Abstract
The scarcity of freshwater resources is a serious problem in arid regions, such as Tunisia, and marginal quality water is gradually being used in agriculture. This study aims to study the impact of treated urban wastewater for reuse in agriculture on the health of soil and food crops. The key findings are that the effluents of Sfax wastewater treatment plant (WWTP) did not meet the relevant guidelines, therefore emitting a range of organic (e.g., up to 90 mg L−1 COD and 30 mg L−1 BOD5) and inorganic pollutants (e.g., up to 0.5 mg L−1 Cu and 0.1 mg L−1 Cd) in the receiving aquatic environments. Greenhouse experiments examining the effects of wastewater reuse on food plants such as tomato, lettuce, and radish showed that the treated effluent adversely affected plant growth, photosynthesis, and antioxidant enzyme contents. However, the pollution burden and biological effects on plants were substantially reduced by using a 50 % dilution of treated sewage effluent, suggesting the potential of reusing treated effluent in agriculture so long as appropriate monitoring and control is in place.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quality fresh water for agriculture is becoming an increasingly scarce resource due to climate change effects (Milano et al. 2012) and increased demand from the agricultural sector (Pedrero et al. 2012; Mesa-Jurado et al. 2012). Hence, wastewater reuse for irrigation represents a sustainable option and an advantageous alternative for the mitigation of the ever-increasing irrigation water scarcity and demand in arid and semiarid regions around the world (Hamilton et al. 2007; Sharma et al. 2007; Angelakis and Durham 2008; Travis et al. 2010). One benefit of such practice is the plant’s uptake of wastewater nutrients, and therefore, a reduction in the pollution load that wastewater contributes to the surface water supply (Liu et al. 2005; Chen et al. 2008; Khurana and Singh 2012). However, depending upon its sources and treatments, sewage effluent may contain undesirable pollutants, and the reclaimed wastewater application may generate undesirable effects on soils, plants, and groundwater resources (Mapanda et al. 2005; Walker and Lin 2008; Drechsel et al. 2010). Among those pollutants, heavy metals are common in urban ecosystem (Li et al. 2009; Xu et al. 2010) and are one of the main anthropogenic toxic chemicals which pose a number of potential environmental and health risks (Singh et al. 2010; Wang et al. 2012; Szkup-Jablonska et al. 2012).
Heavy metal accumulation is known to produce significant physiological and biochemical responses in vascular plants (Mangabeira et al. 2001). The toxicity of heavy metals is generally thought to be due to uncontrolled and excessive production of reactive oxygen species (ROS) which damage cell membranes (Janicka et al. 2008). Removal of ROS is strictly controlled by an assortment of nonenzymatic and enzymatic antioxidant mechanisms in plants. Enzymatic ROS scavenging includes catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD), ascorbate peroxidase (APX), and nonenzymatic scavengers, e.g., glutathione (GSH), carotenoids, and ascorbate (ASC) (Srivastava et al. 2009).
The current study focused on the industrial city of Sfax (Central-Eastern Tunisia) known worldwide for their tanneries, pharmaceuticals, and olives mill industries. South Sfax streams in this area receive industrial effluents, urban and domestic sewage, municipal wastes, and agricultural runoff. Furthermore, the significant increase in wastewater volume, mainly in winter season, causes alterations in hydraulics inside the wastewater treatment plants (WWTPs). This may lead to an enhanced toxicity as a result of the remobilization and release of toxic substances from in-sewer deposits (Gasperi et al. 2010). These highly toxic substances in wastewater can negatively affect the purification efficiency of STPs by inhibiting the metabolic processes of the microorganisms in the biological treatment step (Belhaj et al. 2014). In addition, the toxic chemicals in the influent affect the quality of treated wastewater. Once treated, WWTP effluents may be used for irrigation in agricultural land, as is the case in a suburb of Sfax, Tunisia.
The effects of treated wastewater (TWW) irrigation on the physicochemical properties of soil have been studied in detail (Tarchouna et al. 2010; Belaid et al. 2012; Bedbabis et al. 2014); nevertheless, research on the effects of TWW irrigation soil quality and plant on physiology has not been conducted in detail so far. Consequently, it is important to conduct the risk assessment of this site, which is irrigated with water diverted from polluted streams, to ensure that contamination is not adversely affecting the environment.
The present study aims to investigate the short-term effects of both diluted and undiluted TWW, overloaded with toxic metals, compared with fresh water, on the agriculture land and food crops. The impacts of irrigation treatments on plant health and soil including plant growth, photosynthetic pigments, activities of stress responsible enzymes, and ecotoxicological indicators were examined.
Materials and methods
Experimental design
The current study was conducted in winter 2013 in a greenhouse located at the National Engineering School of Sfax, Tunisia.
