Abstract
In order to understand the biodegradability of algal-derived organic matter, biodegradation experiments were conducted with 13C and 15N-labeled natural phytoplankton and periphytic algal populations in experimental conditions for 60 days. Qualitative changes in the dissolved organic matter were also determined using parallel factor analysis and the stable carbon isotopic composition of the hydrophobic dissolved organic matter through the experimental period. Although algal-derived organic matter is considered to be easily biodegradable, the initial amounts of total organic carbon newly produced by phytoplankton and periphytic algae remained approximately 16 and 44 % after 60 days, respectively, and about 22 and 43 % of newly produced particulate nitrogen remained. Further, the dissolved organic carbon derived from both algal populations increased significantly after 60 days. Although the dissolved organic matter gradually became refractory, the contributions of the algal-derived organic matter to the dissolved organic matter and hydrophobic dissolved organic matter increased. Our laboratory experimental results suggest that algal-derived organic matter produced by phytoplankton and periphytic algae could contribute significantly to the non-biodegradable organic matter through microbial transformations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The organic matter concentrations in water resources have been reported to increase in the UK, North America, Europe, and Japan (Driscoll et al. 2003; Evans et al. 2005; Hejzlar et al. 2003; Imai et al. 2001; Le et al. 2010; Worrall et al. 2003; Yamada et al. 2012). Apart from leading to increased water purification costs (Le et al. 2010), this also causes changes in the fate and transport of pollutants such as heavy metals in water bodies (Tessier and Turner 1995). This phenomenon has been observed in the Han River, which is one of the most important water sources in Korea (Lee and Choi 2009). In order to determine the reasons for this phenomenon, the Korean government is trying to understand the sources and long-term trends of organic matter.
In Lake Paldang, which is a large artificial lake located in the central region of the Han River, an annual rise of chemical oxygen demand (COD) has been observed since 1995. However, the biochemical oxygen demand (BOD) values are nearly constant (Fig. S1). On the other hand, the BOD/COD ratio has shown a decreasing trend since 1999, indicating increased contribution of refractory organic matter to the total organic matter (Badawy and Ali 2006; Vollertsen and Hvitved-Jacobsen 2002). These long-term trends support the accumulation of non-biodegradable organic matter (viz. recalcitrant organic matter) in natural water resources. However, limited information is available on the main source of non-biodegradable organic matter.
Although algal-derived organic matter is considered to be easily biodegradable, accumulating evidence suggests that a substantial fraction of algal-derived organic matter persists after several months of biodegradation (Kragh and Søndergaard 2009; Kristensen et al. 1995; Hanamachi et al. 2008). The labile organic matter can be converted to refractory matter, which remains for a long period in the water column, by microbial degradation. Ogawa et al. (2001) reported that glucose and glutamate, which are easily biodegradable, were rapidly consumed within 2 days by microbial transformation. They further stated that the refractory dissolved organic matter was released within a few days, after the addition of labile organic matter. In addition, Kragh and Søndergaard (2009) demonstrated the production of refractory organic components directly by marine algae or indirectly by food web processes. These results indicate that algal-derived organic matter in the water supply could be an important source of refractory organic matter, which increases the cost of water treatment.
Aquatic organic matter consists of autochthonous as well as allochthonous substances. Hence, it is not easy to separate the fate of organic matter produced by primary producers in a natural sample. In the case of periphytons, it is all the more difficult to distinguish newly produced organic matter from the complex mixture of algae, heterotrophic microbes, and detritus. However, this can be overcome by using isotopically enriched inorganic carbon and/or nitrogen. Stable isotope labeling has been used as a powerful tool to elucidate the fates and dynamics of algal-derived organic matter (Kritzberg et al. 2006; Pace et al. 2004; Veuger et al. 2004; Yoshimura et al. 2009).
During the biodegradation process, the organic matter preservation potential depends on the nature of the organic sources (Owen and Lee 2004). Combined excitation emission matrix fluorescence techniques with parallel factor analysis (EEM-PARAFAC) have been extensively used to infer changes in the qualitative composition of dissolved organic matter during biodegradation (Stedmon and Markager 2005; Yamashita et al. 2008). This new approach has a competitive advantage over conventional methods, i.e., it aids in understanding large EEM data sets (Kowalczuk et al. 2009).
