Abstract
A comparative evaluation of the removal of benzo(a)pyrene (BaP) by sorption and degradation by two microalgal species, Selenastrum capricornutum and Scenedesmus acutus was performed. The monitoring of the amount of BaP remaining in the liquid culture media and the biomass along with the appearance of three metabolites (4,5 dihydrodiol-BaP; 7,8-dihydrodiol-BaP; and 9,10 dihydrodiol-BaP) at short time periods (from 0.25 to 72 h) in cultures exposed to BaP was made by high-performance liquid chromatography (HPLC) with fluorescence and UV detection. Complete removal of BaP was achieved by the two live microalgal species: S. capricornutum at 15 h of exposure (99 %) and S. acutus at 72 h of exposure (95 %). Sorption is an important phenomenon for BaP removal by S. capricornutum but biodegradation is the principal means of removing BaP in live cells. The formation of metabolites by S. capricornutum is rapid and seems to be proportional to the amount of the BaP added to cultures. In contrast, in these bioassays, most of the BaP removal of S. acutus is due to sorption rather than degradation. The appearance of metabolites in the cultures is very slow and at a low amount compared to cultures of S. capricornutum. The similarities and differences existing between the two microalgae are important for the establishment of the conditions for bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benzo(a)pyrene (BaP) is a high molecular weight polycyclic aromatic hydrocarbon (PAH) considered to be a dangerous pollutant because of its carcinogenicity, teratogenicity, and toxicity. BaP has a wide occurrence in environmental matrices such as water and has been observed to bioaccumulate in organisms, which could indirectly cause human exposure via food consumption (Juhasz and Ravendra 2000). BaP is considered the most carcinogenic and toxic PAH. As for all PAHs, BaP may undergo adsorption, volatilization, photolysis, and chemical degradation in the environment, but microbial degradation is the major degradation process (Haritash and Kaushik 2009). Certainly, microbial degradation is the major mechanism for the ecological recovery of PAH-contaminated sites. For this reason, the removal and biodegradation of low molecular weight PAH by diverse types of microorganisms have been reported extensively, and even aerobic bacterial metabolic pathways for compounds such as naphthalene, anthracene, and phenanthrene have been proposed and meticulously described (Peng et al. 2008). In contrast, reports about biodegradation of heavy PAHs, such as BaP, are sparse in the literature. Moreover, the studies related to the microalgal degradation of PAH are scarce compared with those by bacteria and fungi. The use of the metabolic engineering to improve the efficiency of the degradation of toxic compounds such as PAHs (particularly by bacteria), has been considered for bioremediation (Samanta et al. 2002). Although there are few reports, microalgae can provide a good alternative to bacterial and fungal bioremediation systems (Gao et al. 2011).
Microalgae have been reported as effective candidates for the removal of inorganic compounds such as nitrogen and phosphorous (Doria et al. 2012), heavy metals (Kumar et al. 2015), and organic pollutants. Microalgae can contribute to the degradation of environmental pollutants either by increasing the degradation capability of the microbial community or by directly transforming the pollutant in question (Ghasemi et al. 2011). Therefore, several microalgal species have been demonstrated to degrade or remove by sorption very different organic pollutants such as steroids (Faramarzi et al. 2008), the persistent organochlorine pesticide lindane by Scenedesmus intermedius and Dyctiosphaerium chrolledoides (Gonzalez et al. 2012), and nonylphenol by Chlorella vulgaris and Selenastrum capricornutum (Gao and Tam 2011). The herbicide fluroxypyr was significantly accumulated and then rapidly degraded in the cells of Chlamydomonas reinhardtii (Zhang et al. 2011). Other pesticides such as diclofop-methyl and tributyltin were degraded by Chlorella pyrenoidosa, C. vulgaris, Scenedesmus obliquus (Cai et al. 2007), Chlorella miniata, Scenedesmus dimorphus, and Scenedesmus platydiscus (Tam et al. 2002), respectively. Scenedesmus obliquus and Scenedesmus quadricauda were used for the removal of two fungicides (dimethomorph and pyrimethanil) and one herbicide (isoproturon) from their medium (Dosnon-Olette et al. 2010). P-nitrophenol was degraded by a microalgal consortium constituted by C. vulgaris and Coenochloris pyrenoidosa—these organisms were obtained from a waste container fed with the remains and by-products of a culture medium containing substituted aromatic compounds (nitrophenols, chlorophenols, fluorobenzene) (Lima et al. 2003).