The statistical design used included three sources of irrigation, freshwater as the control (C), TWW (T 1), and diluted TWW (T 2) which is TWW diluted by fresh water (1:1), and three vegetable crops, tomato (Solanum lycopersicum L.), lettuce (Lactuca sativa L.), and radish (Raphanus Sativus L.) with 10 replicates. These vegetables were selected because they differ in edible parts, which are the fruit for tomato, the leaves for lettuce, and the roots for radish.
Seeds of each crop plant under investigation were surface-sterilized with 0.001-M HgCl2 solution for 3 min and washed meticulously with sterilized double distilled water. Afterward, seeds were germinated on wet filter paper in darkness at 25 ± 2 °C through 1 week.
Previously germinated seedlings under investigation (tomato, lettuce, and radish) were then transplanted into three groups of 10 plastic bottles (2 L) each (Fig. 1) filled with 2.3 kg of sandy clay top soil (73.5 % sand, 17.24 % clay, and 9.26 % loam), collected from a rural area of Sfax (34° 43 N, 10° 41 E). Bottles were irrigated with freshwater in the first week. Afterward, the first group continued to be irrigated by freshwater (control); the second and third groups were irrigated by TWW and diluted TWW collected from the outlet of Sfax WWTP that has an activated sludge treatment process. This plant serves a population of 526,800 and is designed to purify the urban wastewater with a daily average flow rate of 49,500 m3 day−1. Domestic sources account for approximately 65 % of influent, while industrial sources account for 35 % of influent.
Bottles were maintained in a greenhouse designed as growth chamber programmed for a 12-h photoperiod with photosynthetic photon flux density of 300 μmol m−2 s−1, temperature of 24 ± 1/18 ± 1 °C day/night, and relative humidity of 60/70 ± 3 %.
Samples were taken at the vegetative phase (60 days after sowing for tomato plants and 30 days after sowing for lettuce and radish plants). After washing with distilled water, the plants were separate into roots and shoots. Different growth attributes studied were the number of leaves per plant and area of leaves per plant. After measuring fresh weights, plants were oven dried at 70 °C for 3 days, and the root and shoot dry weights were recorded. The oven-dried plant tissues were ground by mortar into a fine powder and passed through a 0.5-mm sieve for further analysis.
Other samples were taken before the flowering phase (100 days after sowing for tomato and 70 days after sowing for lettuce and radish) to assess components of the antioxidant system in leaves (antioxidant enzymes including CAT and POD and antioxidant compounds including reduced GSH and ASC).
After the plants had been harvested, the soil in each bottle was air dried in open plastic ziplock bags at room temperature before the bags were sealed and stored until required at 20 ± 2 °C. Before analysis, soil was passed through a 1-mm sieve.
Analysis of irrigation waters
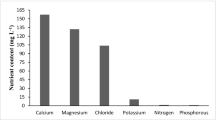
Characteristics of each water type used for irrigation are shown in Table 1. The pH and electrical conductivity (EC) were measured using a pH-meter and conductivity meter, respectively. Total nitrogen was determined by the Kjeldahl method. Total phosphorus was measured by direct colorimetric analysis. Chemical oxygen demand (COD) was determined according to the method by Knechtel (1978). Five-day biochemical oxygen demand (BOD5) was measured using the respirometric method. Macronutrients (Na, Mg, Ca, and K) and heavy metals were determined using atomic absorption spectrometry (Thermo Scientific ICE 3200).
Plant analysis
Photosynthetic pigments and protein content
Fresh leaves (about 0.5 g) were homogenized using a pestle and mortar in 5 mL of 95 % (v/v) ethanol. The homogenate was filtered through pre-ashed filter paper and made up to 25 mL with 95 % ethanol. The filtered solution was used for chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid estimation. Absorbance at 470, 665, and 649 nm was measured using a method described by Lou et al. (2004). The pigment contents were calculated using the following equations (Lichtenthaler and Wellburn 1983):
Chlorophyll and carotenoid contents were expressed as micrograms per gram dry weight (DW) of leaves
Heavy metal contents
Both dry shoot and root samples (0.5 g) for each plant were dry-ashed at 450 °C and digested in three replicates to minimize error, with 1 M HNO3. Plant digests were filtered and transferred first into 25-mL volumetric flasks, brought to volume with Milli-Q water, and then into polyethylene flasks for analysis. The metal concentrations were determined directly in the extract solution by means of atomic absorption spectrometry (Thermo Scientific ICE 3200). Depending on the concentration, a flame atomic absorption spectrometer was introduced to measure Zn, Fe, Pb, Mn, and Cr, whereas the Ni and Cu were measured with a graphite furnace atomic absorption spectrometer. The instrument response was periodically checked versus known standards. An air acetylene flame and hollow cathode lamp were used for all samples. Calibration curves were prepared from dilutions of stock solutions, and all analyses were conducted in triplicate to ensure the accuracy of experimental data/results. The recovery rates for all heavy metals from the plant tissues were found to be more than 98.7 % as determined by digesting three samples each from an untreated plant with known amount of metals. The blanks were run in triplicate to check the precision of the method with each set of samples.
