day 17, r f suddenly rose to impractical levels (>65 Pa/s), resulting in numerous plant shutdowns due to the high TMP reached. For membrane recovery, several chemical cleanings were periodically performed on days 18, 20 and 22 and 23. On the 27th day, the system started to recover its normal operation due to r f decline. Nevertheless, the high organic loads in the feedwater from the 44th to 57th days significantly increased r f again, leading to another unstable period which lasted until the end of phase II. These results suggest that environmental stresses due to raised oxygen concentration and organic load have a profound effect on membrane fouling. Both environmental factors have been identified as causes for biopolymer release and fouling propensity in MBRs (Le-Clech et al. 2006). In accordance with this, r f showed a similar trend to that observed for BPCs during phase II (Fig. 4b). After that, during phase III (61st–84th day), r f gradually dropped to operative values (~1 Pa/s) (Drews 2010). During the same period, TMP i slightly decreased from 26 to 23 kPa confirming the linkage between reversible fouling rate and residual fouling. Yet again, r f decline coincided with a similar BPC decay. Thus, the results indicate that biomass progressively acclimatised after a couple of days, decreasing its BPC levels and fouling potential. Subsequent phases (III and IV) were characterised by stable membrane performance. Phase IV (85th–157th day) started with a chemical cleaning which removed most of the residual fouling. TMP i and r f remained at nearly constant and low values. Another chemical cleaning was performed in phase V, achieving a TMP value similar to that at the beginning of the experimental period. Although r f showed some variability during phase V, it remained between operative values. However, this variability induced a slow TMP i increase at a constant rate of 0.10 Pa/s. This fouling cannot be directly related to BPCs since their concentration remained very low during this phase (Fig. 4b). This behaviour can be justified by other factors such as a slight sludge deflocculation and the rise in MLSS and apparent viscosity with operation time (see Table 2).
Influence of biomass characteristics on membrane fouling rate
As previously reported in many studies, no single biomass parameter can explain membrane fouling, and thus, its origin must be explored in combination with several parameters (Van den Broeck et al. 2011). For this reason, four parameters (BPCs, MLSS, supernatant turbidity and TTF) that describe key suspension properties, such us colloidal biopolymer content, suspended solid concentration, bioflocculation state and sludge filterability, were assessed. Table 4 summarises Pearson’s coefficient of the parameters derived from the fouling rate data obtained during all experimental phases. Figure 6 also shows how the fouling rates change with these parameters.
The highest correlation for r f was found for BPCs, with a coefficient of 0.868, where they ranged from 0.54 to 28.2 mg DOC/L (Fig. 6a). This high correlation is in agreement with some previous studies (Wu and Huang 2009) and differs with others considering components of BPCs such as polysaccharides or proteins (Drews 2010). Discrepancies may be due to the complexity of fouling phenomenon at different operation parameters and hydrodynamic conditions. In subcritical flux operation, where TMP often shows a three-stage profile, the colloidal biopolymers have often been identified as the main foulants during the second stage, corresponding to a prolonged TMP rise (Charfi et al. 2015). During this period, fouling rate increases with foulant load (i.e. permeate flux and biopolymer concentration) but very low fouling rates are commonly reported (Le-Clech et al. 2006). In contrast, supracritical flux operation leads to high fouling rates due to rapid deposition of suspended solids on membrane surfaces, forming a cake layer. Colloidal biopolymers can influence caking by enhancing sludge floc attachment to the membrane (Lin et al. 2014), reducing cake-layer porosity (Charfi et al. 2015) and increasing specific cake resistance due to an osmotic pressure mechanism (Chen et al. 2012). Biopolymers are commonly considered as a whole, without distinguishing soluble and colloidal fractions. However, soluble biopolymers should pass through the membrane with the effluent and thus not significantly contribute to fouling. In fact, membrane pore constriction can also be induced by this soluble fraction but many studies have shown that internal fouling is less than surface fouling in MBRs (Shen et al. 2015). In the present study, the