Abstract
Within the last few years, the presence of bentazone herbicide has been observed in many water resources. For the first time, removal of bentazone using mesoporous silica was investigated revealing reversible adsorption. The adsorption isotherm was well described using the Freundlich model. The affinity towards bentazone is strongly affected by pH in the range of 2–7, decreasing with the increase of the pH, becoming negligible at the neutrality. Regeneration of the adsorbent was possible, and a recovery as high as 70 % was obtained using CH3OH-NaOH solution. Furthermore, appreciable recovery (47 %) was also obtained using water. Applications on the purification of lake water and wastewaters, both characterized by a significant organic carbon load, spiked with 2 mg L−1 bentazone were tested, observing removal yields in the range of 61–73 %. Taking advantage of the fast adsorption kinetics observed, an in-flow purification treatment was set-up, with quantitative removal of bentazone from polluted water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of herbicides and insecticides in agriculture has become a common practice for increasing crop yields and food production; as a consequence, in the last decades, they have been commonly found in water resources, arousing attention of the scientific community (Salman et al. 2011).
Bentazone is a post-emergence herbicide belonging to the thiadiazine class used for selective control of broadleaf weeds and sedges (World Health Organization 2014).
Deposition from aerial applications, leaching or run-off from agricultural lands (Li et al. 2003), and indiscriminate discharge of industrial wastewaters have caused the contamination of water resources by bentazone. Bentazone is highly soluble in water and very mobile in soil (log k ow = 2.8 (Chemical Abstracts Service 2015), and it is resistant to hydrolysis and not subjected to abiotic degradation. Moreover, toxicological studies showed that bentazone exhibits acute and chronic toxicity (World Health Organization 2014).
The occurrence of bentazone in both surface and groundwater has been highlighted in various European countries, including Italy, at concentrations higher than the limit established by the 98/83/CE EU Directive for groundwater (i.e., 0.1 μg L−1) (Loos et al. 2010). High bentazone concentrations, up to 14 μg L−1, have been also reported in Asian rice field pertaining groundwaters (World Health Organization 2014).
The few studies dealing with the occurrence and behaviour of bentazone in wastewater treatment plants (WWTPs) indicated that this species is poorly removed by common activated sludge systems (Majewsky et al. 2013) and that, in some cases, WWTPs can act as source of bentazone (Köck-Schulmeyer et al. 2013).
Photocatalysis (Mir et al. 2014; Seck et al. 2013), adsorption (Otero et al. 2014) and membrane filtration (Hofman et al. 1993) are the most investigated approaches for the removal of bentazone from aqueous matrices.
Although photocatalytic degradation rate of bentazone can be high, its removal efficiency is strongly influenced by a number of operating parameters, such as irradiation time, pH and concentrations of both photocatalyst and oxidizing agent. Furthermore, toxicity reduction after the treatment depends on bentazone concentration and cannot be quantitative (Seck et al. 2012).
Nanofiltration is a promising approach for bentazone removal, as well. However, a cascade of nanofiltration stages are usually requested for satisfying low limits in the presence of bentazone concentrations of few micrograms per litre (Caus et al. 2009).
Adsorption is currently the common physicochemical approach for pollutant removal because of its low cost, efficiency and large-scale applicability. Activated carbons are the widest used materials for this purpose, and recently, they have been investigated also for bentazone removal (Salman and Al-Saad 2012; Salman et al. 2011). Adsorption of bentazone onto mineral surfaces has been also studied to investigate the fate of pesticides in aquifers (Clausen et al. 2001).
Ordered mesoporous materials have been largely investigated as adsorbents (Wu and Zhao 2011), being them very versatile due to the possibility of controlling their porosity and surface area through properly tailored synthesis conditions. Differently from activated carbons, no investigation on the adsorption of bentazone using these materials is available in literature. Ordered mesoporous silica-based sorbents (MS), belonging to the families of SBA-15 and MCM-41 materials, have been extensively studied by our research group for the retention of several classes of environmental pollutants with different physicochemical characteristics, such as volatile organic compounds (Bruzzoniti et al. 2000), metals (Bruzzoniti et al. 2009; Bruzzoniti et al. 2007; Bruzzoniti et al. 2011; Caldarola et al. 2014) and haloacetic acids (Bonelli et al. 2002; Bruzzoniti et al. 2012; Bruzzoniti et al. 2000). The retention of this last class of compounds was attained through the use of the precursor of a mesoporous silica which contained the cationic templating agent that enhanced interactions with the ionizable haloacetic acids. The retention behaviour observed, interpreted as a function of the properties of the sorbents, indicated that MS met the requirements not only as promising adsorbents but also as interesting materials for stationary phases for the implementation of chromatographic analytical methods (Bruzzoniti et al. 2009; Bruzzoniti et al. 2007; Bruzzoniti et al. 2011).
