Abstract
Dissolved organic matter (DOM) is considered to be one of active organic carbon components in river water, and its characteristics would affect quality of drinking water if such a river is used for the purpose. DOM in the Pearl River around metropolitan Guangzhou and its six fractions obtained by sequential resins separation and their percentage distribution of total organic carbon (TOC), the UV absorbance at 254 nm (UV254), and the specific ultraviolet absorbance (SUVA254) were determined. Meanwhile, fluorescence spectroscopy and Fourier transform infrared (FTIR) spectroscopy were used to examine the biodegradable and structural characteristics of DOM. The results showed that the values of TOC, UV254, and SUVA254 changed with season. Especially, SUVA254 was lower than 3 L (mg m)−1, indicating that the hydrophilic fractions were the major components of the DOM. Furthermore, fluorescence spectroscopy revealed the dominant presence of humic-like, fulvic-like, and protein-like fluorophores. Fluorescence index (FI) in four seasons was associated with allochthonous DOM sources and biological DOM. FTIR spectroscopy suggested the feature of DOM with some specific groups (e.g., carbohydrate C–O, amid C═O).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dissolved organic matter (DOM) is considered to be one of the largest numbers of organic constituents in all aquatic ecosystems and includes fulvic acid, humic acid, carbohydrate, and amino acid (He and Wu 2015), and its particle in natural water can pass through a 0.45-mm membrane (Buffle and Leppard 1995). If less affected by anthropogenic activities, DOM can be generally defined as the natural organic matter (NOM), which mainly includes hydrophilic, acidic, and humus substances (Koch et al. 2005), and is released from living and decaying biota (Mopper et al. 1996). In contrast, DOM strongly affected by wastewater has various and uncertain origins (Meng et al. 2013) and increases the solubility and hydrophilicity of organic pollutants, to enhance the environmental risk of organic pollutants (He and Wu 2015). As a result of chlorination in the water treatment processes, DOM is considered to be a precursor in the production of potentially carcinogenic disinfection by-products (DBPs), such as trihalomethanes (THMs), haloacetic acids (HAAs), and haloacetonitriles (HANs) (Panyapinyopol et al. 2005). Especially, THMs negatively affect the liver, the kidney, and the nervous and reproductive systems (Navalon et al. 2008) and induces DNA damage in human-derived hepatoma line to be linked with the increase of cancer risk (Ma et al. 2013; Pifer and Fairey 2012). Therefore, DOM imposes adverse impacts on the quality of drinking water and human health (Chen et al. 2013).
The characterizations of DOM in many globally large rivers have already gained increasing concern (Chen et al. 2013). The Pearl River (Zhujiang) is the fourth longest river (ca. 2320 km in length) and the second largest river in terms of water discharge in China. The Pearl River delivers much freshwater and constitutes one of the major drinking water sources of Guangzhou metropolis, where about 3 million cubic meters of potable water is pumped to water plants every day. In recent years, industrial land use in the downstream district of the Pearl River has substantially contributed toward the increase of DOM levels, due to that large amounts of wastewater are discharged to the river. These are proved by the results of anthropogenic input in urbanized rivers (Meng et al. 2013). Fortunately, many efforts have been devoted to understand the roles of DOM in the aquatic environment of the Pearl River, e.g., the distribution of DOM in the estuary (the Pearl River Delta region) (Callahan et al. 2004) and the origin and fate of DOM (from its main tributaries to the estuary) (Meng et al. 2013). Obviously, these researches focus on the larger and various sections of the Pearl River. Moreover, due to the complexity of DOM in the Pearl River, some researches provide information about the molecular weight distribution for each fraction of DOM with different size membranes to study its characteristics (Zhao et al. 2006). But, with resin adsorption, it would be chemically separated into other six organic fractions prior to analysis: hydrophobic acid (HPOA), hydrophobic base (HPOB), hydrophobic neutral (HPON), hydrophilic acid (HPIA), hydrophilic base (HPIB), and hydrophilic neutral (HPIN), of which the hydrophobic fractions were considered to have higher relativities of THM formation than the hydrophilic fractions (Panyapinyopol et al. 2005). To predict DOM fractions, total organic carbon (TOC) is an aggregate parameter and not a direct indicator of the organic character of the source water (USEPA 1989), and the UV absorbance at 254 nm (UV254) represents the aromatic character of organic matters (Marhaba et al. 2000). Further, specific ultraviolet absorbance (SUVA254) is a good surrogate parameter for aromaticity and humic contents of waters (Drews 2010; Wong et al. 2007) and calculated as SUVA254 = UV254 / dissolved organic carbon (DOC) (Pifer and Fairey 2012). The three-dimensional excitation emission matrix (EEM) reveals seasonal variations of DOM and identifies the source of water (Callahan et al. 2004). Fourier transform infrared (FTIR) spectroscopy further provides valuable information on the structural and functional groups (e.g., the amide, carboxyl, and aliphatic ester) of DOM (Abdulla et al. 2010).