To better understand the biodegradability of algal-derived organic matter, its properties must be determined in natural aquatic environments. Aside from phytoplankton, periphytic algal-derived organic matter is also an important source in aquatic systems. However, there is limited or no information available on the role of periphytic algae in the formation of refractory organic matter. Given that a considerable proportion of the organic matter newly produced by phytoplankton persists after biodegradation, periphytic algal-derived organic matter could also participate in the formation of refractory organic matter. Therefore, in this investigation, an effort to understand the biodegradability of algal-derived organic matter originating from natural phytoplankton and periphytic algal populations was undertaken under laboratory conditions.
The present study was conducted to (i) determine the contribution of algal-derived organic matter during microbial degradation using stable carbon and nitrogen isotope tracers and (ii) understand the qualitative changes in dissolved organic matter during biodegradation.
Materials and methods
Sampling sites and sample preparation
Lake Paldang, constructed in 1973, is a major source of drinking water for the 20 million people residing in the Seoul metropolitan area of Korea (Fig. S2). Although the lake is a protected drinking water resource, its water quality is reported to have deteriorated because of increased algal production (Lee et al. 2015; Na and Park 2006). In order to determine the biodegradability of algal-derived organic matter, natural phytoplankton and periphyton populations were collected from Lake Paldang on May 24, 2011. Surface water containing natural phytoplankton communities was sampled at site P1 (Fig. S1), and the macro-zooplankton were removed by filtration through a 100-μm mesh (Hama et al. 2004; Yoshimura et al. 2009). The water samples were transferred to polycarbonate (Nalgene) bottles that were acid-washed. Several plastic bars (37.5 cm2) affixed to trays were placed underwater at site P2 to collect natural periphyton populations on April 28, 2011. After 1 month (viz. May 24, 2011), each plastic bar with the attached periphyton was placed directly into acid-washed polycarbonate bottles filled with filtrate (pre-combusted GF/F). These natural phytoplankton and periphyton populations were transferred to the laboratory within one-and-a-half hours of collection.
C and N labeling experiment
In order to separate the organic matter newly produced by algae from the bulk pool, natural algal populations were incubated with NaH13CO3 and K15NO3 under fluorescent light (viz. ∼140 μmol m−2 s−1) for 14 h at 20 °C (which corresponds to the in situ temperature). A sub-sample was collected after 14 h (day 0), and the remaining samples were incubated in the dark at 20 °C for 60 days. Sub-samples for both algal populations were collected in triplicate at each sampling date (viz. days 5, 15, 28, and 60). After collecting the sub-sample, the periphyton was scraped off into the water and filtered. After 60 days, the concentrations of dissolved oxygen in the bottles containing natural phytoplankton and periphyton populations were 5.6 and 5.9 mgO2 L−1, respectively. These results indicate that the organic matter had been decomposed under oxic conditions. The particulate fractions in the samples were collected onto pre-combusted (4 h at 450 °C) Whatman GF/F filter, and the pigment composition, carbon, and nitrogen isotopic compositions and their concentrations were determined using HPLC (1200 series, Agilent Technologies, San Jose, CA, USA) and elemental analyzer–isotopic ratio mass spectrometer (EA–IRMS), respectively. The details of pigment analysis are described in Ha et al. (2014), and the detailed methodology for EA-IRMS analysis is provided below. The filtrate samples were analyzed to measure their nutrient concentration, concentrations of dissolved inorganic carbon (DIC) and dissolved organic carbon (DOC), fluorescence property, and carbon and nitrogen isotopic compositions of hydrophobic dissolved organic matter. The concentrations of nutrients (nitrate + nitrite, phosphate, and silicate) were analyzed using a continuous flow nutrient analyzer (Bran + Luebbe, QuAAtro). The MOOS-2 was used as certified reference material. The concentrations of DIC and DOC were measured using a total organic carbon analyzer (TOC-VCPH, Shimadzu, Japan). This method has been validated against a certified reference material (41–44 μmol L−1; Hansell Laboratory, University of Miami).