Antioxidant enzymes content
Preparation of samples for enzyme extraction followed the method described by Mukherjee and Choudhury (1983). SOD activity was measured in accordance with the method of Dhindsa et al. (1981). One unit of SOD activity was defined as the amount of enzyme required to inhibit 50 % of the initial reduction of nitro-blue tetrazolium chloride at 560 nm under the experimental conditions. CAT activity was estimated by the decrease in absorbance at 240 nm over 1 min as a result of H2O2 consumption (Verma and Dubey 2003). POD activity was determined using guaiacol as substrate (Wu and VonTiedemann 2002). One unit of POD activity was calculated by the change in absorbance at 470 nm min−1 g−1 fresh weights at 25 °C (Klapheck et al. 1990). APX activity was assayed in accordance with the method of Asada (1992) by measuring the decrease in absorbance at 290 nm over 1 min as a result of oxidation of ASC using UV spectrophotometer.
The activities of CAT, POD, SOD, and APX were expressed as enzyme units per gram fresh weight (U g−1 FW).
GSH was extracted and measured by the method adopted by Tanaka et al. (1985) and expressed as units per gram FW. Reduced ASC was quantified by the bi-pyridyl method (Knorzer et al. 1996) and expressed as nanomoles per milligram FW.
Soil ecotoxicity analysis
Terrestrial avoidance behavior is proposed as a fast and cost-effective method for assessing toxic effects generated by hazardous chemicals in soils (OECD 2004; Römbke et al. 2005). This rapid and sensitive method is suitable for evaluating the habitat function of soils for soil invertebrates. It is a sublethal test reflecting the bioavailability of a mixture of contaminants in natural soils or contaminants/chemicals in spiked soils. To check whether the studied TWW is suitable for irrigation, the earthworm Eisenia andrei andrei was tested (ISO 2007). The worm avoidance assay (adapted from De Silva and van Gestel 2009; ISO 2007) was conducted by means of disposable aluminum containers (30 × 22 × 6 cm). Each one was separated into two with a cardboard screen, 150 g of air-dried soil placed on each area and afterward the screen gently removed. In one area, experimental soil from one of the irrigation treatments was placed, and in the other area, second soil (OECD soil) that had not been used in the trial, composed of 70 % sand, 20 % kaolin, and 10 % sphagnum, was used. Each of the 90 containers was watered with 48 mL of tap water and left to absorb the water. Ten adult worms were placed in the center of each container at the junction of the two soils. A cardboard cover was placed on top and the containers conserved. Worms were incubated for 48 h at 26 ± 2 °C in the dark. At the end of the assay, screen was replaced between the two soils and each soil removed separately from the container. Worm numbers in each soil were determined by hand sorting. The worms found on the separating line were counted by locating the direction of the head. For each replicate, the avoidance response (%) was calculated using Eq. 4:
where NR is the net avoidance response, C is the number of worms in control soil, T is the number of worms in the treatment soil, and N is the total number of worms exposed (De Silva and van Gestel 2009). Avoidance is indicated by a positive response and attraction by a negative response. When avoidance was ≥80 %, the treatment could be considered to have limited or reduced habitat function (De Silva and van Gestel 2009).
Statistical evaluation of the data
Statistical analyses were performed using the SPSS program (version 17.0) to compare treatment effects. Plant, photosynthetic pigment content, heavy metals, and enzyme assay means were compared by analysis of variance and multiple comparison tests (post hoc Fishers least significant differences). Net avoidance response for the earthworms was evaluated using a two-tailed t test on combined radish, tomato, and lettuce soil data. Differences were considered significant at P < 0.05 for all statistical tests.