However, these techniques have not used to determine DOM fractions in the Pearl River, especially for the drinking water sources’ section of the Guangzhou city. Therefore, the main objectives of the study were to explore the seasonal variations of the DOM characteristics and provide a more detailed description for six organic fractions of DOM in water sample collected from back channel of the Pearl River.
Materials and methods
Sampling and pretreatment
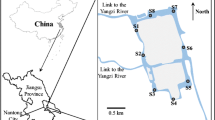
The Pearl River in Guangzhou city is mainly divided into front channel, back channel, and Huangpu channel. Among them, the back channel section (from Baietan to Luoxi ferry) belongs to the drinking water source area. The sampling site was located at Luoxi ferry (between the source area and the populous area) and close to Nanzhou drinking water supply plant, which is one of the most important water services in Guangzhou city (Fig. 1), and sample collections were conducted in spring (March, April, and May), summer (June, July, and August), autumn (September, October, and November), and winter (December, January, and February). The sampling was conducted in three replicates in each season. Samples were collected from the surface of river water (<0.8 m) using glass bottles of 5 L, then filtered immediately through a 0.45-μm cellulose acetate (CA) membrane (acid washed and prerinsed with pure water to remove the remaining compounds within the membrane pores prior to use), and stored in the dark at 4 °C before and after fractionation.
Fractionation
The fractionation of DOM was performed through adsorption using three types of resin adsorbents, i.e., nonionic resin (DAX-8), strong cationic resin (Amberlyst 15), and weak anionic resin (WA-10), following the procedure described by Marhaba et al. (2003), and then, DOM was classified into six fractions: HPOA, HPOB, HPON, HPIA, HPIB, and HPIN, respectively. The fractionation procedure is listed in Fig. 2.
Analysis
All fractions were adjusted to a neutral pH prior to the measure of TOC and UV254. TOC analysis was conducted by the high-temperature combustion method using Shimadzu VCPH TOC analyzer equipment. And, UV254 was measured with a UV-2550 thermo-spectronic (Shimadzu, Japan) with a 1-cm quartz cell.
Water samples were placed in a freezer for freezing about 1.5 h and then in the condition of vacuum freeze for being dried more than 24 h. The samples of 2 mg and dry KBr of 200 mg were mixed and pressed into thin slices under the pressure of 10 t cm−2. The final samples were scanned from 400 to 4000 cm−1 by FTIR spectrometer to determine their transmittance.
Fluorescence of the samples was measured at 25 °C using spectrofluorometer equipped with two monochromators for both the excitation light source and the emission detector (Hitachi F-2500, Japan), and then, the chosen parameters were as follows: (1) the excitation light source: the 150-W xenon lamps, (2) voltage of 700 V, (3) scanning speed of 3000 nm min−1, (4) crack width: λx − λm of 2.5/2.5, (5) the excitation wavelengths (Ex) of 225–525 nm, and (6) the emission wavelengths (Em) of 240–633 nm. Three-dimensional emission/excitation matrix (EEM) fluorescence was drawn by Origin 8.0 software. In addition, the independent-samples test was used to determine the significance of the measured parameter, and p < 0.05 was considered to be statistically significant.