Notably, only few examples of unicellular algae degrading polycyclic aromatic compounds have been reported until now, despite that it has been known for a long time that eukaryotic algae can biotransform and biodegrade aromatic pollutants commonly found in natural and wastewaters (Semple et al. 1999). For example, live cells from S. capricornutum were shown to remove and degrade phenanthrene (PHE), fluoranthene (FLA), and pyrene (PYR), producing hydroxylated and dihydroxylated derivatives that might indicate the employment of the monooxygenase and dioxygenase enzymatic pathways, respectively (Chan et al. 2006). Likewise, C. vulgaris, S. platydiscus, and S. quadricauda have been reported to degrade FLA and PYR by species-specific and toxicant-dependent removal processes (Lei et al. 2007). Chlamydomonas sp., C. miniata, and Synechosystis sp. were tested with the aforementioned microalgae, showing an efficiency to remove PYR that varied from species to species (Lei et al. 2002). The accumulation and biodegradation of PHE and FLA by the diatoms Skeletonema costatum and Nitzschia sp. enriched from a mangrove aquatic ecosystem were also observed (Hong et al. 2008).
Two detailed studies concerning the degradation of BaP by microalgae indicated that S. capricornutum, S. acutus, and Ankistrodesmus braunnii can completely metabolize this compound to cis-dihydrodiols (4,5-; 7,8-; 9,10-; and 11,12-BaP), suggesting the use of a dioxygenase enzyme system (Warshawsky et al. 1995), and that S. capricornutum also removed parent PAHs such as benzo(a)pyrene and others (fluorine, phenanthrene, fluoranthrene, and pyrene) via bioaccumulation and/or biodegradation with a cytochrome P-450 monooxygenase system as the detoxification mechanism. The latest assumption was based on the predominant PAH intermediates found which were not the dihydrodiols metabolites, but the 9-fluorenone and monohydroxylated products of PHE, FLA, PYR, and BaP (Ke et al. 2010). Another study using bioassays observed that S. capricornutum favored the production of BaP dihydrodiols after exposure (Olmos-Espejel et al. 2012). This result is in agreement with reports that the microalgae preferentially metabolize PAHs via dioxygenases. In any case, the controversial suggestions concerning the enzymatic mechanisms used for the degradation of BaP by microalgae indicate that further research is necessary to elucidate the degradative metabolic pathways involved.
Certainly, the chosen experimental design greatly influences our understanding of the removal of organic compounds, either by sorption or degradation. Relevant factors include exposure levels, the number of microalgae, the species, and especially the sampling times for the monitoring of parent and intermediate PAHs in the bioassays and analysis of the media and the biomass. When microalgae are in contact with BaP, it is almost certain that this lipophilic compound is retained on the external cell surface and then internalized for metabolism. It has been demonstrated that BaP molecules penetrate cells of Chlorella kessleri-fluorescence microscopic images show their degradation (disappearance) over time (Takácová et al. 2014). Thus, the purpose of this study is a comparative evaluation of the removal of BaP by sorption and degradation by two microalgal species: S. capricornutum and S. acutus. The first was selected because it is well known to metabolize BaP, and the second was selected because it has been less investigated for degradation of PAHs. In particular, we monitored for the first time the BaP- dihydrodiol metabolites formed by these species in the order in which they appear in the culture (medium and cells). The analysis of compounds at short exposure times (from 0.25 to 72 h) allows a better understanding of the beginning of the biodegradation process of BaP.
Materials and methods
Materials and reagents
Benzo(a)pyrene with a certified purity >99 % was purchased from Chem. Service (West Chester, PA, USA). The BaP metabolite standards benzo(a)pyrene cis-4,5 dihydrodiol (4,5-diol); benzo(a)pyrene trans-9,10 dihydrodiol (9,10-diol); and benzo(a)pyrene trans 7,8 dihydrodiol (7,8-diol) were acquired from the NCI Chemical Carcinogen Reference Standards Repository (Kansas, MO, USA). Methanol, acetonitrile, and isopropanol were HPLC grade from JT Baker (Phillipsburg, NJ, USA). Water (18.2 MΩ cm−1 resistivity) was obtained from a Millipore Simplicity UV deionizer (Bedford MA, USA). Silica C18 Supelclean with average particle size of 53.9 μm and average pore diameter of 73 Å (SUPELCO, Bellefonte, PA, USA) was used as a sorbent to perform solid phase extraction (SPE) and matrix solid phase dispersion (MSPD). The Bristol culture medium contained sodium nitrate (NaNO3), calcium chloride dihydrate (CaCl2·2H2O), magnesium sulfate heptahydrate (MgSO4·7H2O), dibasic sodium phosphate (Na2HPO4), monobasic sodium phosphate (NaH2PO4), sodium chloride (NaCl) (all from JT Baker, Phillisburg, NJ USA), and the ingredient proteose-peptone acquired from MCD LAB (Tlanepantla, MEX, Mexico).