results revealed that colloidal biopolymers (i.e. BPCs) were a crucial factor that regulated membrane fouling. On the other hand, since the membrane was operated at high permeate flux and low shear rates (i.e. without air scouring at the membrane surface), a major contribution from MLSS was expected in contrast with previous studies (Bae and Tak 2005) that remark the importance of colloidal/soluble biopolymer rate. Nevertheless, the level of MLSS appeared not to have a significant effect on membrane fouling since a negative correlation (r = −0.765) between MLSS and r f was calculated (Table 4). This trend is in fact inconsistent with the cake filtration model in dead-end conditions (i.e. without shear rate), where the solid concentration of the cake tends to rise with MLSS concentration (Le-Clech et al. 2006). A deeper analysis showed different trends according to the MLSS range (Fig. 6b). At low to moderate values (<3000 mg/L), a negative correlation was clearly observed, while a slight positive correlation resulted from increasing MLSS above ~5000 mg/L. Therefore, results seem to indicate that the low shear rate due to bioreactor mixing was enough to induce some back-transport of suspended solids from the membrane. It is known that back-transport due to shear-induced diffusion and inertial lift increases with shear rate and particle size (Zeman and Zydney 1996), which justifies a selective deposition of colloidal biopolymers onto the membrane, as previously reported (Pan et al. 2010). This explains the importance of BPCs for membrane fouling at MLSS lower than 3000 mg/L. On the other hand, as discussed in “Evolution of sludge characteristics and process performance”, apparent viscosity significantly increased when MLSS became higher than 3500 mg/L. Since shear rate decreases with viscosity (Trussell et al. 2007), this may be reason for the slight rise in r f when MLSS are above 5000 mg/L. From the results it can be deduced that optimum operating conditions involve concentrations of BPC below ~5 mg/L and MLSS approximately within the range between 3000 and 5000 mg/L. It should be noted that similar results have been reported with secondary MBRs treating raw municipal wastewater. Several studies have suggested that low F/M ratios may result in a low metabolic activity, suitable sludge morphology and low soluble biopolymer concentration, which was correlated to a less fouling potential (Meng et al. 2009, Van den Broeck et al. 2011, Sabia et al. 2013).
The sludge filterability, measured by TTF, did not show a significant correlation with fouling rate (Table 4 and Fig. 6c). As TTF is based on cake filtration in dead-end regime, this trend is consistent with some degree of suspended solids back-transport during filtration in the tMBR. However, supernatant turbidity showed a similar trend to that obtained for TTF, revealing a poor correlation with fouling rate (Table 4 and Fig. 6d). It can thus be concluded that BPCs have a predominant role when compared with other sludge properties as indicators of its fouling potential.
Conclusions
The effect of sludge characteristics on membrane fouling in a tMBR was assessed in this study; the following may be concluded:
-
Environmental stress affected sludge characteristics and thus membrane fouling. A sudden oxygen increase resulted in temporary biomass deflocculation, BPC release and, consequently, severe induced membrane fouling. Organic shock loads also increased BPC content but their effect on membrane fouling seemed to depend on MLSS range. While at MLSS lower than 3000 mg/L, the effect was significant, at higher concentrations, it was not noticeable. This behaviour was attributed to the development of the slow-growing microbial population able to degrade BPCs.
-
At MLSS higher than 3000 mg/L, a low stable biomass growth (0.04 ± 0.002 kg MLVSS/kg COD) was observed, regardless of organic shock loads or feeding failures. This stable growth period was characterised by low bioactivity, BPC content and membrane fouling.
-
Statistical multicollinearity of the sludge characteristics showed a significant correlation between MLSS and a set of parameters (endogenous SOUR, apparent viscosity and BPCs). In contrast, results indicated that MLSS were not directly correlated to sludge filterability, measured as TTF.
-
In the hydrodynamic conditions assessed (J = 35 L/h m2 without air scouring in the filtration phase), incompressible cake-layer formation was found to be the predominant fouling mechanism. In these conditions, it was demonstrated that BPCs have a predominant role, compared with other sludge properties, as indicators of its fouling potential.