Based on these considerations, the aim of this work was to evaluate the sorption properties towards bentazone of a MS material, commercialized as MCM-41 type (following identified as MS1). Accordingly, adsorption kinetics was investigated. Additionally, adsorption studies were completed by fitting the experimental data with three adsorption isotherms (i.e., Langmuir, Freundlich and Temkin). Recoveries of bentazone were evaluated by high-performance liquid chromatography with ultraviolet spectrophotometric detection (HPLC-UV), according to a procedure recently developed (De Carlo et al. 2014; Rivoira et al. 2015). The retention behaviour of the MS1 material was then compared with those of (i) another MS material, commercialized as SBA-type (following identified as MS2), (ii) pillared montmorillonite, chosen as model structure of soil minerals (Tunega and Meleshyn 2010), and (iii) with black carbon, chosen for its unselective retention properties which are fully exploited for analytical applications, such as clean-up of food and environmental matrices extracts in pesticide analysis by the QuECHERS methodology (Bruzzoniti et al. 2014).
A regeneration study of the MS1 sorbent was accomplished, in order to check if its reuse for the removal of bentazone from water is allowed.
Removal properties of MS1 were also verified in the case of water matrices with high organic load, such as lake water and wastewater. Applications of the MS sorbent under flow conditions were also tested showing the feasibility of quantitative removal of bentazone. To the best of our knowledge, this work represents the first study on the use of mesoporous silica materials for the removal of bentazone from water matrices.
Experimental
Chemicals and materials
Stock solution (100 mg L−1) of bentazone was prepared from a Pestanal® analytical standard (Sigma-Aldrich, MO, USA). HNO3 was from Fluka (Sigma); HCOONa and HPLC grade CH3CN, used for the preparation of eluent (see the “Instrumentation” section), were from Merck (Darmstadt, Germany) and Sigma-Aldrich respectively. For the recovery study, methanol, ethanol, 2-propanol (Sigma-Aldrich) and NaOH (49.5–50.5 %, Mallinckrodt Baker B.V., Deventer, Netherlands) were used. High-purity water (18.2 MΩ cm−1 resistivity at 25 °C), produced by an Elix-Milli Q Academic system (Millipore, Vimodrone, MI, Italy), was used for the preparation of eluent and standards.
Eluents and sample solutions were filtered by 0.45 μm mixed cellulose ester membrane filters. MS1 and MS2 materials were purchased from ACS Material, LLC (Medford, MA, USA). Pillared montmorillonite (Aluminium Pillared Clay) from Sigma-Aldrich and Carbon SPE Bulk Sorbent from Agilent Technologies (CA, United States) were used.
Instrumentation
An ion chromatograph ICS3000 model (Dionex, Thermo Fisher Scientific Inc., Sunnyvale, CA, USA), equipped with a 10 μL loop, a LiChro-Cart PuroSphere RP-18, 125 mm × 3.0 mm, 5-μm (Merck), was used for bentazone determination. Detection was performed by spectrophotometric detection (λ = 252 nm) by an AD25 Absorbance Detector (Dionex, Thermo Fisher). Bentazone was eluted by 17.5 mM HCOONa, pH 3 by addition of HNO3 and 35 % CH3CN, according to Rivoira et al. (2015). Eluent flow rate was set at 0.5 mL min−1.
Limits of detection and limits of quantification, evaluated as the concentration referred to a signal S m = S b + 3sb and S m = S b + 10sb, respectively (S b = average signal for blank; s b = standard deviation, for 14 repeated injections), were 12.5 and 41.7 μg L−1, respectively.
Inter-day and intra-day reproducibility of the method (expressed as relative standard deviation,% RSD) was determined both for retention times and peak areas by repeatedly injecting a 125 μg L−1 bentazone solution. For intra-day (within day, n = 13) measurements, % RSD for retention time is 0.2 %, whereas for peak area, % RSD = 5.3 %, for inter-day measurements (n = 52), % RSD is 1.0 % for retention time and 13.1 % for peak area. For pH measurements, an Ag/AgCl pH meter (Hamilton, GR, Switzerland) was used.
Materials characterization
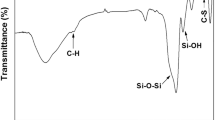
All the sorbents were characterized by nitrogen adsorption-desorption measurements with a Quantachrome Autosorb1 (Quantachrome Instruments, Boynton Beach, FL, USA), to evaluate the Brunauer-Emmet-Teller (BET)-specific surface area. The pore volume was calculated using the NLDFT model (N2 on silica, cylindrical pore for MS1, MS2 and pillared montmorillonite, N2 on carbon, for carbon black). Morphology was studied by Field Emission Scanning Electron Microscopy (FE-SEM). MS1 was characterized by Fourier Transform Infrared Spectroscopy (FT-IR), using a Bruker Equinox 55 equipped with a cryogenic MCT detector (resolution of 2 cm−1). Spectrum was recorded after outgassing the sample at room temperature in an infrared cell connected with a vacuum ramp (residual pressure lower than 1 × 10−3 mbar).