Results and discussion
Irrigation water quality
The physicochemical parameters and the heavy metal levels of TWW (T 1) and diluted TWW (T 2) used for irrigation purposes as well as the control fresh water (C) were appraised and compared with Tunisian standards for wastewater reuse in crop irrigation. In general, the quality of the T 1 and T 2 was not very good due to dysfunction of the Sfax WWTP. As shown in Table 1, the EC of T 1 and T 2 was found to be above the threshold of 7 mS cm−1 defined as the water salinity beyond which severe restrictions for crop irrigation may occur. Treated waste water’s high salinity may be attributed to the deterioration of groundwater quality, due to persistent groundwater pumping for irrigation and the consequent salt water intrusion (Werner et al. 2009; Zhao et al. 2015). Thus, both T 1 and T 2 reuse for irrigation purposes may contribute to the amelioration of groundwater over-abstraction and to its qualitative restoration. High contents of BOD5 and total N were also recorded. This may be ascribed to the fact that Sfax WWTP under various incoming load and residence time during this period which makes the quality of TWW unsteady. The excess of nitrogen may be risky due to possible groundwater pollution. Lubello et al. (2004) reported that in many investigations, a negative effect of high concentrations of ammonia on crop growth was observed. In the bulk of the soil, ammonia goes through nitrification process. Nitrates indeed migrate into the deep soil layers and can be hazardous for shallow groundwater. On the other hand, higher concentrations of COD and heavy metals were also detected in T 1 and T 2. This could be attributed to an increase of illegal discharge of industrial effluents and high loading rate of organic matter (Belhaj et al. 2013; 2014). P, Mn, Fe, Cd, and Ni concentrations in T 1 and T 2 exceeded the limits recommended by the Tunisian Government (NT 106.002 1989) and the Food and Agriculture Organization (FAO) of the United Nations (wastewater quality guidelines for agriculture reuse) for trace metals in irrigation water. In all treatments, P, Mn, and Fe contents in plant tissues (Table 3) are within the safe limit and are not in toxic range (Kabata-Pendias and Pendias 1986). Cd concentration, in both T 1 and T 2, was 6.9- and 3.1-fold higher than the limit permissible level (0.1 mg L−1), respectively. Ni concentrations were 4.6- and 2.65-fold higher than the maximum permissible level in T 1 and T 2. Given the fact that these metals could be accumulated in soil and hence crops with continuous reuse of wastewater in irrigation, therefore, their periodic monitoring should be an important component of wastewater management.
Plant growth, dry matter, and photosynthetic pigment content
A major precursor for increasing plant yield is an increase in biomass production in terms of dry weight mass. Considerable decreases in leaf area in the T 1 and T 2 of shoots and roots were detected in the three studied plants (Table 2); the decreases were positively correlated with the amounts of heavy metals detected in the irrigation waters and was calculated by 52, 49, and 65 % inhibition in leaf area of radish, lettuce, and tomato irrigated with diluted TWW (T 2) as compared with control plants irrigated with fresh water (C). Similar results were reported in studies on Kandelia candel and Bruguiera gymnorrhiza (Huang and Wang 2010), Brassica campestris and Apium graveolens (Yang et al. 2011), and Taraxacum officinale (Bini et al. 2012a, b). The growth responses are probably due to high metal concentrations that damaged plant roots and inhibited uptake of nutrients, thus inhibiting normal plant growth. The level of reduction differed among the studied plants; the maximum inhibition of the shoot system was detected in radish plants and calculated as 66.4 % inhibition of the FW and 76.8 % of the DW of the control plants. Alternatively, the shoot system of lettuce was less affected, with only 14.3 and 18 % inhibition in FW and DW, respectively, of controls. For the root system, the maximum inhibition in FW was found in lettuce plants, followed by radish and afterward tomato, whereas the maximum decrease in DW was detected in tomato plants, followed by radish and then lettuce, which showed the minimum inhibition in root DW in response to heavy metals. This could be attributed to the variable rates of uptake and transport of heavy metals by these three plants (Yang et al. 1995).
Pigment concentration is directly linked to photosynthetic activity, and photosynthetic rates and pigment content, i.e., chl a/chl b ratio, have been found to be correlated (Das et al. 2002).
In accordance with the growth responses, the studied plants showed a significant and progressive decline in chl a, chl b, and carotenoid pigment contents with increase in heavy metal content in the irrigation water (Fig. 2). The decline in photosynthetic amount can be the result of increase of chlorophyll as activity or inhibition of delta-aminolevulinic acid dehydratase (Singh et al. 2012; Aldoobie and Beltagi 2013). In lettuce and tomato, chl b was affected more than chl a by increasing amounts of heavy metals in irrigation water, which resulted in marked increase in the chl a/chl b ratio in plants treated with TWW and diluted TWW, compared with fresh water. Increase in chl a/chl b ratio after heavy metal treatment was reported also by Zengin and Munzuroglu (2005) and Gomes et al. (2011). Subsequent chlorosis progression was described by Ebbs and Uchil (2008). These observations suggest a specific pattern of Chl loss during metal-induced chlorosis, representing either a direct effect of metals on the two Chl pools or an indirect effect via oxidative stress.
Carotenoids are essential components of the photosynthetic apparatus in a wide range of organisms, which participate in the adaptation of plastids to changing environmental light conditions and prevent photooxidative damage of the photosynthetic apparatus by detoxifying ROS (Simkin et al. 2008). Previous studies have reported increase (Drazkiewicz and Baszynski 2005), decrease (Ekmekc et al. 2008), or no change (Mishra et al. 2006) in the content of carotenoids in plants in response to heavy metal stress. In our study, carotenoid content in the three plants decreased significantly at the highest concentration of heavy metals. As a result, the lowest carotenoid content in radish leaves could lead to a diminished capacity to protect photosystems against photooxidation (Di Toppi et al. 2009).
