Abstract
Pollution effects were assessed by means of biochemical biomarkers (catalase, glutathione S-transferase and acetylcholinesterase activities, and metallothioneins content) in five species at selected coastal sites across the Eastern Mediterranean and the Black Sea. The mussel Mytilus galloprovincialis, a well-established sentinel species, was investigated in the Adriatic Sea, Aegean Sea, and Black Sea. The mussel Brachidontes pharaonis and the striped red mullet Mullus surmuletus were used in the Levantine Sea where M. galloprovincialis is not present. The white seabream Diplodus sargus sargus and the gastropod Rapana venosa were additionally sampled in the Adriatic and the Black Sea, respectively. Mussels showed catalase, glutathione S-transferase, and acetylcholinesterase responses to pollution in most geographical areas while the response of metallothioneins was restricted to a few sites. R. venosa showed marked responses of catalase and metallothioneins whereas both fish species did not generally exhibit variations in biomarker values among sites. The approach based on the reference deviation concept using the “Integrated Biological Responses version 2” index was useful for the interpretation of overall biomarker responses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last few decades, the evaluation of the effects of pollution in marine coastal and estuarine areas has been a growing concern worldwide. In the European Union, they resulted in two main directives, the EU Water Framework Directive (WFD, Directive 2000/60/EC) and the Marine Strategy Framework Directive (MSFD, 2008/56/EC). While the former had already pointed out the relevance of biological monitoring for the evaluation of water quality, biomarkers as tools for assessing pollution effects at the individual level have mainly been proposed for the MSFD (Lyons et al. 2010; Giltrap et al. 2013; Bellas et al. 2014). The recognition of the value of biomarkers in the evaluation of pollution effects in the marine environment is a step forward for assessing pollution effects. Depending on the intensity and persistence of the pollution source, the effects of pollution can be manifested at different levels of biological organization (e.g., Richardson et al. 2011; Pereira et al. 2012). The first signs will be most likely observed at lower organization levels, i.e., gene, proteins, and up to the organism level, and these changes could then lead to changes at the population and community levels (Moore et al. 2004). Biomarkers at the biochemical level can provide information on the qualitative and quantitative relationships between pollutant exposure and biological responses, and some of them can predict responses at higher levels of biological organization (Hyne and Maher 2003; Seabra Pereira et al. 2014). Such early warnings of marine pollution are extremely important, as timely detection will allow corrective measures to be undertaken, avoiding irreversible effects on the entire ecosystem.
The ability of different biomarkers to detect biological effects of pollutants in marine organisms has been shown by numerous studies, under different disturbance scenarios and across different geographical regions (e.g., Galloway et al. 2004; Lehtonen et al. 2006; Zorita et al. 2007; Gagné et al. 2008; Bellas et al. 2014). Nevertheless, studies analyzing trends over large spatial scales, particularly in the Eastern Mediterranean and Black Sea, are scarce. Although some biomarkers have been included in international environmental monitoring programs, across large geographical areas, the lack of comparability of the data is a flaw of the biomarker approach (Sanchez and Porcher 2009). Bivalves, including mussels of the genus Mytilus (e.g., Mytilus galloprovincialis in the Mediterranean Sea versus Mytilus edulis in the North Sea), as well as fish (e.g., Mullus sp., Platichthys flesus, Zoarces viviparus, and Perca sp.) are the most commonly used sentinel species for monitoring pollutant effects in coastal environments. This is primarily due to their wide geographical distribution, abundance, and accessibility in the field (bivalves), as well as position in the trophic chain and their key role in human nutrition (fish) (Viarengo et al. 2007; Thain et al. 2008). Considering the geographical scale encompassed by the MSFD, it is imperative that countries involved validate common indicators (e.g., suite of biomarkers) and approaches (e.g., sentinel species, methodologies) for the evaluation of the effects of pollutants in the marine ecosystem. However, the use of common sentinel species for large geographical areas may not be feasible as many of them are not cosmopolitan. On the other hand, congener species may respond differently to pollution (Moschino et al. 2011). In this regard, it is essential to analyze response patterns of a common set of biomarkers in a variety of species.
The present study assessed the effects of pollution in the Eastern Mediterranean (Adriatic Sea, Aegean Sea, Levantine Sea) and the Black Sea using a suite of biochemical biomarkers. The study utilized the well-recognized sentinel species M. galloprovincialis and also the alternative sentinel species Brachidontes pharaonis, Rapana venosa, Mullus surmuletus, and Diplodus sargus sargus. In each geographical area, reference and impacted sites differing in contamination levels were selected. The mussel M. galloprovincialis was investigated in the Adriatic, Aegean, and Black Sea. Since M. galloprovincialis is not present in the Levantine Sea, alternative sentinel species (the mussel B. pharaonis and the fish M. surmuletus) were investigated in this area. The fish D. sargus sargus and the gastropod R. venosa were additionally applied in the Adriatic and the Black Sea, respectively. Activities of the antioxidant enzyme catalase (CAT), the phase II biotransformation enzyme glutathione S-transferase (GST), and the enzyme of neurotransmission acetylcholinesterase (AChE) as well as levels of the metal binding proteins metallothioneins (MTs) were used as biochemical biomarkers. These biomarkers are among the most widely used and proposed as suitable for biomonitoring in the Mediterranean Sea (Viarengo et al. 2007). Moreover, as supporting parameters, the condition index in molluscs and the condition factor in fish were evaluated to highlight the general physiological condition and the nutritive status of the selected organisms.