Adsorption kinetics on MS1 sorbent
Batch experiments were performed in triplicate using 1 g of MS1 in contact with 50 mL of 2 mg L−1 bentazone, pH 2.5, for defined period of times. A blank was run in parallel. The pH value chosen allowed maximum adsorption percentage, while ensuring material stability, whereas initial bentazone concentration, being representative of medium-high level environmental contamination, was chosen to allow reliable determination of bentazone remained in the solution after adsorption, according to the method performance detailed in the “Instrumentation” section. Samples were withdrawn at fixed time intervals (0, 1, 3, 5, 10, 20, 30, 45, 60, 180, 330 min) and filtered before HPLC-UV analysis to determine the residual concentration of bentazone in the solution (C e , mg L−1).
Adsorption isotherm on MS1 sorbent
Triplicate experiments were performed with 0.2 g of MS1 per 10 mL of bentazone solutions (pH 2.5). Eight bentazone solutions (concentration range 0.5–100 mg L−1) were stirred at room temperature for 26 h (after verifying that the equilibrium conditions were achieved). The concentration range explored allowed reliable determination of bentazone remained in the solution after adsorption, according to the method performance detailed in the “Instrumentation” section. A control was processed in parallel.
The amount of bentazone adsorbed at equilibrium (q e in mg g−1) was determined as follows:
where V (L) is the volume of solution, m (g) is the mass of the adsorbent and C 0 (mg L−1) is the initial concentration of the analyte.
Comparison of bentazone adsorption on different substrates
For these experiments, 0.2 g of each material was stirred for 26 h, in order to reach equilibrium conditions, in a vessel containing 2 mg L−1 bentazone in a 10-mL solution. Experiments were performed, in triplicate, at pH values included in the range of 2–7. The pH of the solution was corrected with HNO3 or NaOH. In parallel, a control (blank) was performed in the same experimental conditions but in the absence of bentazone. For each material, bentazone concentration not retained (C e) was determined by HPLC-UV as detailed in the “Instrumentation” section. The percentage of bentazone absorbed (R ads ) was calculated as follows:
Recovery of bentazone from MS1 sorbent
Recovery procedures for a possible regeneration of the material were also investigated. Based on the retention mechanisms hypothesized (hydrogen bond and van der Waals interactions, see discussion in the text), desorption experiments with different aqueous solutions (H2O, pH 7) and organic solvents (CH3OH, CH3CN, ethanol, isopropanol) or mixtures (0.1 M NaOH, 80 % CH3OH) were performed. In detail, 0.1 g MS1, preliminarily loaded with known concentration of bentazone, was treated with 5 mL of solvent (in order to maintain the same solid/liquid ratio used throughout the previous experiments) and stirred at room temperature for 60 min. The sample was then filtered, diluted 1:1 with water and analysed by HPLC-UV. All the tests were in triplicate, and a blank control (MS1 not containing bentazone) was run in parallel for each extraction solution.
The recovery percentage was calculated as follows:
where C d is the concentration (mg L−1) of bentazone measured in solution after the desorption test, C i (mg g−1) is the amount of bentazone adsorbed on that portion of material, and V and m are respectively the volume (L) of the solvent and the mass (g) of material used for the recovery study.
Water samples and characterization
For real sample analysis, lake water and wastewater from an intermediate treatment stage of a WWTP were tested.
Lake water was sampled from the epilimnion of Avigliana lake (Turin, Italy) in July 2013, as described by Marchisio et al. (2015). After sampling, the lake water was frozen until further processing. Inorganic ions were determined by ion chromatography, whereas dissolved organic carbon (DOC) and inorganic carbon (IC) were measured by a Shimadzu TOC-VCSH instrument (Marchisio et al. 2015).
Wastewater from aerated mixed septic tank and landfill leachate was sampled at the inlet and outlet of a membrane biological reactor (MBR) including nitrification, denitrification and ultrafiltration stages. The samples were characterized for chemical oxygen demand (COD) and 5-day biochemical oxygen demand (BOD5), total suspended solids and N content.
Lake water and wastewater samples were analysed in order to verify the absence of bentazone at the quantitation limit of the method. Subsequently, 0.2 g MS1 was put in contact for 20 min, under stirring, with 10 mL of the two samples spiked with 2 mg L−1 bentazone, and the pH was corrected to 2.1 with HNO3. This value was chosen according to the effect of pH observed on sorption behaviour for MS1 (see “Results and Discussion” section). The samples were filtered (0.22 μm) and analysed by HPLC to evaluate the percentage of bentazone absorbed (R ads ) by Eq. (2).
Column tests
To evaluate the performance of MS1 under flow conditions, column tests were performed. Poly-Prep® Chromatography Columns from Bio-Rad (Segrate, MI, Italy), 9 cm high, conical 0.8 × 4 cm polypropylene columns were filled with 0.2 g MS1.
Column experiments were performed at recycling conditions by repeatedly flowing 10 ml of 2 mg L−1 bentazone solution, pH 2.1 (0.25 mL min−1 flow rate), on the same column for four cycles (duration of each cycle 40 min).
Additionally, a purification treatment with four columns posed in series was also tested, under the same pH conditions (3.0 mL min−1).
The samples were filtered (0.22 μm) and analysed by HPLC to evaluate the percentage of bentazone absorbed (R ads ) by Eq. (2).