Algal cultures
Selenastrum capricornutum and Scenedesmus acutus were obtained from the Culture Collection of Algae (UTEX) at the University of Texas (Austin, TX, USA). The axenic microalgal culture of each species was cultivated at room temperature (23–25 °C) in a 250-mL Erlenmeyer flask containing 150 mL of Bristol’s medium (NaNO3 2.94 mM, CaCl2·2H2O 0.17 mM, MgSO4·7H2O 0.3 mM, Na2HPO4 0.43 mM, NaH2PO4 1.29 mM, NaCl 0.43 mM) supplemented with 0.5 g L−1 of proteose-peptone. The cultures were placed in a chamber illuminated with a T5 14 W cool white fluorescence lamp under a 16:8-h light/dark cycle. Continuous shaking at 100 rpm was accomplished by a Thermo Scientific model 2346 (Dubuque, IA, USA) orbital shaker. For the exposure bioassays, the cells were harvested at mid-exponential growth (at approximately 10 days), and the cell numbers were counted in a Neubauer hemocytometer with improved rulings (Boeco, Germany) corresponding to 5 × 106 cells mL−1. At this stage, the absorbance of the cultures was measured at 685 nm with a Spectronic Genesys 5 UV–Vis spectrophotometer (ThermoScientific, USA), giving a 1.0 ± 0.2 AU value.
Bioassays
Cultures containing 15 mL of a liquid culture of S. capricornutum or S. acutus (initially with 5 × 106 cells mL−1) were prepared in 50-mL Erlenmeyer flasks. BaP was added at 266 μg L−1 to the cultures of S. acutus and at 5, 50, and 266 μg L−1 to the cultures of S. capricornutum. The flasks assigned to the exposure test were designated as “Live Microalgae Cultures” (LMC) and were shaken on a rotary shaker (100 rpm) at room temperature, with the same photoperiod (16:8-h light/dark) used for algal growth, but illuminated with yellow light provided by a 40 W incandescent lamp. All experiments were repeated in triplicate at 0.75, 1.5, 3, 6, 15, and 24 h of exposure for S. capricornutum and 0.25, 0.5, 0.75, 2, 6, 15, 24, 48, and 72 h for S. acutus. The residual concentrations of BaP and the dihydrodiol metabolites (4,5-; 7.8-; and 9,10-BaP) were determined using analytical procedures. Simultaneously, three different controls were carried out for each microalgae specie: (a) dead microalgal culture (DMC medium and biomass) to monitor passive sorption by cells—with the same cell number as live cultures but killed by autoclaving and the same concentration of BaP; (b) Blank (B) to monitor the abiotic loss of BaP—consisting of liquid media with BaP but without algae; and (c) blank microalgal culture (BMC) to monitor the effect of the acetonitrile used to dissolve the BaP and interferences from the matrix—consisting of liquid media with the same cell density as the other live cultures, but without BaP.
Sample preparation
Each exposed culture (15 mL) was centrifuged for 20 min at 3900 rpm in a SIGMA 2–5 centrifuge (Osterode am Harz, Germany). The cell pellet (biomass) was air-dried, and the supernatant (the liquid culture medium) was also retained. Both components, liquid media and dry biomass, were treated off-line by solid phase extraction (SPE) and by matrix solid phase dispersion (MSPD), respectively. The recoveries of BaP were 75 % (medium) and 85 % (biomass). All extracts were analyzed by high-performance liquid chromatography with fluorescence detection (metabolites) or spectrophotometric UV detection (BaP). Quantification was accomplished at a very low amount (ng/pg). The extracts containing the metabolites (M1 and B1) were diluted with water to 10 % acetonitrile and then they were preconcentrated online in a 20 × 2 mm i.d. stainless steel SPE precolumn packed with a solid phase Nucleosil 100–10 C18 (Macherey Nagel, Düren, Germany) as described by Olmos-Espejel et al. (2012). The diluted extracts were loaded with an Eldex Duros Series 1000 C pump (Napa, CA, USA). The mobile phase flow to desorb the analytes from the precolumn, was controlled by a Rheodyne model 7000 six-port switching valve (Berkeley, CA, USA).
Extraction of BaP and their metabolites from liquid media
Extraction of the analytes from the centrifuged liquid medium was performed by an SPE method using a cartridge packed with 300 mg of C18 silica previously conditioned with 4 mL of acetonitrile and 8 mL of water. Then, 15 mL of the sample was mixed with enough isopropanol to obtain 20 % in this mixture for its application through the SPE cartridge. The following sequence of cleaning solvents was then passed through this cartridge: 5 mL of water, 4 mL of 10:90 v/v acetonitrile/water mixture and 1 mL of 30:70 v/v acetonitrile/water mixture. Finally, the analytes were eluted from the cartridge in two different extracts designated as M1 (3 mL of 55:45 v/v acetonitrile/water mixture) and M2 (4 mL of acetonitrile). The two extracts were analyzed by HPLC; M2 (containing the BaP) was injected directly by a loop of 20 μL, and M1 (containing the metabolites) was preconcentrated and analyzed online.