EA–IRMS analysis
After removal of inorganic carbon (using 12N hydrochloric acid fumes), the isotopic composition and concentrations of particulate organic carbon (POC) were determined using an isotope ratio mass spectrometer (Isoprime, GV Instruments, Manchester, UK) with an elemental analyzer (Euro EA3028, EuroVector, Milan, Italy). The isotopic composition and concentrations of particulate nitrogen (PN) were also determined by a procedure similar to POC, barring the acidification step. The coefficients of variations of POC and PN concentrations using standard materials were 3.3 and 3.4 %, respectively. The analytical precisions for the carbon and nitrogen isotopic ratios, estimated by running standards (IAEA standards CH6 and N1), were 0.05 and 0.1 ‰, respectively. The standard reference materials for carbon and nitrogen were Vienna Pee Dee Belemnite (VPDB) and atmospheric N2, respectively. The carbon isotope ratio of DOC was determined in the freeze-dried material after exposure to acid fumes to remove inorganic carbon (Urban et al. 2005). The removal of inorganic carbon with 12N hydrochloric acid fumes was verified using NaHCO3.
Fluorescence analysis of DOM
The fluorescence EEM was measured with a luminescence spectrometer (LS-55, Perkin-Elmer, Liantrisant, UK) by scanning the emission spectra from 280 to 550 nm at 0.5 nm increments and stepping through excitation wavelengths from 250 to 500 nm at 5 nm increments. The methods of measuring the EEM and normalizing the fluorescence intensity were described in detail by Phong et al. 2014. After data acquisition, PARAFAC modeling was conducted using MATLAB 7.0 (Math Works, Natick, MA, USA) with the DOMFluor Toolbox (http://www.models.life.ku.dk), and the identified components and numbers were validated by split-half analysis. The details of the modeling have been provided elsewhere (Kowalczuk et al. 2009; Nguyen et al. 2013; Stedmon et al. 2003). The fluorescence emission spectra of the samples were recorded at 300 to 480 nm (under 254 nm exCitation) to calculate the humification index (HIX). The HIX was estimated based on the equation proposed by Ohno (2002) and ranged from zero to one.
C and N isotopic compositions of hydrophobic DOM
The hydrophobic DOM was extracted using a solid phase C18 extraction disk (3M Empore) following the method of Kim et al. (2003). The disks were conditioned using methanol before extracting the water samples. The water samples were passed through a GF/F filter, and the pH of the filtrates was adjusted to two using hydrochloric acid prior to extraction. Aliquots of 800–1000 ml of the filtrate water were loaded onto the SPE disk. The retentate containing hydrophobic DOM was eluted with methanol and dried using a centrifugal evaporator (EYELA, Tokyo, Japan). The carbon and nitrogen isotopic compositions of hydrophobic DOM absorbed onto the glass fiber filters (Whatman GF/F) were measured using an EA–IRMS as described above. However, the nitrogen isotopic composition of all hydrophobic DOM showed values less than the detection limit. The carbon isotopic composition of hydrophobic DOM is described in the “Results and discussion” section.
Data analysis
The concentrations of algal-derived organic matter in the sub-samples were calculated according to the equation (Hama and Yanagi 2001; Hama et al. 2004) below:
where a is is the 13C (or 15N) atom percentage in an incubated sample, a ns is the 13C (or 15N) atom percentage in a natural (non-incubated) sample, a ic is the 13C (or 15N) atom percentage in DIC (or DIN), and C (or N) is the concentration of organic carbon (viz. POC and DOC) or PN in the incubated samples.
The kinetics of DOM degradation was expressed by a first-order exponential decay model taking the non-biodegradable pool into consideration, using the following equation:
where OM(t) is the amount of OM remaining at time t, BOM is the biodegradable pool, k is the degradation rate constant (day−1), t is the time (days), and NBOM is the non-biodegradable pool (Lønborg et al. 2009). Curve fitting was carried out using the software SIGMA PLOT 10.0 (Chabbi et al. 2006; Lønborg et al. 2009).
Statistical analysis
The statistical differences between day 0 and day t (viz. day 5, 15, 28, and 60) were tested using the Student’s t test or Mann-Whitney U test. These statistical analyses were performed using the IBM SPSS Statistics 21 software (SPSS Inc., Chicago, IL).