Results and discussion
Bentazone displays a keto-enol tautomerism (Ania and Béguin 2007) and has a pKa value of 3.3 (Chemical Abstracts Service 2015) (see Fig. 1); therefore, according to pH, bentazone can be present in the undissociated or dissociated form.
Bentazone adsorption on MS1 was studied at pH 2.5, in order to keep bentazone mainly in the undissociated form and MS1 silica at an approximatively surface zero charge.
Adsorption kinetics
The adsorption kinetics profile obtained for bentazone is reported in Fig. 2, where the ratio between the concentration of bentazone remaining in solution (C e , mg L−1) and the initial concentration (C 0 , mg L−1) is plotted as a function of contact time (t = 0 is also shown). The data obtained show that removal of bentazone from solution is very rapid and occurs within the first minutes of contact, reaching a percentage as high as 86 %.
After a contact time of 30 min, a desorption of bentazone apparently occurs, giving a removal percentage of 74 % after about 6 h. The release of bentazone from MS1 may be interpreted as the result of competitive adsorption of water molecules on MS1 surface through the formation of H bonding with silanol groups on silica surface (Grünberg et al. 2004; Kocherbitov and Alfredsson 2007). We suggest that in contact with water solution, the surface becomes more hydrophilic due to hydration and formation of surface hydroxyls. Figure 3 reports the FT-IR spectra in the hydroxyl stretching region of MS1 (outgassed at room temperature) before and after contact with bentazone solution. An increase of the intensity of the band at about 3500 cm−1, due to H-bonded hydroxyls, is observed, confirming the suggested surface modification.
Adsorption isotherm
Adsorption isotherm at pH 2.5 (room temperature) is shown in Fig. 4, whereas values of adsorbed amount q e (mg g−1) and equilibrium concentration C e (mg L−1) are listed in Table 1 together with initial bentazone concentration C 0 (mg L−1) and corresponding removal percentage (R ads). The removal percentage never reaches 100 % even at low initial bentazone concentration, and the adsorbed amount q e increases with increasing equilibrium concentration, confirming that the adsorption is an equilibrium process and that saturation is not reached in these experimental conditions.
Adsorption isotherm for bentazone onto MS1. Experimental (inverted triangle) and calculated (square) values according to linearized Freundlich model. Experimental conditions: 0.2 g of MS1 in contact with 10 mL of bentazone solutions (pH 2.5, range 0.5–100 mg L−1), stirred at room temperature for 26 h
The experimental data obtained were fitted with the three models hereafter described:
-
The Langmuir model (Liu 2006), linearized in Eq. 4, which describes the formation of a monolayer of adsorbate under the following assumptions: (i) The surface is homogeneous and constituted of identical binding sites; (ii) the sites are independent (no interaction between molecules adsorbed); and (iii) each site on the surface can hold at most one molecule of adsorbate
$$ \frac{C_e}{q_e}=\frac{1}{K_L\cdot {Q}_0}+\frac{C_e}{Q_0} $$(4)- K L :
-
adsorption equilibrium constant (L mg−1)
- Q 0 :
-
maximum monolayer coverage capacities (mg g−1)
-
The Freundlich isotherm (Foo and Hameed 2010; Freundlich 1906), linearized in Eq. 5, which is the most applied empirical model to describe a non-ideal and reversible adsorption and is not limited to the formation of a monolayer but describes multilayer adsorption in heterogeneous systems.
$$ \ln {q}_e= \ln {K}_F+\frac{1}{n} \ln {C}_e $$(5)- K F (mg1 − 1/n L1/n g−1):
-
adsorption coefficient
1/n is a measure of surface heterogeneity
-
The Temkin (Temkin and Pyzhev 1940) model, Eq. 6, which postulates that the heat of adsorption varies linearly with coverage, unlike the Freundlich method, in which a more complex dependence of the heat of adsorption on the coverage is assumed (i.e. the heat of adsorption decreases exponentially with increasing coverage)
$$ {q}_e=a+b \ln {C}_e $$(6)where a and b are empirical constants.
The Langmuir model, which was found describing bentazone adsorption onto activated carbon, does not explain bentazone adsorption onto MS1 (R 2 = 0.6956).
The Temkin model, which was found to explain adsorption data for naphthalene on periodic mesoporous organosilica (Vidal et al. 2011), does not explain the system under investigation (R 2 = 0.7510).
Conversely, linearized Freundlich isotherm model provided a good fitting (R 2 = 0.9701) with adsorption data.
The values of K F (0.0586) and n (1.2703) were calculated from the intercept and the slope of the plot. The value obtained for n, a measure of the surface heterogeneity, is in the range (1.04–1.34) reported for the adsorption onto mesoporous silica SBA-15 (Bui and Choi 2009) of pharmaceutical compounds of medium polarity with logK ow values similar to those of bentazone. The isotherm calculated using the above-mentioned parameters showed a very good agreement with the experimental one (Fig. 4).