Extraction of BaP and their metabolites from biomass
Samples containing 5 mg of dried biomass were blended in an agate mortar with 100 mg of dry C18 silica (preconditioned with 2 mL of acetonitrile) until a homogeneous mixture was obtained. The mixture was placed into a 1-mL polypropylene cartridge with a polyethylene frit in the bottom, compressed and covered with another polyethylene frit. Then, 10 mL of deionized water and 5 mL of a 20:80 v/v acetonitrile/water mixture were added as clean-up eluents. Finally, the analytes were eluted from the cartridge in two different fractions designated as B1 (1.5 mL of 40:60 v/v acetonitrile/water mixture) and B2 (0.5 mL of 90:10 v/v acetonitrile/water mixture). The two extracts were analyzed by HPLC; B2 (containing the BaP) was injected directly into the analytical column in a 20-μL loop and B1 (containing the metabolites) was preconcentrated online and analyzed.
Chromatographic analysis
The remaining BaP and its metabolites in the exposed cultures were analyzed by HPLC on a Varian model 210 liquid chromatograph, coupled to a model 363 fluorescence detector and a model Polychrom 9065 UV diode array detector (Palo Alto, CA, USA) using STAR Workstation multi-instrument Version 6.20 software. This HPLC system was equipped with a manual injection port and a Rheodyne 7725i switching valve with a 20-μL loop. A Nucleosyl 100-5-C18 stainless steel analytical column (150 mm × 4.6 mm I.D.) connected to a guard column (20 mm × 2 mm I.D., packed with the same stationary phase) (Macherey Nagel, Düren, Germany) was used.
The chromatographic conditions consisted of two mobile phases at a flow rate of 1 mL min−1: 100 % methanol (for benzo[a]pyrene) and 65 % methanol:35 % water (for the metabolites). The detection wavelengths for BaP was 263 nm (UV detection), and those for metabolites were λ ex = 263 nm and λ em = 430 nm (fluorescence detection). The extracts with metabolites were simultaneously sent to confirmative analysis of dihydrodiols with an HPLC-MS (Q-TOF) system. An Alltima HPC18 AQ column, with a particle diameter of 3 μm and dimensions of 100 mm × 2.1 mm was used with a mobile phase composed of 50 % acetonitrile with 0.01 % formic acid and 50 % water with 0.01 % formic acid at a flow rate of 0.3 mL min−1. The Q-TOF had an ion source Agilent ESI-Jet Stream Thermal Focusing, Polarity (+). Gas/N2, flow 13 L min−1, collision energy 140 eV, TOF mass range m/z 50–350.
Results and discussion
Residual BaP in microalgal cultures
The amounts of BaP recovered from the liquid media and biomass of live microalgal cultures (LMC), dead microalgal cultures (DMC), blank microalgae cultures (BMC), and the blank (B) are shown in Fig. 1.
BaP gradually disappeared from the two LMC media that contained live cells and the residual amount decreased more rapidly in the media that contained S. capricornutum (Fig. 1b) than from the S. acutus media (Fig. 1a). At 0.75 h, the amount of BaP in the liquid medium from S. capricornutum decreased from 4000 to 849 ± 99 ng, while the amount of BaP recovered from the S. acutus medium was 2133 ± 95 ng, approximately two and a half-fold greater. This high value indicates that the disappearance of BaP is slower in S. acutus cultures. In fact, the S. acutus liquid media did not contain a similar amount of BaP as for Se. capricornutum at 0.75 until 15 h of exposure (824 ± 34 ng).
Even greater differences in BaP in the biomass of the two LMC were noted. Figure 1a shows that the S. acutus cells slowly accumulate BaP: at 0.25 h, the amount accumulated is only 231 ± 37 ng; at 48 h, it is 1848 ± 106 ng. In contrast, S. capricornutum accumulate all of the BaP, very rapidly, probably in the first few minutes because the residual amount was 1213 ± 70 ng at 0.75 h. However, Fig. 1b indicates that at longer times, this value did not gradually increase as for S. acutus, but rapidly decreased until the residual amount was 29 ± 9 ng in the biomass at 24-h exposure. These results clearly indicate that the initial BaP sorption process (adsorption and/or absorption) on the S. capricornutum cells is almost immediately completed. Moreover, the rapid decrease in the cell-sorbed BaP recovered at 15–24 h strongly indicates that the BaP is degraded very quickly, probably beginning just after the first molecules are adsorbed. Due to the rapid sorption and degradation, the S. capricornutum cultures were difficult to monitor at times shorter than 0.75 h in liquid media, because the measured values of BaP showed very high variability.