Results and discussion
Biodegradability of algal-derived organic matter
The labeled DIC and DIN were successfully incorporated into the phytoplankton and periphytic algae over 14 h under light. Moreover, using enriched stable isotope tracers, it was possible to separate the newly produced organic matter from the bulk pool (Table 1). The initial (viz. day 0) concentrations of bulk POC, PN, DOC, and pigments were higher in the periphyton than the phytoplankton. Among the pigments, the highest concentrations recorded in both algal populations were fucoxanthin, which is a diatom marker pigment (Jeffrey et al. 1997), followed by chlorophyll a. These results suggest that the phytoplankton and periphytic algal communities were dominated by diatom taxa. The newly produced POC, PN, and DOC comprised 8.2, 5.0, and 0.43 % of the bulk pools, respectively, in the phytoplankton. On the other hand, in the periphytic algae, they comprised 2.6, 3.4, and 0.56 % of the bulk pools, respectively. The DOC fractions derived from both algal populations were 6.0 and 8.1 % of the newly produced total organic carbon for phytoplankton and periphytic algae, respectively. These values were comparable with those reported by Baines and Pace (1991) using 14C as a tracer. The concentrations of nitrate + nitrite, phosphate, and silicate were 119, 0.27, and 77 μmol L−1, respectively, in bottles containing phytoplankton populations, and 105, 0.65, and 77 μmol L−1, respectively, in bottles containing periphyton populations on day 0 (i.e., after 14 h of light incubation).
Time-dependent changes in concentrations of chlorophyll a and newly produced organic matter are shown in Fig. 1. The concentration of Chl a decreased rapidly during the initial 5 days in the phytoplankton-derived organic matter, while it decomposed from day 0 to day 28 in the periphytic algal-derived organic matter. The decay constant (k) of Chl a was three times greater in the phytoplankton than in the periphytic algae. Similar values of PO13C were observed in both samples on day 0, but the degradation rate (k) was also faster in the phytoplankton-derived organic matter than in the periphytic algae. Louda et al. (1998) stated that during the senescence and death of algal cells, Chl a is converted to a degraded form. However, in contrast to phytoplankton, the Chl a of periphytic algae remained at 23 % of the initial value until day 15. Sekar et al. (2002) reported that the Chl a concentrations in biofilm were sustained for 15 days under dark conditions. They also observed a succession of algal communities in light-grown biofilms (Chlorophyceae–diatom–cyanobacteria), while the diatom mostly dominated the dark-grown biofilms for 15 days. Thus, as previous reports verify that periphytic diatom can survive until day 15, this could explain the slow degradation rates of periphytic algal-derived organic matter. The decay constant (k) of the POC in phytoplankton was comparable with those reported by a previous investigator (Pett 1989).
Changes in a Chl a, b newly produced PO13C, c DO13C, and d P15N of phytoplankton and periphytic algae. The plots represent observed mean values with standard deviations, and the solid line represents values predicted by the exponential decay model. The “k” is the degradation rate constant of each compound. Curve fitting was not attempted on DO13C
The concentrations of particulate organic matter were decreased in both populations, while the DOC either remained stable or increased. The initial DO13C concentration was retained even after 60 days in the bottles containing phytoplankton populations. Further, its concentration was dramatically increased on day 5. This was probably caused by a significant decrease in Chl a and PO13C (i.e., released as a labile fraction). In the case of periphytic algae, there was a 73 % decrease in PO13C as compared to its initial value, but the DO13C increased about three times compared to day 0. This increase coincided with 18 % of the biodegradable POC over 60 days.
The newly produced P15N by both algal-populations declined rapidly within the first 5 days and continued to decline further until day 28. Although they showed different concentrations, the degradation rates were the same. Generally, organic nitrogen is preferentially degraded compared to organic carbon through the decomposition of organic matter during early diagenesis (Hecky et al. 1993; Talbot and Lærdal 2000). However, the disparate results were found in this study. The organic matter preservation has been known to be affected by the phytoplankton source (Hanamachi et al. 2008; Nguyen and Harvey 1997). In an oxic microbial degradation experiment using Skeletonema costatum (diatom), Kristensen et al. (1995) reported concentrations of POC and PON on day 38 to be 66 and 72 % of the initial concentration, respectively. In a decomposition experiment using Thalassiosira weissflogii (diatom), Harvey et al. (1995) also reported that the POC was preferentially degraded rather than protein over 20 days. Our results correspond to these previous findings involving degradation experiments using a single cell diatom.