Comparison of bentazone adsorption on different substrates. Effect of pH
The affinity of the MS1 material towards bentazone was compared with that of three different substrates (carbon black, pillared montmorillonite and an additional MS sorbent, named MS2) as a function of solution pH. Data (Fig. 5) are reported as average retention as a function of pH. According to bentazone pKa value, three pH values (2, 3.5 and 7) were investigated in order to have bentazone in different dissociation conditions. The pH values chosen are representative of soil acidity conditions and of pH of wastewaters and groundwater.
Concerning the choice of substrates, pillared montmorillonite was chosen due to its similarity with clay component of soil, whereas carbon black was chosen as unselective sorbent widely used for matrix removal in pesticides analysis. Finally, MS2 sorbent with larger pore size than MS1 (4.5 nm for MS1 and 8.5 nm for MS2) was chosen for comparison.
Specific surface area (SSA) and pore volumes of all materials are summarized in Table 2.
The results shown in Fig. 5 indicate that, with the exception of carbon black, the affinity of the materials for bentazone is pH dependent; in fact, acidic pH values enhance retention on both the MS materials, whereas neutral pH values do not allow adsorption on MS materials (retention was equal to zero) while maintaining a certain retention (5.2 %), for pillared montmorillonite.
The retention behaviour observed for montmorillonite allows to ascribe the interaction pollutant-substrate to hydrogen-bonding and ionic interactions rather than to van der Waals interactions, since the last ones are expected to be less influenced by the pH and by the ionic forms of analyte and substrate. The modest adsorption of bentazone from aqueous solutions on montmorillonite is in agreement with the destabilization effect of water which decreases analyte-sorbent interactions as elucidated by calculation of adsorption potential profiles by theoretical methods (Meleshyn and Tunega 2009).
As regards the two MS sorbents, at all the pH values investigated, they exhibit similar retention capabilities, with MS1 being slightly more performing than MS2 type.
As previously highlighted for montmorillonite, also for the two mesoporous silicas, the decrease of retention at increasing pH suggests that hydrogen-bonding and ionic interactions strongly affects the pollutant-sorbent interaction. More in detail, the higher retention is obtained at pH 2 at which bentazone is present in its undissociated form and silica is supposed to have a surface charge close to zero. Increasing the pH, bentazone is mainly present in the ionized form, and silica surface is expected to become negatively charged (Braga et al. 2011; Vallet-Regí et al. 2007). The repulsion between ionic bentazone and negatively charged silica disadvantages the adsorption which becomes negligible at neutral pH. The results obtained let us hypothesize that specific surface area (which is almost double for MS1) is not a limiting factor for these substrates, provided that a certain mesoporosity is present for the accessibility of the active surface of the sorbent; as the case of MS2, carbon black exhibits the highest retention even at low pH despite the lowest SSA, so showing the highest affinity towards bentazone among the materials tested. This may be ascribed to hydrophobic interactions due to the π-electrons of bentazone.
When comparing MS materials with montmorillonite, the lower removal observed at acidic pH for the latter may be ascribed to a stronger affinity for water adsorption and at least partially to the lower pore volume and SSA of the clay.
Recovery of bentazone from MS1
The recovery of bentazone from MS1 was investigated in view of possible regeneration process of the adsorbent. Solvents that exhibit different polarity and H-bonding interactions were chosen. In fact, both polarity of the solvent, which affects the solubility (and possibly tautomeric and dissociation equilibria), and H-bonding interactions are expected to play key role in partition equilibrium.
As H bonding is concerned, on the one hand, the competition of the solvent with the herbicide in the formation of H bonding with the substrate may be used to favour bentazone desorption. On the other hand, the formation of H bonding with bentazone by the solvent may shift the partition equilibria towards the solution.
Desorption experiments were performed with aqueous solutions (H2O, pH 7) and organic solvents (CH3OH, CH3CN, ethanol, 2-propanol) or mixtures (20 mM NaOH, 80 % CH3OH).
As shown (Fig. 6), among the organic solvents tested, ethanol gives the highest recovery percentages (67.2 ± 16.0 %) but with relevant standard deviation, as also observed for 2-propanol.
For the sake of simplicity, the two contributions from polarity and H bonding will be hereafter considered separately.
As far as polarity is concerned, Table 3 reports the relative polarity values for the used solvents (Reichardt 2003).
Although a correlation between the recovery of bentazone and solvent polarity was not observed, CH3CN and 2-propanol, which have the lowest relative polarity values, provided the lowest recoveries (42.5 ± 0.1 and 42.4 ± 18.5 %, respectively). This behaviour is coherent with the medium-high polarity of bentazone (logK ow = 2.80).
As regards H bonding, the solvent may be envisaged to act through (i) bentazone-solvent interactions and (ii) silica surface-solvent interactions.
Concerning the former interactions, for protic solvents (CH3OH, ethanol and 2-propanol), H bonding can occur in the following ways:
-
1.
Bentazone behaves as hydrogen-acceptor (through N and O atoms) and organic protic solvent as hydrogen donor (through the –OH group)
-
2.