On the other hand, the BaP recovered from the liquid media and biomass of the two DMC seemed to be relatively constant for all the studied exposure times. However, the average amount of residual BaP in the biomass was higher than the amount found in the liquid media (2254 ± 180 vs. 935 ± 53 for S. acutus and 1456 ± 170 vs. 477 ± 50 ng for S. capricornutum), which suggests that strong passive sorption occurred on dead cells. The sorption of BaP by the dead microalgae can be favored by the affinity existing between the non-polar components of the cell wall and cell membrane with the lipophilic BaP, which has an octanol-water partition coefficient (log P) of 5.97. Some authors have found that the bioaccumulation of hydrophobic organic compounds by some phytoplanktonic algae is consistent with the partitioning from water into the lipids of cell walls and membranes, but this process is slow (Swackhamer and Skoglund 1993). Additionally, Luo et al. (2014) observed that Chlorella sp. heat-killed cells were more adsorptive to BaP than live cells.
Comparing the behavior of residual BaP in biomass from DMC and LMC of S. acutus, the average value attained by the DMC was always greater than the maximal value obtained at 48 h from the LMC (2254 ± 180 vs. 1848 ± 106 ng). This difference supports the idea that even thought sorption is an important phenomenon for BaP removal by this microalgae, it is not the only mechanism for BaP removal from aqueous media by LMC; cellular metabolism might also contribute. For the S. capricornutum cultures, the continuous decrease in residual BaP in the medium and biomass of the LMC indicates that biodegradation is the principal means of removing BaP in live cells. This is different than the DMC, for which the amount of BaP detected was constant at all the exposure times.
In any case, a clear decrease in the amount of BaP with time in LMC liquid media for both microalgal species was seen, contrary to DMC which remained constant. Thus, continuous removal by biodegradation is indicated in both species because the gradual loss of BaP in liquid medium was always much lower than in the blank (B) that measured abiotic removal, principally by photo-oxidation (Nadal et al. 2006). The BMC did not show problems of viability, neither by interferences from the matrix in the chromatograms at the same retention times of the analytes.
Additionally, S. acutus accumulated more BaP in dead cells than S. capricornutum; possibly because the two microalgae cells have different shapes and sizes. S. acutus grown in Bristol medium can be either oblong single cells with dimensions of 5–6 μm in length and 2 μm in width or an aggregates such as the characteristic tetrads, with more fusiform cells with respect to the single cells (Giovanardi et al. 2014). S. capricornutum has the typical croissant-form or half-moon appearance of solitary cells with an average size of 9.1 μm in length and 3.2 μm in width. S. capricornutum is also known as Pseudokirchneriella subcapitata, and their cells can sustain morphological alterations such as modification of cells volume when they are exposed to metals (Machado and Soares 2014).
Removal and degradation of BaP
It was mentioned in the previous section that the disappearance of BaP from liquid medium was faster in LMC of S. capricornutum than in LMC of S. acutus. Figure 2a shows this phenomenon by comparing the curves of the variation in recovered BaP from the medium with exposure time between the two LMC.
The percentage removal (amount of BaP added to LMC − amount of residual BaP in LMC medium/amount of BaP added to LMC) and the percentage degradation (amount of BaP added to LMC − total amount of residual BaP in LMC (medium + biomass)/amount of BaP added to LMC) were calculated. Figure 2b, c show the percentage of the removal and degradation vs. exposure time for two LMC. It is worth noting that the removal of BaP occurred very rapidly in the S. capricornutum live cultures because the removal at 0.75 h was 80 % while the removal in S. acutus live cultures was 45 % at the same exposure time. Complete removal of BaP was achieved in the two microalgae species live cultures at a later time: by S. capricornutum at 15 h of exposure (99 % removal) and S. acutus at 72 h of exposure (95 % removal). Figure 2b, c show also the evolution of the percentage degradation with time for S. capricornutum and S. acutus LMC, respectively. S. capricornutum degraded rapidly: 50 % degradation is evident at 0.75 h, 98 % at 15 h of exposure, and the percentage degradation and percentage removal had the same value of 99 % at 24-h exposure. This behavior suggests that S. capricornutum uses metabolism as the principal means of removing BaP. Contrary, as seen in Fig. 2c, S. acutus shows different behavior, with low degradation of 30 % at the initial time of 0.75 h; this percentage increased thereafter, but had a very slow velocity and always lower that the percentage removal over the entire time range, reaching a maximum value of 51 % degradation at 72 h. It is clear that this microalgae presents a slower degradation rate than S. capricornutum and it varied at low values more than the values for percentage removal. This suggests that in these bioassays, most of the BaP removal from the medium of S. acutus is due to sorption rather than degradation, but even considering the low percentage degradation at the 72 h, degradation seems to slowly and continuously increase and at a longer exposure time, complete degradation of the BaP will probably be realized. This hypothesis is well supported by the later appearance of metabolites.