Nguyen and Harvey (1997) also reported that almost half of the dinoflagellate-produced PN attributable to polypeptides and/or proteins was of a refractory nature. The algal-derived DOC remained at more than 70 % after biodegradation (Kragh and Søndergaard 2009; Yoshimura et al. 2009). Although quite different proportions of biodegradable fraction of phytoplankton sources have been described in previous reports (Hanamachi et al. 2008; Nguyen and Harvey 1997), in this study as well, considerable photosynthetic organic matter could remain as non-biodegradable organic matter after the degradation process. On the other hand, the high proportions of newly produced periphytic algal organic matter persisted after 60 days. The periphyton not only consisted of algae but also comprised bacteria, fungi, and detritus (Carr et al. 2005). Kuehn et al. (2014) reported that photosynthetic products synthesized by periphytic algae were quickly transferred to and/or assimilated by heterotrophic microbial communities. In this context, the newly produced organic matter derived from periphytic algae could persist not only as detritus (which is of refractory nature) but also partially in incorporated form in the heterotrophic microbial biomass. In the present study, after 60 days, most of the Chl a was degraded in both algal populations, but some newly produced POC, DOC, and PN persisted.
Characterization of dissolved organic matter
The concentrations of bulk dissolved organic carbon were quite similar in the bottles containing phytoplankton populations for 60 days. However, it was significantly altered in the bottles containing periphytic algae (Table 2). After 28 and 60 days, the concentrations of newly produced dissolved organic carbon by phytoplankton and periphytic algae increased compared to day 0. The HIX, which is an indicator of the degree of degradation (Ohno 2002), also increased in both bottles containing phytoplankton and periphytic algae communities during the degradation experiment. Generally, large amounts of organic matter produced by phytoplankton lead to an accumulation of dissolved organic carbon, which is labile in nature (Engel et al. 2012; Ittekkot et al. 1981). However, in the present study, it could be stated that the increase of newly produced DOC in both algal populations after 60 days was not related to the accumulation of labile DOC, considering the elevated HIX values. In addition, although the concentration of bulk DOC remained unchanged, the humification degree of DOC increased.
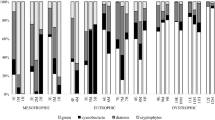
Using the EEM-PARAFAC, the four fluorescence components (C1, C2, C3, and C4) were extracted from the EEM measurements for all 30 samples (Fig. 2 and Table S1). These components were similar to the peaks M, A, C, and T, respectively, described by Coble (1996). The filtrate samples containing organic matter derived from natural phytoplankton communities, wherein the relative abundances of C1 and C2 gradually increased until day 60, but the relative abundance of C4 showed a decreasing trend (Fig. 3). Although no statistically significant differences were observed, a similar pattern was recorded in the case of the filtrate samples comprising organic matter derived from natural periphyton populations. The Fmax values of four components also showed a pattern similar to relative abundance of each fluorescence component (Table S2). These results indicate qualitative changes in dissolved organic matter during the degradation experiments. Previous researchers have reported C1 to be assigned to the microbial humic-like component, and C2 was similarly assigned to the terrestrial fluorescent component (Cory and McKnight 2005; Yamashita et al. 2008). Stedmon and Markager (2005) demonstrated that C3 was ubiquitous in all environments, while Cory and McKnight (2005) explained it as an unknown terrestrial component. On the other hand, the C4 is reported to be attributable to proteinaceous material related to recent biological activity (Determann et al. 1998). Parlanti et al. (2000) reported that a protein-like peak appeared first, followed by the β component (similar to C1 in this study, Table S1) during the first stages of algal degradation. Furthermore, the fluorescence intensity of the β peak increased, while the protein-like peak declined. Similar results were obtained in the present study. The dissolved organic matter in both samples seems to become increasingly refractory over the incubation time.
PARAFAC model outputs representing the four fluorescent components (upper panel) and split-half validation results of four components (lower panel). The lines indicate excitation/emission loadings (blue dotted lines indicate two independent halves of the dataset, while red line indicates the complete dataset)
Contribution of algal-derived organic matter to hydrophobic DOM
The carbon isotope ratios of hydrophobic DOM were gradually enriched in both samples during the 60 days, even though they showed different trends (Fig. 4). The DOM composition changed as the degradation progressed; the protein-like component decreased significantly, while the humic-like components were increased. In addition, the humification index also increased. These results indicate that the dissolved organic matter changed into refractory organic matter as the decomposition advanced. According to Ogawa et al. (2001), labile organic matter can be transformed to a refractory form by heterotrophic bacteria. Lara and Thomas (1995) also found that a considerable fraction of algal-derived organic matter remained as hydrophobic DOM after 267 days. They also stated that 14 % of the newly produced POC was accumulated as hydrophobic DOC fractions. In general, hydrophobic organic matter is less prone to microbial degradation and is more recalcitrant (Jandl and Sletten 1999; Jandl and Sollins 1997). In addition, Aoki et al. (2008) reported that the hydrophobic acids consisted mainly of humic substances. Therefore, these results suggest that, after rearrangement, the algal-derived organic matter could probably be attributed to non-biodegradable organic matter in the lake.