Heavy metal accumulation in plants
The concentrations of Fe, Ni, Cd, Cu, Mn, and Pb (mg kg−1 FW) in shoots and roots of radish, tomato, and lettuce are shown in Table 3. Significant increases in the accumulated FE, Mn, Zn, and Cu were detected in both shoots and roots of the three studied plants irrigated with T 1 and T 2 compared with the control plants irrigated with fresh water. In addition, other toxic heavy metals (Ni, Cd, and Pb) were detected only in plants irrigated with TWW. Although, heavy metals accumulated primarily in the roots plants receiving TWW for irrigation. The restriction of metal absorption and translocation to the shoots may be related to the avoidance mechanism in the roots. In this respect, El-Beltagi et al. (2010) found that in plants that are not hyperaccumulators of Cd, roots have been shown to be the major site of phytochelatin synthesis and hence Cd accumulation.
It was observed that lettuce shoots accumulated much more amounts of all the detected heavy metals as compared with the shoots of radish and tomato plants. Leafy vegetables tend to accumulate high amounts of Cd, Ni, and Pb due not only to their large leaf area and high transpiration rate but also to the fast growth rate of these plants as observed by Weldegebriel et al. (2012). Surprisingly, the results (Table 3) showed that Cd contents in radish plant and Pb contents, in both lettuce and radish, irrigated with T 1 and T 2 surpassed the maximum limit. In these cases, consuming the vegetables may pose health risk due to the high Cd and Pb concentrations (Codex Alimentarius Commission 2001).
Antioxidant defense system
In order to elucidate the response of antioxidant system to heavy metals, activities of antioxidative enzymes were followed. The three studied plants irrigated with wastewater exhibited higher superoxide dismutases (SOD), enzymes-catalases (CAT), peroxidases (POD), and ascorbate peroxidase (APX) activities compared to the same plants irrigated with fresh water (Fig. 3). Measurement of the activity of antioxidant enzymes (CAT, POD, and SOD) can provide useful information about the stress level in plants. Significant increase in SOD, CAT, and POD activities under heavy metals exposure has been observed in many plant species treated with different metals (Najeeb et al. 2011; Wang et al. 2014). In addition, a significant amount of GSH accumulated in the plants in response to all treatments, with the highest accumulation in plants irrigated with T 1 relative to controls of the same species. The accumulation was the lowest in radish (118 %), intermediate in lettuce (124 %), and highest in tomato (129 %). The induction of the synthesis of glutathione, a substrate in the synthesis of phytochelatins, was observed in plants in response to heavy metals (Petrova et al. 2012). In contrast, the amount of detected ASC decreased in plants irrigated with contaminated wastewater relative to control plants. Rao and Sresty (2000) also reported that the ASC content of roots and shoots of two pigeon pea cultivars showed a significant negative correlation with increasing concentrations of metal ions (Zn and Ni).
Ecological risk evaluation
After irrigation with wastewater, some contaminants may persist in soil leading to high-risk exposure of soil organisms to the product. Among them, the earthworms may be in direct contact and ingest the contaminated soil particles. However, by having sensory tubercles on their body surfaces, depending on the pollutant concentration, they can detect and avoid the contaminated soil. Evidently, the avoidance test is considered a sensitive tool in risk assessment as earthworms detect a wide range of contaminants including polyaromatic hydrocarbons, heavy metals, explosives, crude oil, and pesticides (ISO 2007).
The sensitive response of earthworms, especially to contaminants, makes them one of the most suitable animals for use as bioindicators of soil quality (Reinecke and Reinecke 2004; Lukkari et al. 2005). Species numbers, abundance, and biomass can easily give measurable elements. In this study, the net response (Amorim et al. 2005) was used as an intuitive parameter due to its simple scale varying between avoidance of (+1), preference (−1), and no avoidance (0) for the test soil. For all treatments, net avoidance was positive indicating that worms were avoiding the treated soils (Fig. 4). Furthermore, there was no significant difference on worm avoidance due to the plant species grown in the soil (P = 0.769) or the irrigation treatment (P = 0.542). Indeed, according to the 80 % avoidance criterion for the habitat function proposed by Mangala et al. (2009), soils treated with wastewater T 1, with a significantly avoidance percentages (84.6 ± 9.12 %), presented a limited habitat quality. A clear preference pattern for soil irrigated with diluted wastewater (T 2) and freshwater (C) was observed. Therefore, these soils would be a better environment for these ground-living invertebrates than the soils treated with wastewater (T 1).