Bentazone behaves as hydrogen donor (through the –OH group) and organic solvent as hydrogen acceptor (through the –OH group)
As regards the silica surface-solvent interaction, for the same protic solvents, various H bonding may occur, where the case in which the MS1 behaves as hydrogen donor (through the Si–OH group) and the organic solvent behaves as hydrogen acceptor (through the –OH group) is expected to be favoured (Natal-Santiago and Dumesic 1998).
For the aprotic solvent CH3CN, the interactions involved are of the N···H type and only two of them are possible, namely between bentazone as hydrogen donor (through the –OH group) and CH3CN as hydrogen acceptor (through the –CN group) and between MS1 as hydrogen donor (through the –OH group) and CH3CN as hydrogen acceptor (through the –CN group). This is in line with the lower recovery obtained for CH3CN.
Among the organic solvents tested, methanol provides the best results in terms of recovery (R = 56.4 ± 9.1 %) with acceptable reproducibility.
In consideration of the strong dependence of bentazone retention on pH, a solution of 20 mM NaOH, 80 % CH3OH was used, with the aim to promote the recovery of bentazone through dissociation. Indeed, the highest recovery percentage (69.6 ± 10.3 %) was obtained. Following the same rationale, water (pH 7, which ensures dissociation of bentazone and a negative charge of silica surface to provide repulsion between analyte and sorbent) was tested, observing a recovery of 46.8 ± 4.6 %. The lower recovery obtained, if compared with the mixture NaOH/CH3OH, must be ascribed to the lower concentration of competing ions (−OH−) and to the absence of hydrophobic interactions provided by the organic solvent, which assist the desorption of bentazone. Nevertheless, it should be highlighted that the possibility to significantly recover bentazone from MS1 with water is an important finding, due to the simplicity and the low cost of the procedure. Moreover, this procedure would allow the application of the water solution containing the recovered bentazone for agriculture reuse practice.
It should be here mentioned that none of the solvents tested were effective to remove bentazone from carbon black.
Applications
MS1 was tested for the removal of bentazone from real waters (lake and wastewater). The rationale of this choice was the selection of natural and treated waters with high carbon load, in order to evaluate possible matrix interferences. The chemical parameters of lake and wastewater samples are detailed in Tables 4 and 5, respectively, together with the removal percentages obtained for bentazone.
Lake water
As shown, lake water is characterized by relatively high amounts of DOC, and it is therefore a representative matrix which can provide competitive interactions with the MS1 substrate.
As reported in Table 4, the removal percentage obtained was 61 %, indicating efficacious interactions of bentazone with MS1 sites, which are not deployed by the DOC.
Wastewater samples
In order to envisage the integration of MS1 inside wastewater treatment trains, bentazone was spiked into a wastewater sample, composed by aerated mixed septic tank and landfill leachate, before and after an MBR treatment.
Although both MBR influent and effluent are characterized by discrete amounts of BOD, COD and suspended solids (TSS), MS1 still exhibits satisfactory removal percentages (72 and 77 %), indicating a low impact of the matrix on bentazone/MS1 interactions. As expected, the best removal performance in the case of the effluent water in respect to the influent water can be explained by the lower load of the matrix.
The highest removal percentages obtained for wastewater samples in respect to lake water is here interpreted in terms of matrix composition. Lake water is expected to be rich in humic acids which can shield the surface of MS1 through hydrogen-bond interactions, differently from wastewater samples where large hydrophilic molecules should not be present or partially degraded by the primary aerobic treatment prior to the MBR step.
Column tests
The performance of MS1 was tested under flow conditions, in order to simulate the operating procedures inside a treatment plant.
The results obtained showed that also at recycling conditions, which can be a favourable process for separating solid and liquid phases, the removal percentage was significantly high (73 %), under a total contact time of 160 min.
Additionally, the removal performance of MS1 was enhanced through the flowing of the same solution containing bentazone onto four columns posed in series. In Fig. 7, the chromatogram obtained for the injection of the solution collected at the outlet of the fourth column is compared with the chromatogram of the inlet solution. As shown, after the whole treatment, quantitative removal of bentazone was observed since residual bentazone concentration was below the quantitation limit, supporting the applicability of the MS1 sorbent in plant for bentazone removal.
Conclusions
In this study, the adsorption of bentazone from aqueous solutions by MS was investigated for the first time.
The adsorption process, well-described by the Freundlich model, is reversible and affected by pH, in the range of 2–7, decreasing with pH increase. The affinity of the silica surface towards bentazone is mainly explained by ionic and intermolecular interactions. The performance observed for the sorbent (86 % removal after 5 min contact time) is worth of note, mostly considering that the active surface did not need any functionalization.
In view of adsorbent regeneration, recovery of bentazone from MS, investigated with several solvents, can be rationalized and optimized in terms of solvent polarity and H bonding in bentazone/solvent, bentazone/silica and silica/solvent. The sorbent can be regenerated (recovery about 70 %) with methanol-NaOH solution and, appreciably, simply with water.
The sorbent, applied in batch-tests for the removal of bentazone from lake waters and inlet and outlet waters of a MBR of a WWTP, allowed significant removal percentages in the range of 61–73 %, which can be enhanced by the set-up of an in-flow cartridge procedure.