For the DMC, the removal was always efficient and constant at all exposure times and was rapid during the first hour because at 0.75 h, S. acutus had already removed 75 % and S. capricornutum had removed 83 %; the average removal values achieved by dead cells during the entire time were 77 and 88 %, respectively, and also the percentage degradation rates were constant at 20 and 50 %, respectively. These average percentages of removal and degradation did not change greatly from those at the initial times in the DMC. The removal efficiency of dead cells from S. acutus is higher than such of live cells (47 %) at 0.25 h; thus, the DMC remove BaP more efficiently than the LMC within the first hour. Indeed, some other dead microalgae species were found to be more efficient than live microalgae for removing organic compounds; for example, the removal of tributyltin from artificial wastewater (Tam et al. 2002). Thus, in these cases, biosorption was more important than degradation over a short exposure time. In contrast, live and dead cells of S. capricornutum showed little difference in values of percentage removal at 0.75 h (79 and 83 %, respectively). In this case, degradation begins at the initiation of exposure.
Metabolites
Selenastrum capricornutum
The metabolites detected and quantified in the S. capricornutum LMC medium and biomass were benzo(a)pyrene 4,5 dihydrodiol (4,5-diol); benzo(a)pyrene 7,8 dihydrodiol (7,8-diol); and benzo(a)pyrene 9,10 dihydrodiol (9,10-diol). Figure 3 shows the amounts of these metabolites in the media and biomass at different exposure times. Figure 3b shows that the 4,5-diol and the 7,8- diol appeared in the biomass first, at the beginning of the bioassay. This demonstrated that the degradation process occurs immediately as indicated by variations of the residual BaP in the live cultures. Once the two metabolites are formed inside the cells at 0.75–1.5 h, they are excreted from the cells into the liquid medium and appear later at 1.5–3 h of exposure, as seen in Fig. 3a. These metabolites attain maximal production in cells at 3 h of exposure for the 7,8 diol and at 6 h for the 4,5-diol; afterwards, the amount of the two metabolites decreased, and they finally disappeared from the biomass and the liquid media at 24 h of exposure, as indicated in Fig. 3.1 and 3.3. On the other hand, Fig. 3a, b also show that the appearance of the 9,10-diol begins at 6 h of exposure for both liquid medium and biomass and its production increases gradually and reaches a maximum at 15 h of exposure. It is interesting to note that while the 9,10 diol appears in the culture, the amount of the other metabolites decreases with the exposure time. Figure 3.2 shows that the 9,10-diol reaches a maximum amount in culture (biomass + medium) at 15 h of exposure, but later, at 24 h, it can be found only in the liquid medium with no other dihydrodiols present. Warshawsky et al. (1995) also detected the three aforementioned metabolites in cultures of S. capricornutum exposed to BaP along with the 11,12-diol, which was considered as the major metabolite under yellow light exposure. But unlike us, they reported the amount of 4,5-diol and 11,12-diol together. In this work, the 4,5-diol was measured separately from the 11,12-diol. The latter compound was not quantified because of the lack of a standard, but the chromatogram of a LMC extract at 6 h presented in Fig. 4 shows that the peak between the 4,5- and the 7,8-diols was thought to correspond to 11,12-diol. A subsequent confirmative analysis of the extract by HPLC/MS/MS shows that in effect, this compound had the same spectrum as the others metabolites. Figure 5 shows the chromatogram and the mass spectra obtained for all the four peaks having the same molecular ion (269.09) corresponding to the dihydrodiol metabolites. It interesting to note that the compound identified as the 11,12-diol behaved in a similar fashion to the 4,5 and 7,8 diol metabolites in the BaP exposure tests.
HPLC chromatograms of Selenastrum capricornutum B1 extracts at 6 h of exposure. Conditions: Nucleosyl 100-5-C18 column (150 mm × 4.6 mm); mobile phase constituted by 65 % methanol and 35 % water at a flow rate of 1 mL min−1. Fluorescence detection with λ ex = 263 nm and λ em = 430 nm. LMC live microalgae cultures, BMC blank microalgae cultures, B blank
HPLC-MS (Q-TOF) chromatogram of metabolites in Selenastrum capricornutum B1 extract and mass spectra. Conditions: Alltima HPC18 AQ column, 3 μm, (100 mm × 2.1 mm); mobile phase constituted by 50 % acetonitrile with 0.01 % formic acid and 50 % water with 0.01 % formic acid at a flow rate of 0.3 mL min−1. a 9,10-diol. b 4,5-diol. c 11,12-diol. d 7,8-diol
Scenedesmus acutus
Figure 6 shows the amount of the metabolites formed by S. acutus in liquid medium and biomass of LMC at the different exposure times. It can be observed that the 4,5 and 7,8 diol appear in the biomass immediately at 0.25 h (Fig. 6b), with a low amount produced. Then, these metabolites are excreted from the cells into the liquid medium at 2–4 h of exposure (Fig. 6a). Additionally, similar to S. capricornutum cultures, the 9,10 diol appears later, at 4 h of exposure and is scarcely present in the biomass (Fig. 6.b) and has a slow rate of formation in cells up to 24 h of exposure. Then, this metabolite is released into liquid medium at 48 h and continues to increase at 72 h in liquid medium and the biomass.