Net avoidance (%) for earthworms between an OECD soil and soil treated with either fresh water (C), treated wastewater (T 1), and diluted treated wastewater (T 2). Avoidance is indicated by a positive response and attraction by a negative response. Each value is the mean (±standard error) of 10 replicates with 10 worms per replicate. Columns with an asterisk above them suggest significant difference to an avoidance of zero (P < 0.05)
Conclusions
In summary, this study showed that the performance of Sfax WWTP was less than satisfactory; hence, the effluent quality was less than desirable. The concentrations of many pollutants such as COD, BOD5, Cd, and Ni in the effluent exceeded the maximum recommended values, hence presenting a pollution problem to the local rivers and agriculture land. The results showed that tomato, lettuce, and radish plants grown in effluent caused harmful effects on their growth and photosynthetic activity. In addition, the plants were found to accumulate higher amounts of Cd and Pb than the permissible limits, thereby presenting that a potential human health risk resulted from the consumption of vegetables irrigated with such wastewater. Besides, a decrease in enzymatic activities in plants depending on the irrigation with TWW was detected. The positive correlation of the stimulation measurements of the antioxidant system with the amounts of heavy metal in irrigation water proved that these two parameters are efficient biomarkers for the degree of water pollution with heavy metals. Indeed, polluted water irrigation was more likely to have direct toxic impact on soil habitat quality after short-term usage, with implications for human health from the consumption of food crops.
References
Aldoobie NF, Beltagi MS (2013) Physiological, biochemical and molecular responses of common bean (Phaseolus vulgaris L.) plants to heavy metals stress. Afr J Biotechnol 12(29):4614–4622
Amorim MJB, Römbke J, Soares AMVM (2005) Avoidance behaviour of Enchytraeus albidus: effects of benomyl, carbendazim, phenmedipham and different soil types. Chemosphere 59:501–510
Angelakis AN, Durham B (2008) Water recycling and reuse in EUREAU countries: trends and challenges. Desalination 218:3–12
Asada K (1992) Ascorbate peroxidase: ahydrogen peroxides cavenging enzyme in plants. Physiol Plant 85:235–241
Bedbabis S, Ben Rouina B, Boukhris M, Ferrara G (2014) Effect of irrigation with treated wastewater on soil chemical properties and infiltration rate. Environ Manag 133:45–50
Belaid N, Lenain JF, Buzier R, Kallel M, Ayoub T, Ayadi A, Baudu M (2012) Assessment of metal accumulation in calcareous soil and forage crops subjected to long-term irrigation using treated wastewater: case of El Hajeb-Sfax, Tunisia N. Agric Ecosyst Environ 158:83–93
Belhaj D, Ghrab S, Medhioub M, Kallel M (2013) Performance evaluation of an industrial wastewater treatment plant in South-Eastern Tunisia. Deswater:1-6
Belhaj D, Jaabiri I, Turki N, Azri C, Kallel M, Ayadi H (2014) Descriptive and multivariable analysis of the water parameters quality of Sfax sewage treatment plant after rehabilitation. IOSR-JCE 16:81–91
Bini C, Wahsha M, Fontana S, Maleci L (2012a) Effects of heavy metals on morphological characteristics of Taraxacum officinale growing on mine soils in NE Italy. J Geogr Explor 123:101–108
Bini C, Wahsha M, Fontana S, Maleci L (2012b) Effects of heavy metals on morphological characteristics of Taraxacum officinale Web growing on mine soils in NE Italy. J Geoch Explor 123:101–108
Chen W, Wu L, Frankenberger WT, Chang AC (2008) Soil enzyme activities of long-term reclaimed wastewater-irrigated soils. J Environ Qual 37:36–42
Codex Alimentarius Commission (2001) Food additives and contaminants. Jt. FAO/ WHO Food Stand. Program. ALINORM 01/12A, pp 1-289
Das AB, Parida A, Basak UC, Das P (2002) Studies on pigments, proteins and photosynthetic rates in some mangroves and mangrove associates from Bhitarkanika, Orissa. Mar Biol 141:415–422
De Silva PM, van Gestel CA (2009) Comparative sensitivity of Eisenia Andrei and Perionyx excavatus in earthworm avoidance tests using two soil types in the tropics. Chemosphere 77:1609–1613
Dhindsa RS, Plumb-Dhinsda P, Thorne PA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Di Toppi LS, Castagna A, Andreozzi E, Careri M, Predieri G, Vurro E, Ranieri A (2009) Occurrence of different inter-varietal and inter-organ defence strategies towards supra-optimal zinc concentrations in two cultivars of Triticum aestivum L. Environ Exp Bot 66:220–229
Drazkiewicz M, Baszynski T (2005) Growth parameters and photosynthetic pigments in leaf segments of Zea mays exposed to cadmium, as related to protection mechanisms. J Plant Physiol 162:1013–1021