Results and discussion
Seasonal characteristics of total organic carbon, UV absorbance at 254 nm, and specific ultraviolet absorbance
After fractionation, six fractions of DOM were determined as follows: (1) HPOA: carboxylic acid with five to nine carbon atoms, aromatic carboxylic acid with one to two rings, and phenol and tannin with one to two rings; (2) HPOB: protein, aromatic amines with one to two rings, and alkanes; (3) HPON: hydrocarbons, fatty alcohols, alkyl alcohols, esters, ketones, aldehydes, carboxylic acids, and aliphatic amine with >5 carbon atoms, aromatic carboxylic acid with >9 carbon atoms, and aromatic amines ≥3 rings; (4) HPIA: fatty acid <5 carbon atoms, hydroxy acids, sugar, alkyl (low molecular weight), monobasic acid, and dibasic acid; (5) HPIB: fatty amine with <9 carbon atoms, amino acids, purines, and pyrimidines; and (6) HPIN: fatty amine, alcohol, aldehyde, ester, ketone with <5 carbon atoms, and polysaccharide (Kanokkantapong et al. 2006).
As shown in Fig. 3a, HPIN was the major component and followed by HPOA in four seasons. Winter and summer seasons in Guangzhou belong to dry and wet seasons, which these two fractions constituted as much as 70 and 42 % of TOC. These were consistent with previous reports (Marhaba and Van 1999; Bruchet et al. 1987). Last four fractions (HPIA, HPIB, HPON, and HPOB) existed from high to low as follows: HPIB, HPOB, HPON, and HPIA in wet season, whilst HPON, HPIB, HPIA, and HPOB in dry season, respectively. In addition, hydrophilic fractions (HPIA, HPIB, HPIN) were more than the hydrophobic fractions (HPOA, HPOB, HPON) according to the distribution percentages of TOC. Figure 3b displays the seasonal variation of UV254 for six fractions. HPOA had the highest values in four seasons and had been followed by HPON and HPIB fractions in order, but HPIN, HPIA, and HPOB still existed in very slight quantity, which indicated that the dissolved aromatic compounds and unsaturated compounds with C═C bonds appeared more frequently. On the contrary, the hydrophobic fractions were more than the hydrophilic fractions comparing with TOC results. These were explained that the values of UV254 only represent the aromatic character of organic matters and are not considerable relationship with TOC. Figure 3c illustrates the seasonal variation of SUVA254 for each fraction. It is generally accepted that the SUVA254 corresponds to the nature of organic matter in the water source. Samples with an SUVA254 ≥4 L (mg m)−1 have a relatively high content of hydrophobic organic compounds, while samples with an SUVA254 ≤3 L (mg m)−1 are largely hydrophilic (Kitis et al. 2001; Chaiket et al. 2002; Karanfil et al. 2002). Obviously, SUVA254 was lower than 3 L (mg m)−1 in four seasons, suggesting that the hydrophilic components were the main fraction in the DOM. Actually, SUVA254 values for most of the hydrophilic fractions in summer, autumn, and winter were higher, but less than the hydrophobic fractions in spring. Moreover, the higher values for HPOA in autumn and spring, HPIA in winter, and HOIN in summer indicated that the DOM of these samples had higher aromaticity (Mash et al. 2004) and more aromatic organic carbon or organic compounds with conjugated unsaturated double bond.