Since the structure and physicochemical properties of bentazone are similar to the ones of some other molecules patented as herbicides (e.g. 3-ethyl-2,1,3-benzothiadiazin-4-one 2,2-dioxide; bicyclothiadiazinones as 2H-1,2,6-thiadiazine-3,5(4H,6H)-dione, 2,4-diphenyl-1,1-dioxide), which can be found in wastewater as the result of their application to soil, the results obtained herein are not only important per se but may have also wider significance.
References
Ania CO, Béguin F (2007) Mechanism of adsorption and electrosorption of bentazone on activated carbon cloth in aqueous solutions. Water Res 41:3372–3380
Bonelli B, Bruzzoniti MC, Garrone E, Mentasti E, Onida B, Sarzanini C, Serafino V, Tarasco E (2002) Mesoporous materials for the retention and separation of haloacetic acids. Chromatographia 56:S189–S191
Braga PRS, Costa AA, De MacEdo JL, Ghesti GF, De Souza MP, Dias JA, Dias SCL (2011) Liquid phase calorimetric-adsorption analysis of Si-MCM-41: evidence of strong hydrogen-bonding sites. Microporous Mesoporous Mater 139:74–80
Bruzzoniti MC, Mentasti E, Sarzanini C, Onida B, Bonelli B, Garrone E (2000) Retention properties of mesoporous silica-based materials. Anal Chim Acta 422:231–238
Bruzzoniti MC, Prelle A, Sarzanini C, Onida B, Fiorilli S, Garrone E (2007) Retention of heavy metal ions on SBA-15 mesoporous silica functionalised with carboxylic groups. J Sep Sci 30:2414–2420
Bruzzoniti MC, De Carlo RM, Fiorilli S, Onida B, Sarzanini C (2009) Functionalized SBA-15 mesoporous silica in ion chromatography of alkali, alkaline earths, ammonium and transition metal ions. J Chromatogr A 1216:5540–5547
Bruzzoniti MC, Sarzanini C, Torchia AM, Teodoro M, Testa F, Virga A, Onida B (2011) MCM41 functionalized with ethylenediaminetriacetic acid for ion-exchange chromatography. J Mater Chem 21:369–376
Bruzzoniti MC, de Carlo RM, Sarzanini C, Caldarola D, Onida B (2012) Novel insights in Al-MCM-41 precursor as adsorbent for regulated haloacetic acids and nitrate from water. Environ Sci Pollut Res 19:4176–4183
Bruzzoniti MC, Checchini L, De Carlo RM, Orlandini S, Rivoira L, Del Bubba M (2014) QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: a critical review. Anal Bioanal Chem 406:4089–4116
Bui TX, Choi H (2009) Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J Hazard Mater 168:602–608
Caldarola D, Mitev DP, Marlin L, Nesterenko EP, Paull B, Onida B, Bruzzoniti MC, Carlo RMD, Sarzanini C, Nesterenko PN (2014) Functionalisation of mesoporous silica gel with 2-[(phosphonomethyl)-amino] acetic acid functional groups. Characterisation and application. Appl Surf Sci 288:373–380
Caus A, Vanderhaegen S, Braeken L, Van der Bruggen B (2009) Integrated nanofiltration cascades with low salt rejection for complete removal of pesticides in drinking water production. Desalination 241:111–117
Chemical Abstracts Service (2015) Scifinder, version 2015. Available at https://scifinder.cas.org. Accessed July 2015
Clausen L, Fabricius I, Madsen L (2001) Adsorption of pesticides onto quartz, calcite, kaolinite, and α-alumina. J Environ Qual 30:846–857
De Carlo RM, Rivoira L, Ciofi L, Ancillotti C, Checchini L, Del Bubba M, Bruzzoniti MC (2014) Evaluation of different QuEChERS procedures for the recovery of selected drugs and herbicides from soil using LC coupled with UV and pulsed amperometry for their detection. Analyt Bioanalytic Chem 407:1217–1229
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Grünberg B, Emmler T, Gedat E, Shenderovich I, Findenegg GH, Limbach HH, Buntkowsky G (2004) Hydrogen bonding of water confined in mesoporous silica MCM-41 and SBA-15 studied by 1H solid-state NMR. Chem Eur J 10:5689–5696
Hofman JAMH, Noij Th HM, Kruithof JC, Schippers JC (1993) Removal of pesticides and other micropollutants with membrane filtration. Water Supp 11:259–269
Kocherbitov V, Alfredsson V (2007) Hydration of MCM-41 studied by sorption calorimetry. J Phys Chem C 111:12906–12913
Köck-Schulmeyer M, Villagrasa M, López de Alda M, Céspedes-Sánchez R, Ventura F, Barceló D (2013) Occurrence and behavior of pesticides in wastewater treatment plants and their environmental impact. Sci Total Environ 458–460:466–476
Li K, Liu W, Xu D, Lee S (2003) Influence of organic matter and pH on bentazone sorption in soils. J Agric Food Chem 51:5362–5366
Liu Y (2006) Some consideration on the Langmuir isotherm equation. Colloids Surf A Physicochem Eng Asp 274:34–36