Drechsel P, Scott CA, Raschid-Sally L, Redwood M, Bahri A (2010) Wastewater irrigation and health: assessing and mitigating risks in low-income countries. Earth scan, London
Ebbs S, Uchil S (2008) Cadmium and zinc induced chlorosis in Indian mustard (Brassica juncea L) involves preferential loss of chlorophyll b. Photosynthetica 46(1):49–55
Ekmekc Y, Tanyolac D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165:600–611
El-Beltagi HS, Mohamed AA, Rashed MM (2010) Response of antioxidative enzymes to cadmium stress in leaves and roots of radish (Raphanus sativus L.). Not Sci Biol 2(4):76–82
Gasperi J, Gromaire MC, Kafi M, Moilleron R, Chebbo G (2010) Contributions of wastewater, runoff and sewer deposit erosion to wet weather pollutant loads in combined sewer systems. Water Res 44:5875–5886
Gomes MP, Marques TC, Nogueira MO, Castro EM, Soares AM (2011) Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci Agric 68:566–573
Hamilton AJ, Stagnitti F, Xiong X, Kreidl SL, Benke KK, Maher P (2007) Waste-water irrigation: the state of play. Vadose Zone J 6:823–840
Huang GY, Wang YS (2010) Physiological and biochemical responses in the leaves of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) exposed to multiple heavy metals. J Hazard Mater 182:848–854
ISO (2007) Soil Quality: Avoidance Test for Testing the Quality of Soils and Effects of Chemicals on Behavior-Part 1: Test with Earthworms (Eisenia fetida and Eisenia andrei). ISO/DIS 17512-1.2. International Organization for Standardization, Geneva
Janicka R, Katarzyna K, Marek B, Grazyna K (2008) Response of plasma membrane H+ ATPase to heavy metal stress in Cucumis sativus roots. J Exp Bot 59:3721–3728
Kabata-Pendias A, Pendias H (1986) Trace elements in soils and plants. CRC Press Inc, Florida
Khurana MP, Singh P (2012) Waste water use in crop production. Resour Environ 2:116–131
Klapheck S, Zimmer I, Cosse H (1990) Scavenging of hydrogen peroxide in endosperm of Ricinus communis by ascorbate peroxidase. Plant Cell Physiol 31:1005–1013
Knechtel RJ (1978) A more economical method for the determination of chemical oxygen demand. Water Pollut Control:25-29
Knorzer OC, Durner J, Boger P (1996) Alterations in the antioxidative system of suspension cultured soy bean cells (Glycine max) induced by oxidative stress. Physiol Plant 97:388–396
Li P, Wang X, Allinson G, Li X, Xiong X (2009) Risk assessment of heavy metals in soil previously irrigated with industrial wastewater in Shenyang, China. J Hazard Mater 161:516–521
Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophyls a and b of leaf extracts in different solvents. Biochem Soc Trans 603:591–592
Liu WH, Zhao JZ, Ouyang ZY, Solderland L, Liu GH (2005) Impacts of sewage irrigation on heavy metal distribution and contamination in Beijing, China. Environ Int 32:805–812
Lou LQ, Shen ZG, Li XD (2004) The copper tolerance mechanisms of Elsholtzia haichowensis, a plant from copper-enriched soils. Environ Exp Bot 51:111–120
Lubello C, Gori R, Nicesse FP, Ferrini F (2004) Municipal-treated wastewater reuse for plant nurseries irrigation. Water Res 38(12):2939–2947
Lukkari T, Aatsinki M, Väisänen A, Haimi J (2005) Toxicity of copper and zinc assessed with three different earthworm tests. Appl Soil Ecol 30:133–146
Mangabeira P, Almeida AA, Mielke M, Gomes FP, Mushrifah I, Escaig F, Laffray D, Severo MI, Oliveira AH, Galle P (2001) Ultrastructural investigations and electron probe X-ray microanalysis of chromium-treated plants. Proc. VI ICOBTE, Guelph, p 555
Mapanda F, Mangwayana EN, Nyamangara J, Giller KE (2005) The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric Ecosyst Environ 107:151–165
Mesa-Jurado MA, Martin-Ortega J, Ruto E, Berbel J (2012) The economic value of guaranteed water supply for irrigation under scarcity conditions. Agr Water Manage 113:10–18
Milano M, Ruelland D, Fernandez S, Dezetter A, Fabre J, Servat E (2012) Facing climatic and anthropogenic changes in the Mediterranean basin: what will be the medium-term impact on water stress? C R Geosci 344:432–440
Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MN (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Mukherjee SP, Choudhury MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou W (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574
NT 106.002 (1989) Normes tunisienne de la réutilisation des eaux usées traitées en agriculture
OECD (2004) Guideline for Testing of Chemicals No 222, Earthworm Reproduction Test (Eisenia fetida/andrei). Paris, Organization for Economic Cooperation and Development