In summary, the appearance of metabolites in the cultures of S. acutus is very slow compared to cultures of S. capricornutum. It is noteworthy that in Fig. 6.1–3, the occurrence of metabolites seems to be in a “slow motion,” clearly showing that the first metabolites formed are the 7,8 and 4,5 diols, almost at the same time. The production of the 7,8 diol is finished before the others and it attains a maximal value at 15 h and decreases afterwards until 72 h (Fig. 6.3). The 4,5 diol appeared at 0.25 h and continuously increased up to 72 h (Fig. 6.1). The 9,10-diol took longer to appear in the biomass at 4 h, and is excreted into the liquid medium at 48 h (Fig. 6.2). The slow biodegradation of BaP by S. acutus explains why the sorption process is more important for removal at the initial exposure times. However, even if degradation is slow, it is always continuous and contributes to the complete transformation of BaP, but requires a longer time.
Effect of the concentration of BaP
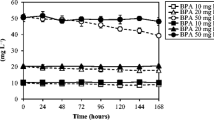
The production of benzo(a)pyrene 4,5 dihydrodiol and benzo(a)pyrene 9,10 dihydrodiol was monitored by HPLC and fluorescence detection for three different concentrations of BaP added to the S. capricornutum cultures. The amounts of these metabolites found in the liquid medium and the biomass were determined at the maximum production time for each compound: 6 and 15 h for the 4,5- and 9,10-diols, respectively. Table 1 shows these results and the amounts in the S. acutus liquid media and biomass at 266 μg/L of BaP and 72 h of exposure. The S. acutus cultures were tested only at the highest concentration of exposure of BaP because in this case, the formation of metabolites was considerably weaker than that of S. capricornutum, approximately 10-fold lower for the 4,5-diol and 50-fold lower for the 9,10-diol. The detection limit of the analytical method was not suitable for the determination of such low levels of the metabolites formed at lower concentration of BaP in the exposure bioassays.
Table 1 shows that the amount of metabolite in either the biomass or the liquid medium increased with the concentration of BaP. The increase was linear with a correlation coefficient of r 2 > 0.99 even though the number of cells was the same in all bioassays. These results could indicate that the enzymatic systems are activated to respond proportionately to the amount of pollutant to which the cells are exposed. Further research is required to understand the kinetics of the process and identify all of the metabolites formed during the biodegradation process.
Conclusions
A comparison of the removal of BaP by the two green algal species, S. capricornutum and S. acutus, shows similarities and differences that are important for the establishment of the conditions for bioremediation. The two microalgae are able to quickly and efficiently remove BaP from an aqueous medium. However, BaP disappears more rapidly from S. capricornutum cultures compared to S. acutus cultures. It is important to understand that even if BaP is quickly removed from the medium by S. acutus, it is retained by the cells for a certain period of time because sorption predominates over degradation at short times of exposure. The formation of metabolites by S. capricornutum is rapid and seems to be proportional to the amount of the benzo(a)pyrene added to cultures in the concentration range tested, but further study is necessary to elucidate the entire mechanism and its kinetics.
References
Cai X, Liu W, Jin M, Lin K (2007) Relation of diclofop- methyl toxicity and degradation in algae cultures. Environ Toxicol Chem 26:970–975
Chan SMN, Luan T, Wong MH, Tam NFY (2006) Removal and biodegradation of polycyclic aromatic hydrocarbons by Selenastrum capricornutum. Environ Toxicol Chem 25:1772–1779
Doria E, Longoni P, Scibilia L, Iazzi N, Cella R, Nielsen E (2012) Isolation and characterization of a Scenedesmus acutus strain to be used for bioremediation of urban wastewater. J Appl Phycol 24:375–383
Dosnon-Olette R, Trotel-Aziz P, Couderchet M, EullafFroy P (2010) Fungicides and herbicide removal in Scenedesmus cells suspensions. Chemosphere 79:117–123
Faramarzi MA, Adrangi S, Tabatabaei Y (2008) Microalgal transformation of steroids. J Phycol 44:27–37
Gao QT, Tam NFY (2011) Growth, photosynthesis and antioxidant responses of two microalgal species, Chrorella vulgaris and Selenastrum capricornutum, to nonylphenol stress. Chemosphere 82:346–354
Gao QT, Wong YS, Tam NFY (2011) Removal and biodegradation of nonylphenol by different Chlorella species. Mar Pollut Bull 63:445–451