Loos R, Locoro G, Comero S, Contini S, Schwesig D, Werres F, Balsaa P, Gans O, Weiss S, Blaha L, Bolchi M, Gawlik BM (2010) Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res 44:4115–4126
Majewsky M, Farlin J, Bayerle M, Gallé T (2013) A case-study on the accuracy of mass balances for xenobiotics in full-scale wastewater treatment plants. Environ Sci Proc Impact 15:730–738
Marchisio A, Minella M, Maurino V, Minero C, Vione D (2015) Photogeneration of reactive transient species upon irradiation of natural water samples: formation quantum yields in different spectral intervals, and implications for the photochemistry of surface waters. Water Res 73:145–156
Meleshyn A, Tunega D (2009) Monte Carlo simulation of bentazone and phenanthrene adsorption on the external montmorillonite surface. DFG – IUSS SYMPOSIUM. Advan Molecul Model Biogeochem Interf Perspect Soil Res. Book of Abstracts, p 15. Available at http://www.spp1315.unijena.de/spp1315_multimedia/download/DFG+2009+book+of+abstracts.pdf. Accessed July 2015
Mir NA, Haque MM, Khan A, Muneer M, Vijayalakshmi S (2014) Photocatalytic degradation of herbicide bentazone in aqueous suspension of TiO2: mineralization, identification of intermediates and reaction pathways. Environ Technol 35:407–415
Natal-Santiago MA, Dumesic JA (1998) Microcalorimetric, FTIR, and DFT studies of the adsorption of methanol, ethanol, and 2,2,2-trifluoroethanol on silica. J Catal 175:252–268
Otero R, Esquivel D, Ulibarri MA, Romero-Salguero FJ, Van Der Voort P, Fernández JM (2014) Mesoporous phenolic resin and mesoporous carbon for the removal of S-Metolachlor and Bentazon herbicides. Chem Eng J 251:92–101
Reichardt C (2003) Solvents and solvent effects in organic chemistry, 3rd edn. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Rivoira L, De Carlo RM, Cavalli S, Bruzzoniti MC (2015) Simple SPE-HPLC determination of some common drugs and herbicides of environmental concern by pulsed amperometry. Talanta 131:205–212
Salman JM, Al-Saad K (2012) Batch study for herbicide bentazon adsorption onto palm oil fronds activated carbon. Int J Chem Sci 10:731–740
Salman JM, Njoku VO, Hameed BH (2011) Bentazon and carbofuran adsorption onto date seed activated carbon: kinetics and equilibrium. Chem Eng J 173:361–368
Seck EI, Doña-Rodríguez JM, Fernández-Rodríguez C, González-Díaz OM, Araña J, Pérez-Peña J (2012) Photocatalytical removal of bentazon using commercial and sol–gel synthesized nanocrystalline TiO2: operational parameters optimization and toxicity studies. Chem Eng J 203:52–62
Seck EI, Doña-Rodríguez JM, Fernández-Rodríguez C, Portillo-Carrizo D, Hernández-Rodríguez MJ, González-Díaz OM, Pérez-Peña J (2013) Solar photocatalytic removal of herbicides from real water by using sol–gel synthesized nanocrystalline TiO2: operational parameters optimization and toxicity studies. Sol Energy 87:150–157
Temkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR 12:327–356
Tunega D, Meleshyn A (2010) Bentazone and phenanthrene adsorption on external montmorillonite surface -Monte Carlo modelling. Acta Mineralog Petrograph. Abstract Series 6, p 90. Available at https://www.yumpu.com/en/document/view/32475163/mineralogica-petrographica-abstractseries-ima2010/99. Accessed July 2015
Vallet-Regí M, Balas F, Arcos D (2007) Mesoporous materials for drug delivery. Angew Chem Int Ed 46:7548–7558
Vidal CB, Barros AL, Moura CP, de Lima ACA, Dias FS, Vasconcellos LCG, Fechine PBA, Nascimento RF (2011) Adsorption of polycyclic aromatic hydrocarbons from aqueous solutions by modified periodic mesoporous organosilica. J Colloid Interface Sci 357:466–473
World Health Organization (2014) Bentazone in drinking water, http://www.who.int/water_sanitation_health/dwq/chemicals/Bentazone_background_new.pdf, last accessed 27/4/2014
Wu Z, Zhao D (2011) Ordered mesoporous materials as adsorbents. Chem Commun 47:3332–3338
Acknowledgments
M.C.B. and M.D.B. thank Drs. E. Coppini and D. Fibbi (Gida S.p.A., Prato, Italy) for the supply of the wastewater samples. M.C.B. is grateful to Prof. D. Vione (University of Turin) for the supply of the lake water sample. Financial support from Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR, Italy) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bruzzoniti, M.C., De Carlo, R.M., Rivoira, L. et al. Adsorption of bentazone herbicide onto mesoporous silica: application to environmental water purification. Environ Sci Pollut Res 23, 5399–5409 (2016). https://doi.org/10.1007/s11356-015-5755-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5755-1