The δ13C values of hydrophobic DOM collected before adding tracers were −25.62 and −25.10 ‰ in the bottles containing phytoplankton and periphytic algal populations, respectively. On the other hand, its values were enriched on day 0 compared to natural samples. These results indicate that algal-derived organic matter is rapidly transformed to hydrophobic DOM during photosynthesis. In the case of phytoplankton-derived organic matter, although the highest concentration of DO13C was observed on day 5, the δ13C values of hydrophobic DOM showed a different trend. These results suggest that the carbon isotopic composition in hydrophobic DOM can potentially represent non-biodegradable algal-derived organic matter in the present study. The higher δ13C values in the hydrophobic DOM derived from periphytic algae compared with phytoplankton are clearly related to the fast transformation of algal-derived organic matter by heterotrophic microbial communities in the periphyton.
Generally, the algal-derived organic matter is considered to be easily biodegradable, but the organic matter newly produced by algal populations can be transformed by microbial communities into organic matter that is refractory in nature. Although the present study, as designed, did not provide detailed information of microbial activity, it clearly indicates that algal-derived organic matter produced by phytoplankton and periphytic algae could contribute to the non-biodegradable organic matter in aquatic environments by microbial transformation. Therefore, primary production of phytoplankton and periphytic algae should be monitored and controlled to manage water quality in the water resources.
References
Aoki S, Ohara S, Kimura K, Mizuguchi H, Fuse Y, Yamada E (2008) Characterization of fluorophores released from three kinds of lake phytoplankton using gel chromatography and fluorescence spectrophotometry. Anal Sci 24(11):1461–1467
Badawy MI, Ali MEM (2006) Fenton’s peroxidation and coagulation processes for the treatment of combined industrial and domestic wastewater. J Hazard Mater 136(3):961–966
Baines SB, Pace ML (1991) The production of dissolved organic matter by phytoplankton and its importance to bacteria: patterns across marine and freshwater systems. Limnol Oceanogr 36(6):1078–1090
Carr GM, Morin A, Chambers PA (2005) Bacteria and algae in stream periphyton along a nutrient gradient. Freshwater Biol 50(8):1337–1350
Chabbi A et al (2006) Lignite degradation and mineralization in lignite-containing mine sediment as revealed by 14C activity measurements and molecular analysis. Org Geochem 37(8):957–976
Coble PG (1996) Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar Chem 51(4):325–346
Cory RM, McKnight DM (2005) Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ Sci Technol 39(21):8142–8149
Determann S, Lobbes JOM, Reuter R, Rullkötter JU (1998) Ultraviolet fluorescence excitation and emission spectroscopy of marine algae and bacteria. Mar Chem 62(1):137–156
Driscoll CT, Driscoll KM, Roy KM, Mitchell MJ (2003) Chemical response of lakes in the Adirondack Region of New York to declines in acidic deposition. Environ Sci Technol 37(10):2036–2042
Engel A, Harlay J, Piontek J, Chou L (2012) Contribution of combined carbohydrates to dissolved and particulate organic carbon after the spring bloom in the northern Bay of Biscay (North-Eastern Atlantic Ocean). Cont Shelf Res 45:42–53
Evans CD, Monteith DT, Cooper DM (2005) Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environ Pollut 137(1):55–71
Ha SY, La HS, Min JO, Chung KH, Kang SH, Shin KH (2014) Photoprotective function of mycosporine-like amino acids in a bipolar diatom (Porosira glacialis): evidence from ultraviolet radiation and stable isotope probing. Diatom Res 29(4):399–409
Hama T, Yanagi K (2001) Production and neutral aldose composition of dissolved carbohydrates excreted by natural marine phytoplankton populations. Limnol Oceanogr 46(8):1945–1955
Hama T, Yanagi K, Hama J (2004) Decrease in molecular weight of photosynthetic products of marine phytoplankton during early diagenesis. Limnol Oceanogra 49(2):471–481