Characterization of organic composition by excitation emission matrix analysis
Fluorescence properties of DOM could reveal its chemical characteristics and origin (Lombardi and Jardim 1999). Several components, such as humic like, fulvic like, and protein like, have been already associated with DOM in natural waters (Cuss and Guéguen 2013). Actually, there were two types of humic like: microbial and terrestrial humic like. Microbial humic like with maximum fluorescence excitation wavelength was at 300 or 305 nm and emission wavelength approximately at 376 nm, and terrestrial humic-like had two excitation maxima, at ≤260 and 360 nm, and one emission ranged between 380 and 460 nm, respectively (Maie et al. 2014). Fulvic-like appeared with an excitation wavelength between 390 and 455 nm and emission wavelength between 509 and 521 nm. Protein like was identified as two excitation maxima at 220 and 275, with other emission peaks at 305 and 350 nm (Baker and Inverarity 2004). The protein-like substances such as a tryptophan-like acid could be observed in terrestrial waters (He and Ohno 2012).
The excitation and emission maxima (or ranges) of each component for DOM and its six fractions are listed in Table 1. And then, Fig. 4 presents the identified components from the EEMs of DOM samples. These three fluorescent components were identified in drinking water sources’ section of the Pearl River, and the fluorescence intensities of protein-like materials were shown to be strongest, of which seasonal variation was decreased in the order of summer, autumn, winter, and spring. Meanwhile, the fluorescence index (FI) was also calculated as the ratio of the Em intensity (f 450/500 nm) for the Ex wavelength of 370 nm and then used to differentiate between allochthonous and autochthonous DOM sources (Shafiquzzaman et al. 2014) and also to determine terrigenous DOM (FI 1.4) and biological DOM (FI 1.9) (McKnight et al. 2001). Therefore, FI values of DOM samples were obtained as 2.31 (spring), 1.88 (summer), 2.11 (autumn), and 2.25 (winter), indicating that these FI values were associated with allochthonous DOM sources and biological DOM and attributed to the increase of the amount of wastewater discharged around the back channel section (Haizhu and Panyu districts), which has been become the industrial and residential area. As shown in Fig. 4, protein-like materials were still the major component for both the hydrophilic fractions and the hydrophobic fractions. Among them, the fluorescence intensities of protein-like components for HPIB, HPIN, HPOB, and HPON in summer were higher than those of other seasons, similar as DOM samples, but higher in autumn for HPIA and HPOA. These fractions were mainly composed of humic-like component and dominated by microbial types in summer but less in winter, which explained that the bacteria had better activities in DOM consumption during the summer season. In six fractions, HPOA was deemed to be the greatest precursor for DBPs, particularly THMs (Chang et al. 2001). Thus, it should be paid more attention in water treatment, due to its enrichment in microbial humic-like and protein-like components. However, none of fulvic-like materials exists in the hydrophilic fractions and HPOB.
Fourier transform infrared
Fulvic like and protein like are considered to be associated with the carboxyl group of humic substance structures and DOM in the aromatic ring structure of amino acids, respectively. And, summer is one of the most representative seasons in Guangzhou section. Therefore, the FTIR spectrum of six organic fractions of DOM and raw samples in summer was further analyzed and then is presented in Fig. 5 and Table 2. In Fig. 5, the intensities of several peaks, such as O–H bending, C–O stretching, and N–H vibration groups of the primary amine group, etc., were more significant than some peaks, which included C═O stretching, O–C–C stretching, and N–H vibration groups of secondary amine group, etc. Moreover, some bands, around 1260 cm−1 for instance, were ascribed to the multiple overlapping peaks, implying that it would be corresponded to amide III or C–O stretching of carboxylic acid. And, peaks around 1100 cm−1 were mainly C–O stretching and possibly due to P–O groups. Then, these bands were further identified as P compounds after element analysis. These results revealed that DOM samples had relatively higher concentration of amide, ester, carboxylic acid, and thiols owing to their stronger bands and also had a certain contribution from lignin, fatty acid, waxes, and aliphatic (Table 2). In addition, the major materials of hydrophilic and hydrophobic fractions were identical to those of DOM samples and appeared enriched in amide and ester. These were in good agreement with the calculation of SUVA254 values and the analysis for fluorescence of fulvic like and protein like.