AOB, ammonium-oxidising bacteria; BOD5, 5-day biochemical oxygen demand, mg/L; BPC, biopolymer clusters, mg DOC/L; CIP, chemical cleaning in place; COD, total chemical oxygen demand, mg/L; CODs, soluble chemical oxygen demand, mg/L; d 10, measured particle size at 10 % of volume distribution, μm; d 50, measured particle size at 50 % of volume distribution, μm; d 90, measured particle size at 90 % of volume distribution, μm; DFCm, Delft filtration characterisation method; DOC, dissolved organic carbon, mg/L; EfOM, organic matter present in secondary effluents, mg DOC/L; EPS, extracellular polymeric substances; (F/M)C, carbon feed to microorganism ratio, kg CODs/kg MLVSS day; (F/M)N, nitrogen feed to microorganism ratio, kg N/kg MLVSS day; HRT, hydraulic retention time, h; J, filtrate flux, L/h m2; J B, backwashing flux, L/h m2; MBR, membrane bioreactor; MLSS, mixed liquor total suspended solids, mg/L; MLVSS, mixed liquor volatile suspended solids, mg/L; N–NH3, ammonium–nitrogen, mg/L; N–NO2 −, nitrite–nitrogen, mg/L; N–NO3 −, nitrate–nitrogen, mg/L; NOB, nitrite-oxidising bacteria; OPEX, operating expenditure; PSD, particle size distribution; PVDF, polyvinylidene fluoride; r, Pearson’s coefficient; r f , reversible fouling rate, Pa/s; SMP, soluble microbial products; SOURAOB, specific AOB oxygen uptake rate, mg O2/g MLVSS h; SOURendogenous, specific endogenous oxygen uptake rate, mg O2/g MLVSS h; SOURNOB, specific NOB oxygen uptake rate, mg O2/g MLVSS h; SOUROHO, specific heterotrophic oxygen uptake rate, mg O2/g MLVSS h; SRT, sludge retention time; t B, backwashing phase duration, s; tMBR, tertiary submerged membrane bioreactor; TMP, transmembrane pressure, kPa; TMP f , transmembrane pressure final filtration phase, kPa; TMP i , transmembrane pressure initial filtration phase, kPa; TSS total suspended solids, mg/L; TTF time-to-filter, s; Yobs, low sludge-yield coefficient, kg MLVSS/kg COD
References
Amanatidou E, Samiotis G, Bellos D, Pekridis G, Trikoilidou E (2015) Net biomass production under complete solids retention in high organic load activated sludge process. Bioresour Technol 182:193–199
Aquino SF, Stuckey DC (2008) Integrated model of the production of soluble microbial products (SMP) and extracellular polymeric substances (EPS) in anaerobic chemostats during transient conditions. Biochem Eng J 38:138–146
Bae TH, Tak TM (2005) Interpretation of fouling characteristics of ultrafiltration membranes during the filtration of membrane bioreactor mixed liquor. J Membr Sci 264:151–160
Charfi A, Yang Y, Harmand J, Ben Amar N, Heran M, Grasmick A (2015) Soluble microbial products and suspended solids influence in membrane fouling dynamics and interest of punctual relaxation and/or backwashing. J Membr Sci 475:156–166
Chen J, Zhang M, Wang A, Lin H, Hong H, Lu X (2012) Osmotic pressure effect on membrane fouling in a submerged anaerobic membrane bioreactor and its experimental verification. Bioresour Technol 125:97–101
Cote P, Alam Z, Penny J (2012) Hollow fiber membrane life in membrane bioreactors (MBR). Desalination 288:145–151
Di Bella G, Torregrossa M, Viviani G (2011) The role of EPS concentration in MBR foaming: analysis of a submerged pilot plant. Bioresour Technol 102:1628–1635
Drews A (2010) Membrane fouling in membrane bioreactors—characterisation, contradictions, causes and cures. J Membr Sci 363:1–28
Fenu A, Roels J, Wambecq T, De Gussem K, Thoeye C, De Gueldre G, Van De Steene B (2010) Energy audit of a full scale MBR system. Desalination 262:121–128
Ginestet P, Audic JM, Urbain V, Block JC (1998) Estimation of nitrifying bacterial activities by measuring oxygen uptake in the presence of the metabolic inhibitors allythiourea and azide. Appl Environ Microbiol 64(6):2266–2268
Judd, S., (2010). The MBR book, principles and applications of membrane bioreactors for water and wastewater treatment, 2nd edition. Elsevier
Koch Membrane (2014) Case study. Aquapolo Ambiental Water Reuse Project., http://www.kochmembrane.com/PDFs/Case-Studies/KMS-Sao-Paulo-Brazil-Case-Study.aspx
Le-Clech P, Chen V, Fane AG (2006) Fouling in membrane bioreactors used in wastewater treatment. J Membr Sci 284:17–53
Lin H, Zhang M, Wang F, Meng F, Liao B, Hong H, Chen J, Gao W (2014) A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: characteristics, roles in membrane fouling and control strategies. J Membr Sci 460:110–125
Lin HJ, Gao WJ, Leung KT, Liao BQ (2011) Characteristics of different fractions of microbial flocs and their role in membrane fouling. Water Sci Technol 63:262–269