Hanamachi Y, Hama T, Yanai T (2008) Decomposition process of organic matter derived from freshwater phytoplankton. Limnology 9(1):57–69
Harvey RH, Tuttle JH, Tyler Bell J (1995) Kinetics of phytoplankton decay during simulated sedimentation: changes in biochemical composition and microbial activity under oxic and anoxic conditions. Geochim Cosmochim Ac 59(16):3367–3377
Hecky RE, Cambell P, Hendzel LL (1993) The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol Oceanogra 38(4):709–724
Hejzlar J, Dubrovský M, Buchtele J, Růžička M (2003) The apparent and potential effects of climate change on the inferred concentration of dissolved organic matter in a temperate stream (the Malše River, South Bohemia). Sci Total Environ 310(1):143–152
Imai A, Fukushima T, Matsushige K, Hwan Kim Y (2001) Fractionation and characterization of dissolved organic matter in a shallow eutrophic lake, its inflowing rivers, and other organic matter sources. Water Res 35(17):4019–4028
Ittekkot V, Brockmann U, Michaelis W, Degens E (1981) Dissolved free and combined carbohydrates during a phytoplankton bloom in the northern North Sea. Mar Ecol Prog Ser 4(3):299–305
Jandl R, Sletten RS (1999) Mineralization of forest soil carbon: interactions with metals. J Plant Nutr Soil Sc 162(6):623–629
Jandl R, Sollins P (1997) Water-extractable soil carbon in relation to the belowground carbon cycle. Biol Fert Soils 25(2):196–201
Jeffrey SW, Mantoura RFC, Wright SW (eds) (1997) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO monographs on oceanographic methodology, vol 10. UNESCO, Paris
Kim S, Simpson AJ, Kujawinski EB, Freitas MA, Hatcher PG (2003) High resolution electrospray ionization mass spectrometry and 2D solution NMR for the analysis of DOM extracted by C18 solid phase disk. Org Geochem 34(9):1325–1335
Kowalczuk P, Durako MJ, Young H, Kahn AE, Cooper WJ, Gonsior M (2009) Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: interannual variability. Mar Chem 113(3):182–196
Kragh T, Søndergaard M (2009) Production and decomposition of new DOC by marine plankton communities: carbohydrates, refractory components and nutrient limitation. Biogeochemistry 96(1):177–187
Kristensen E, Ahmed SI, Devol AH (1995) Aerobic and anaerobic decomposition of organic matter in marine sediment: which is fastest? Limnol Oceanogr 40(8):1430–1437
Kritzberg ES, Cole JJ, Pace MM, Granéli W (2006) Bacterial growth on allochthonous carbon in humic and nutrient-enriched lakes: results from whole-lake 13C addition experiments. Ecosystems 9(3):489–499
Kuehn KA, Francoeur SN, Findlay RH, Neely RK (2014) Priming in the microbial landscape: periphytic algal stimulation of litter-associated microbial decomposers. Ecology 95(3):749–762
Lara RJ, Thomas DN (1995) Formation of recalcitrant organic matter: humification dynamics of algal derived dissolved organic carbon and its hydrophobic fractions. Mar Chem 51(3):193–199
Le C, Zha Y, Li Y, Sun D, Lu H, Yin B (2010) Eutrophication of lake waters in China: cost, causes, and control. Environ Manage 45(4):662–668
Lee HW, Choi JH (2009) Temporal analysis of trends in dissolved organic matter in Han River water. Environ Eng Res 14(4):256–260
Lee Y, Ha SY, Park HK, Han MS, Shin KH (2015) Identification of key factors influencing primary productivity in two river-type reservoirs by using principal component regression analysis. Environ Monit Assess 187:213. doi:10.1007/s10661-015-4438-1
Lønborg C, Davidson K, Álvarez-Salgado XA, Miller AEJ (2009) Bioavailability and bacterial degradation rates of dissolved organic matter in a temperate coastal area during an annual cycle. Mar Chem 113(3):219–226
Louda JW, Li J, Liu L, Winfree MN, Baker EW (1998) Chlorophyll-a degradation during cellular senescence and death. Org Geochem 29(5):1233–1251
Na EH, Park SS (2006) A hydrodynamic and water quality modeling study of spatial and temporal patterns of phytoplankton growth in a stratified lake with buoyant incoming flow. Ecol Model 199(3):298–314
Nguyen RT, Harvey HR (1997) Protein and amino acid cycling during phytoplankton decomposition in oxic and anoxic waters. Org Geochem 27(3):115–128