Ghasemi Y, Rasoul-Amini S, Fotooh-Abadi E (2011) The biotransformation, biodegradation and bioremediation of organic compounds by microalgae. J Phycol 47:969–980
Giovanardi M, Baldisserotto C, Daglia M, Ferroni M, Sabia A, Pancaldi S (2014) Morpho-physiological aspects of Scenedesmus acutus PVUW12 cultivated with a dairy industry waste and after starvation. Plant Biosyst 148:1–9
Gonzalez R, García-Balboa C, Rouco M, López Rodas B, Costas E (2012) Adaptation of microalgae to lindane: a new approach for bioremediation. Aquat Toxicol 109:25–32
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Hong YW, Yuan DX, Ling QM, Yang TL (2008) Accumulation and biodegradation of phenantrene and fluoranthene by the algae enriched from a mangrove aquatic ecosystem. Mar Pollut Bull 56:1400–1405
Juhasz AL, Ravendra N (2000) Bioremediation of high molecular weight polycyclic aromatic hydrocarbons. A review of the microbial degradation of benzo(a)pyrene. Int Biodeterior Biodegrad 45:57–88
Ke L, Luo L, Wang P, Luan T, Tam NFY (2010) Effects of metals on biosorption and biodegradation of mixed polyciclic aromatic hydrocarbons by a freshwater green alga Selenastrum capricornutum. Bioresour Technol 101:6950–6961
Kumar KS, Dahms HU, Won EJ, Lee JS, Shin KH (2015) Microalgae-A promising tool for heavy metal remediation. Ecotoxicol J Phycol Environ Safe 113:329–352
Lei AP, Wong YS, Tam NFY (2002) Removal or pyrene by different microalgal species. Water Sci Technol 46:195–201
Lei AP, Hu ZL, Wong WS, Tam NFY (2007) Removal of fluoranthene and pyrene by different microalgal species. Bioresour Technol 98:273–280
Lima SAC, Castro PML, Morais R (2003) Biodegradation of p-nitrophenol by microalgae. J Appl Phycol 15:137–142
Luo L, Wang P, Lin L, Luan T, Ke L, Tam NFY (2014) Removal and transformation of high molecular weight polycyclic aromatic hydrocarbons in water by live and dead microalgae. Process Biochem 49:1723–1732
Machado MD, Soares EV (2014) Modification of cell volume and proliferative capacity of Pseudokirchneriella subcapitata cells exposed to metal stress. Aquat Toxicol 147:1–6
Nadal M, Wargent JJ, Jones KC, Paul ND, Schuhmacher M, Domingo JL (2006) Influence of UV-B radiation and temperature on photodegradation of PAHs: preliminary results. J Atmos Chem 55:241–252
Olmos-Espejel JJ, García de Llasera MP, Velasco-Cruz M (2012) Extraction and analysis of polycyclic aromatic hydrocarbons and benzo[a]pyrene metabolites in microalgae cultures by off-line/on-line methodology based on matrix solid-phase dispersion, solid- phase extraction and high-performance liquid chromatography. J Chromatogr A 1262:138–147
Peng RH, Xiong AS, Xue Y, Fu XY, Gao F, Zhao W, Tian YS, Yao QH (2008) Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev 32:927–955
Samanta SK, Singh OM, Jain RK (2002) Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trend Biotechnol 20:243–248
Semple KT, Cain RB, Schmidt S (1999) Biodegradation of aromatic compounds by microalgae. FEMS Microbiol Lett 170:291–300
Swackhamer DL, Skoglund RS (1993) Bioaccumulation of PCBs by algae: kinetics versus equilibrium. Environ Toxicol Chem 12:831–832
Takácová A, Smolinská M, Ryba J, Mackulak T, Jokrllová J, Hronec P, Cík G (2014) Biodegradation of benzo(a)pyrene through the use of algae. Cent Eur J Chem 12:1133–1143
Tam NFY, Chong AMY, Wong WS (2002) Removal of tributyltin (TBT) by live and dead microalgal cells. Mar Pollut Bull 45:362–371
Warshawsky D, Cody T, Radike M, Reilman R, Schumann B, LaDow K, Scneider J (1995) Biotransformation of benzo(a)pyrene and other polycyclic aromatic hydrocarbons and heterocyclic analogs by several green algae and other algal species under gold and white light. Chem Biol Interact 97:131–148
Zhang S, Qiu CB, Zhou Y, Jin ZP, Yang H (2011) Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green algae Chlamydomonas reinhardtii. Ecotoxicology 20:337–347
Acknowledgments
This work was supported by the Consejo Nacional de Ciencia y Tecnología de México CONACyT, project CB 166389 and the Dirección General de Asuntos de Personal Académico from the Universidad Nacional Autónoma de México DGAPA-UNAM, project PAPIIT IT203214.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
García de Llasera, M.P., Olmos-Espejel, J.d.J., Díaz-Flores, G. et al. Biodegradation of benzo(a)pyrene by two freshwater microalgae Selenastrum capricornutum and Scenedesmus acutus: a comparative study useful for bioremediation. Environ Sci Pollut Res 23, 3365–3375 (2016). https://doi.org/10.1007/s11356-015-5576-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5576-2