Pedrero F, Allende A, Gil MI, Alarcón JJ (2012) Soil chemical properties, leaf mineral status and crop production in a lemon tree orchardirrigated with two types of wastewater. Agr Water Manage 109:54–60
Petrova S, Benesova D, Soudek P, Vanek T (2012) Enhancement of metal (loid)s phytoextraction by Cannabis sativa L. J Food Agric Environ 10(1):631–641
Rao KVM, Sresty TV (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Reinecke AJ, Reinecke SA (2004) Earthworms as test organisms in ecotoxicological assessment of toxicant impacts on ecosystems. In: Edwards CA (ed) Earthworm Ecology, 2nd edn. CRC Press, Boca Raton, pp 299–320
Römbke J, Jänsh S, Didden W (2005) The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicol Environ Saf 62:249–265
Sharma RK, Agrawal M, Marshall F (2007) Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol Environ Saf 66:258–266
Simkin AJ, Moreau H, Kuntz M, Pagny G, Lin C, Tanksley S, McCarthy J (2008) An investigation of carotenoid biosynthesis in Coffea canephora and Coffea arabica. J Plant Physiol 165:1087–1106
Singh A, Sharama RK, Agrawal M, Marshall FM (2010) Health risk assessment of heavy metals via dietary intake of food stuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem Toxicol 48:611–619
Singh G, Agnihotri RK, Reshma RS, Ahmad M (2012) Effect of lead and nickel toxicity on chlorophyll and proline content of Urd (Vigna mungo L.) seedlings. Int J Plant Physiol Biochem 4(6):136–141
Srivastava S, Srivastava AK, Suprasanna PD, Souza SF (2009) Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J Exp Bot 60(12):3419–3431
Szkup-Jablonska M, Karakiewicz B, Grochans E, Jurczak A, Nowak-Starz G, Rotter I, Prokopowicz A (2012) Effects of blood lead and cadmium levels on the functioning of children with behaviour disorders in the family environment. Ann Agric Environ Med 19:241–246
Tanaka K, Suda Y, Kondo N, Sugahara K (1985) O3 tolerance and ascorbate dependent H2O2 decomposing systems in chloroplasts. Plant Cell Physiol 26:1425–1431
Tarchouna LG, Merdy P, Raynaud M, Pfeifer HR, Lucas Y (2010) Effects of long term irrigation with treated wastewater. Part I: evolution of soil-chemical properties. Appl Geochem 25:1703–1710
Travis MJ, Wiel-Shafran A, Weisbrod N, Adar E, Gross A (2010) Grey water reuse for irrigation: effect on soil properties. Sci Total Environ 408:2501–2508
Verma S, Dubey RS (2003) Pb toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Walker C, Lin HS (2008) Soil property changes after four decades of wastewater irrigation: a landscape perspective. Catena 73:63–74
Wang Y, Qiao M, Liu Y, Zhu Y (2012) Health risk assessment of heavy metals in soils and vegetables from wastewater irrigated area, Beijing-Tianjin city cluster, China. J Environ Sci 24:690–698
Wang C, Liu Y, Zeng G, Hu X, Ying Y, Hu X, Zhou L, Wang Y, Li H (2014) Mechanism of exogenous selenium alleviates cadmium induced toxicity in Bechmeria nivea (L.) Gaud (Ramie). Trans Nonferrous Metals Soc China 24:3964–3970
Weldegebriel Y, Chandravanshi BS, Wondimu T (2012) Concentration levels of metals in vegetables grown in soils irrigated with river water in Addis Ababa, Ethiopia. Ecotoxicol Environ Saf 77:57–63
Werner AD, Jakovovic D, Simmons CT (2009) Experimental observations of salt-water up-coning. J Hydrol 373:230–241
Wu XY, VonTiedemann A (2002) Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut 116:37–47
Xu J, Wu L, Chang AC, Zhang Y (2010) Impact of long-term reclaimed wastewater irrigation on agricultural soils: a preliminary assessment. J Hazard Mater 183:780–786
Yang X, Baligar VC, Martens DC, Clark RB (1995) Influx, transport and accumulation of cadmium in plants species grown at different Cd2+ activities. J Environ Sci Health 30:569–583
Yang Y, Nan Z, Zhao Z, Wang Z, Wang S, Wang X, Jin W, Zhao C (2011) Bioaccumulation and translocation of cadmium in cole (Brassica campestris L.) and celery (Apium graveolens) grown in the polluted oasis soil, Northwest China. J Environ Sci 23(8):1368–1374
Zengin FK, Munzuroglu O (2005) Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Cracov Ser Bot 47(2):157–164
Zhao H, Zhang J, Zhou JL (2015) Tidal impact on the dynamic behavior of dissolved pharmaceuticals in the Yangtze Estuary, China. Sci Total Environ 536:946–954
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Belhaj, D., Jerbi, B., Medhioub, M. et al. Impact of treated urban wastewater for reuse in agriculture on crop response and soil ecotoxicity. Environ Sci Pollut Res 23, 15877–15887 (2016). https://doi.org/10.1007/s11356-015-5672-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5672-3