Nguyen HVM, Lee MH, Hur J, Schlautman MA (2013) Variations in spectroscopic characteristics and disinfection byproduct formation potentials of dissolved organic matter for two contrasting storm events. J Hydrol 481:132–142
Ogawa H, Amagai Y, Koike I, Kaiser K, Benner R (2001) Production of refractory dissolved organic matter by bacteria. Science 292(5518):917–920
Ohno T (2002) Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ Sci Technol 36(4):742–746
Owen RB, Lee R (2004) Human impacts on organic matter sedimentation in a proximal shelf setting, Hong Kong. Cont Shelf Res 24(4):583–602
Pace ML et al (2004) Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature 427(6971):240–243
Parlanti E, Wörz K, Geoffroy L, Lamotte M (2000) Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org Geochem 31(12):1765–1781
Pett RJ (1989) Kinetics of microbial mineralization of organic carbon from detrital Skeletonema costatum cells. Mar Ecol Prog Ser 52(2):123–128
Phong DD, Lee Y, Shin KH, Hur J (2014) Spatial variability in chromophoric dissolved organic matter for an artificial coastal lake (Shiwha) and the upstream catchments at two different seasons. Environ Sci Pollut Res 21(12):7678–7688
Sekar R, Nair KVK, Rao VNR, Venugopalan VP (2002) Nutrient dynamics and successional changes in a lentic freshwater biofilm. Freshwater Biol 47(10):1893–1907
Stedmon CA, Markager S (2005) Tracing the production and degradation of autochthonous fractions of dissolved organic matter by fluorescence analysis. Limnol Oceanogr 50(5):1415–1426
Stedmon CA, Markager S, Bro R (2003) Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar Chem 82(3):239–254
Talbot MR, Lærdal T (2000) The Late Pleistocene–Holocene palaeolimnology of Lake Victoria, East Africa, based upon elemental and isotopic analyses of sedimentary organic matter. J Paleolimnol 23(2):141–164
Tessier A, Turner DR (eds) (1995) Metal speciation and bioavailability in aquatic systems. Wiley, New York
Urban NR, Auer MT, Green SA, Lu X, Apul DS, Powell KD, Bub L (2005) Carbon cycling in Lake Superior. J Geophys Res 110:C06S90
Veuger B, Middelburg JJ, Boschker HTS, Nieuwenhuize J, Van Rijswijk P, Rochelle-Newall EJ, Navarro N (2004) Microbial uptake of dissolved organic and inorganic nitrogen in Randers Fjord. Estuar Coast Shelf S 61(3):507–515
Vollertsen J, Hvitved-Jacobsen T (2002) Biodegradability of wastewater—a method for COD-fractionation. Water Sci Technol 45(3):25–34
Worrall F, Burt T, Shedden R (2003) Long term records of riverine dissolved organic matter. Biogeochemistry 64(2):165–178
Yamada E, Ohara S, Uehara T, Hirota T, Hatori N, Fuse Y, Aoki S (2012) Biodegradation of dissolved organic matter (DOM) released from phytoplankton in Lake Biwa. Anal Sci 28(7):675–681
Yamashita Y, Jaffé R, Maie N, Tanoue E (2008) Assessing the dynamics of dissolved organic matter (DOM) in coastal environments by excitation emission matrix fluorescence and parallel factor analysis (EEM-PARAFAC). Limnol Oceanogr 53(5):1900–1908
Yoshimura K, Ogawa T, Hama T (2009) Degradation and dissolution properties of photosynthetically-produced phytoplankton lipid materials in early diagenesis. Mar Chem 114(1–2):11–18
Acknowledgments
The current research was supported by the grants from the National Research Foundation of Korea Grant funded by the Korean government and the Marine Biotechnology Program funded by the Ministry of Oceans and Fisheries, Korea. This research was a part of the project titled “Determining origin and fate of inflow nitrogen into marine environment using nitrogen stable isotope ratio of amino acid” funded by the Ministry of Oceans and Fisheries, Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 6686 kb)
Rights and permissions
About this article
Cite this article
Lee, Y., Lee, B., Hur, J. et al. Biodegradability of algal-derived organic matter in a large artificial lake by using stable isotope tracers. Environ Sci Pollut Res 23, 8358–8366 (2016). https://doi.org/10.1007/s11356-016-6046-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6046-1