Conclusions
In this study, DOM and its fractions in the back channel section of the Pearl River were characterized using multiple analytical tools. The values of TOC, UV254, and SUVA254 were affected by seasonal changes; the hydrophilic fractions were found to be the main components and enriched with aromatic components. For the spectral features, fluorescence analysis found the fluorophore components present in DOM and its fractions and identified the major presence of microbial and terrestrial humic like, fulvic like, and protein like. FTIR spectra indicated that DOM was more enriched in carbohydrate, amide, carboxylic, and aliphatic compounds.
References
Abdulla HAN, Minor EC, Dias RF, Hatcher PG (2010) Changes in the compound classes of dissolved organic matter along an estuarine transect: a study using FTIR and 13C NMR. Geochim Cosmochim Acta 74:3815–3838
Baker A, Inverarity R (2004) Protein-like fluorescence intensity as a possible tool for determining river water quality. Hydrol Process 18(15):2927–2945
Bruchet A, Anselme C, Duguet JP, Mallevialle L (1987) THM formation potential and organic content: a new approach in water chlorination chemistry. Environ Impact Health Effects 6:633–647
Buffle J, Leppard GG (1995) Characterization of aquatic colloids and macromolecules. 2. Key role of physical structures on analytical results. Environ Sci Technol 29(9):2176–2184
Callahan J, Dai M, Chen RF, Li X, Lu Z, Huang W (2004) Distribution of dissolved organic matter in the Pearl River Estuary, China. Mar Chem 89:211–224
Chaiket T, Singer CP, Miles A, Pallotta C (2002) Effectiveness of coagulation, ozonation and biofiltration in controlling DBPs. J Am Water Works Assoc 94(12):81–85
Chang EE, Chiang PC, Ko YW, Lan WH (2001) Characteristics of organic precursors and their relationship with disinfection by-products. Chemosphere 44:1231–1236
Chen H, Meng W, Zheng B, Wang C, Li A (2013) Optical signatures of dissolved organic matter in the watershed of a globally large river (Yangtze River, China). Limnologica 43:482–491
Cuss CW, Guéguen C (2013) Distinguishing dissolved organic matter at its origin: size and optical properties of leaf-litter leachates. Chemosphere 92:1483–1489
Drews A (2010) Membrane fouling in membrane bioreactors—characterisation, contradictions, cause and cures. J Membr Sci 363(1–2):1–28
Guo X, He X, Zhang H, Deng Y, Chen L, Jiang J (2012) Characterization of dissolved organic matter extracted from fermentation effluent of swine manure slurry using spectroscopic techniques and parallel factor analysis (PARAFAC). Microchem J 102:115–122
He Z, Ohno T (2012) Fourier transform infrared and fluorescence spectral features of organic matter in conventional and organic dairy manure. J Environ Qual 41:911–919
He Z, Wu F (2015) Labile organic matter: chemical compositions, function, and significance in soil and the environment, SSSA Special Publication 62, 2015 Published by: Soil Science Society of America, Inc
He Z, Honeycutt CW, Zhang H (2011) Elemental and Fourier transform infrared spectroscopic analysis of water and pyrophosphate extracted soil organic matter. Soil Sci 176:183–189
Kanokkantapong V, Marhaba TF, Pavasant P, Panyapinyophol B (2006) Characterization of haloacetic acid precursors in source water. J Environ Manag 80:214–221
Karanfil T, Schlautman MA, Erdogan I (2002) Survey of DOC and UV measurement practices with implications for SUVA determination. J Am Water Works Assoc 94(12):68–80
Kitis M, Karanfil T, Kilduff JE, Wigton A (2001) The reactivity of natural organic matter to disinfection by-product formation and its relation to specific ultraviolet absorbance. Water Sci Technol 43(2):9–16
Koch BP, Witt MR, Engbrodt R, Dittmar T, Kattner G (2005) Molecular formulae of marine and terrigenous dissolved organic matter detected by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Geochim Cosmochim Acta 69:3299–3308