Marketsandmarkets (2014). Membrane bioreactor systems market by application (municipal wastewater treatment and industrial wastewater treatment), by type (hollow fiber, flat sheet, and multi tubular), by configuration (internal/submerged and external/side stream), and by region—trends & forecasts to 2019. Report CH 2651
Meng F, Chae SR, Drews A, Kraume M, Shin HS, Yang F (2009) Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res 43:1489–1512
Metcalf and Eddy, Inc. (2003) Wastewater engineering: treatment and reuse. McGraw-Hill, New York
Pan JR, Su YC, Huang C, Lee HC (2010) Effect of sludge characteristics on membrane fouling in membrane bioreactors. J Membr Sci 349:287–294
Pollice A, Laera G, Blonda M (2004) Biomass growth and activity in a membrane bioreactor with complete sludge retention. Water Res 38:1799–1808
Pollice A, Laera G, Saturno D, Giordano C (2008) Effects of sludge retention time on the performance of a membrane bioreactor treating municipal sewage. J Membr Sci 317:65–70
Rosenberger S, Kruger U, Witzig R, Manz W, Szewzyk U, Kraume M (2002) Performance of a bioreactor with submerged membranes for aerobic treatment of municipal wastewater. Water Res 36:413–420
Sabia G, Ferraris M, Spagni A (2013) Effect of solid retention time on sludge filterability and biomass activity: long-term experiment on a pilot-scale membrane bioreactor treating municipal wastewater. Chem Eng J 221:176–184
Shen LG, Lei Q, Chen JR, Hong HC, He Y-M, Lin HJ (2015) Membrane fouling in a submerged membrane bioreactor: impacts of floc size. Chem Eng J 269:328–334
Standard methods for the examination of water and wastewater (2005). American Public Health Association/Water Environment Federation, 21st ed. Washington DC
Trussell RS, Merlo R, Hermanowicz SW, Jenkins D (2007) Influence of mixed liquor properties and aeration intensity on membrane fouling in a submerged membrane bioreactor at high mixed liquor suspended solids concentrations. Water Res 41:947–958
Van den Broeck R, Krzeminski P, Van Dierdonck J, Gins G, Lousada-Ferreira M, Van Impe JFM, van der Graaf JHJM, Smets IY, van Lier JB (2011) Activated sludge characteristics affecting sludge filterability in municipal and industrial MBRs: unraveling correlations using multi-component regression analysis. J Membr Sci 378:330–338
Van Loosdrecht M, Henze M (1999) Maintenance, endogenous respiration, lysis, decay and predation. Water Sci Technol 39:107–117
Vera L, González E, Díaz O, Delgado S (2014) Performance of a tertiary submerged membrane bioreactor operated at supra-critical fluxes. J Membr Sci 457:1–8
Villain M, Marrot B (2013) Influence of sludge retention time at constant food to microorganisms ratio on membrane bioreactor performances under stable and unstable state conditions. Bioresour Technol 128:134–144
Wang XM, Li XY (2008) Accumulation of biopolymer clusters in a submerged membrane bioreactor and its effect on membrane fouling. Water Res 42:855–862
Wang Z, Yu H, Ma J, Zheng X, Wu Z (2013) Recent advances in membrane bio-technologies for sludge reduction and treatment. Biotechnol Adv 31:1187–1199
Wilén BM, Keiding K, Nielsen PH (2000) Anaerobic deflocculation and aerobic reflocculation of activated sludge. Water Res 34:3933–3942
Wu J, Huang X (2009) Effect of mixed liquor properties on fouling propensity in membrane bioreactors. J Membr Sci 342:88–96
Zeman LJ, Zydney AL (1996) Microfiltration and ultrafiltration: principles and application. Marcel Dekker, Inc., New York
Acknowledgments
The control system, funded by the N.R.C. (MINECO project CTM2011-27307), was tested for long-term validation during the MBR operation described in this paper. The authors wish to express their gratitude to GE Water & Process Technologies and to BALTEN for their support and also to the staff of the Water Analysis Laboratory of the Chemical Engineering Department at Universidad de La Laguna (ULL) for their analytical advice. We greatly appreciate access to the multiparameter universal controller permitted by the CONVAGUA project of the Canary Agency-ACIISI, sponsored by the European Commission and the Canary Islands Regional Government. This study has been carried out in the framework of MBRgenera-AGUA01 project funded by Fundación Cajacanarias.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Díaz, O., Vera, L., González, E. et al. Effect of sludge characteristics on membrane fouling during start-up of a tertiary submerged membrane bioreactor. Environ Sci Pollut Res 23, 8951–8962 (2016). https://doi.org/10.1007/s11356-016-6138-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6138-y