Lombardi AT, Jardim WJ (1999) Fluorescence spectroscopy of high performance effluent chromatography fractionated marine and terrestrial organic materials. Water Res 33:512–520
Ma D, Gao B, Sun S, Wang Y, Yue Q, Li Q (2013) Effects of dissolved organic matter size fractions on trihalomethanes formation in MBR effluents during chlorine disinfection. Bioresour Technol 136:535–541
Maie N, Sekiguchi S, Watanabe A, Tsutsuki K, Yamashita Y, Melling L, Cawley KM, Shima E, Jaffé R (2014) Dissolved organic matter dynamics in the oligo/meso-haline zone of wetland-influenced coastal rivers. J Sea Res 91:58–69
Marhaba TF, Van D (1999) Chlorinated disinfection by-product formation potential of dissolved organic matter fractions at an ozonation water treatment plant. Environ Res 3(3):255–268
Marhaba TF, Van D, Lippincott RL (2000) Rapid identification of dissolved organic matter fractions in water by spectral fluorescent signatures. Water Res 34:3543–3550
Marhaba TF, Pu Y, Bengraine K (2003) Modified dissolved organic matter fraction technique for natural water. J Hazard Mater 101:43–53
Mash H, Westerhoff PK, Baker LA, Nieman RA, Nguyen ML (2004) Dissolved organic matter in Arizona reservoirs: assessment of carbonaceous sources. Org Geochem 35:831–843
McKnight DM, Boyer EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT (2001) Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic materials and aromaticity. Limnol Oceanogr 46(1):38–48
Meng F, Huang G, Yang X, Li Z, Li J, Cao J, Wang Z, Sun L (2013) Identifying the sources and fate of anthropogenically impacted dissolved organic matter (DOM) in urbanized rivers. Water Res 47:5027–5039
Mopper K, Feng Z, Bentjen SB, Chen RF (1996) Effects of cross-flow filtration on the absorption and fluorescence properties of sea water. Mar Chem 55:53–74
Navalon S, Alvaro M, Garcia H (2008) Carbohydrates as trihalomethanes precursors. Influence of pH and the presence of Cl− and Br− on trihalomethane formation potential. Water Res 42:3990–4000
Panyapinyopol B, Marhab TF, Kanokkantapong V, Pavasant P (2005) Characterization of precursors to trihalomethanes formation in Bangkok source water. J Hazard Mater 120:229–236
Pifer AD, Fairey JL (2012) Improving on SUVA254 using fluorescence-PARAFAC analysis and asymmetric flow-field flow fractionation for assessing disinfection byproduct formation and control. Water Res 46:2927–2936
Shafiquzzaman M, Ahmed AT, Azam MS, Razzak A, Askri B, Hassan HF, Ravikumar BN, Okuda T (2014) Identification and characterization of dissolved organic matter sources in Kushiro River impacted by a wetland. Ecol Eng 70:459–464
USEPA (1989) National Primary Drinking Water Regulations: disinfection/-disinfection by-products (D/DBP) rule. Fed Regist 59:38668
Wong H, Mok KM, Fan XJ (2007) Natural organic matter and formation of trihalomethanes in two water treatment processes. Desalination 210:44–51
Zhao Z, Gua J, Fan X, Li H (2006) Molecular size distribution of dissolved organic matter in water of the Pearl River and trihalomethane formation characteristics with chlorine and chlorine dioxide treatments. J Hazard Mater 134:60–66
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 41471259), Guangdong Technology Research Centre for Ecological Management and Remediation of Urban Water Systems (2012gczxA005), and Foundation for Distinguished Young Talents in Higher Education of Guangdong, China (2013LYM0018).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Céline Guéguen
Rights and permissions
About this article
Cite this article
Zheng, L., Song, Z., Meng, P. et al. Seasonal characterization and identification of dissolved organic matter (DOM) in the Pearl River, China. Environ Sci Pollut Res 23, 7462–7469 (2016). https://doi.org/10.1007/s11356-015-5999-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5999-9