The aims of the present study were (1) to investigate whether the responses to pollution of the suite of biochemical biomarkers are consistent across the study areas in a well-recognized sentinel species (M. galloprovincialis) and (2) to compare the biomarker responses in alternative sentinel species with those observed in M. galloprovincialis. In addition, an attempt was made to compare environmental stress levels at the selected sites across the study areas by integration of biomarker responses using the “Integrated Biological Response version 2” (IBRv2) index. The IBRv2 index has been proposed as an integrative tool that can be used without species limitation in large monitoring programs (Sanchez et al. 2013).
Materials and methods
Sentinel species, study sites, and animal sampling
The Mediterranean mussel M. galloprovincialis occurs in the low intertidal zone of exposed rocky coasts with relatively high wave energy. It is a native of the Mediterranean and Black Seas and is commonly used as sentinel in ecotoxicological investigations (Viarengo et al. 2007). B. pharaonis is an Indo-Pacific mussel that has colonized the Mediterranean Sea via the Suez Canal. It is abundant in midlittoral rocky habitats. Both M. galloprovincialis and B. pharaonis are sedentary filter feeders, attached by byssus threads to rocks and stones. The veined whelk R. venosa is a large predatory gastropod that occurs in the Black Sea down to 30 m depth in areas with sandy bottoms, as well as in rocky and muddy habitats. R. venosa is native of Asian waters; it was introduced into the Black Sea in the 1940s, and has also been reported in the Aegean and Adriatic Seas, North Sea, Uruguay, and Chesapeake Bay (USA). The striped red mullet M. surmuletus is a benthic fish species found in the Mediterranean Sea, eastern North Atlantic Ocean, and the Black Sea. Adults occur on broken and rough grounds but are also found over sand and soft bottoms at depths less than 100 m and feed on benthic organisms. The white sea bream D. sargus sargus is common in the Mediterranean but rare in the Black Sea. It is also present in the East Atlantic coast and South Africa. It is a demersal fish species, inhabiting littoral waters on rocky bottoms and sand close to rocks, up to 50 m depth in the Mediterranean Sea. Adults are carnivorous.
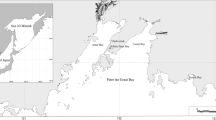
Animal sampling was carried out in seven geographical areas: along the Slovenian and Italian coasts (Venice Lagoon and Apulia coast) of the Adriatic Sea, the Greek coast (Saronikos Gulf) of the Aegean Sea, the southeast Cypriot coast of the Levantine Sea, as well as the Romanian (Constanta area) and Russian coasts (Blue Bay) of the Black Sea (Fig. 1). Two to four sampling sites were selected in each geographical area including one reference site (away from known pollution sources) as well as sites affected by anthropogenic activities (maritime traffic, industrial, agricultural, and urban activities). Specifically, four sites were sampled along the Slovenian coast (SL_S1, SL_S2, SL_S3, SL_Ref), two sites in the Venice Lagoon (IT_S1, IT_Ref1), two sites along the Apulia coast (IT_S2, IT_Ref2), four sites in Saronikos Gulf (EL_S1, EL_S2, EL_S3, EL_Ref), four sites along the Cyprus southeast coast (CY_S1, CY_Ref1, CY_S2, CY_Ref2), two sites in the Constanta area (RO_S1, RO_Ref), and two sites along the Russian coast (RUS_S1, RUS_Ref). The types of anthropogenic pressures, trophic status, temperature, and salinity during samplings as well as sentinel species sampled in each area are shown in Table 1. Hot spot sites such as ports and marinas were included in some geographical areas (the Slovenian, the Greek, and the Romanian coast).
Location of the sampling sites at different areas in the Eastern Mediterranean and Black Sea. Scale bar: 5 km. SL Slovenian coast, IT Italy (Lagoon of Venice and Apulian coast), EL Greece (Saronikos Gulf), RO Romania coast, CY Cyprus southeast coast, RUS Russian coast, R reference sites, S impacted sites
In each geographical area, animals of similar size (Table 1) were collected between April and May 2013. Across all the areas, the length of M. galloprovincialis ranged between 4 and 8 cm. The length of B. pharaonis and R. venosa ranged from 2 to 4 cm and from 4 to 7 cm, respectively. The length of the fish species D. sargus sargus and M. surmuletus ranged from 25 to 29 cm and from 39 to 54 cm, respectively. Molluscs (M. galloprovincialis, B. pharaonis, and R. venosa) were collected by hand (including while diving). Fish were collected by spearfishing (D. sargus sargus) or trawling nets (M. surmuletus). Forty to 45 animals of each species were collected from each site (except B. pharaonis where 115 animals were collected per site due to the small size of individuals). The animals were transferred to the laboratory within a few hours of sampling in moist and cool conditions (molluscs) or in aerated cooled containers with seawater from the sampling site (fish). Whole soft tissues of molluscs were stored at −20 °C for condition index measurements (10 to 15 individuals per site). Fish were anesthetized on ice and sacrificed by decapitation. Fish weight and length were recorded for condition factor determination (15 individuals per site). Gills and digestive glands of molluscs and muscle and liver of fish were sampled for biomarker analyses (30 individuals per site). Tissue samples were pooled (samples of six individuals) and five pooled samples per site were frozen in liquid nitrogen. Due to the small size of B. pharaonis, tissues of 20 individuals were pooled in each sample (five pooled samples per site). Samples were stored at −80 °C and were transported in dry ice to the Hellenic Center for Marine Research (HCMR), Greece, where the biomarker analyses were performed.
To characterize the study sites in terms of chemical pollution, existing data on metal, polycyclic aromatic hydrocarbon (PAHs), and polychlorinated biphenyl (PCBs) concentrations in sediments and in sentinel species (mussels and/or fish) were compiled from the literature. At areas where literature data on contaminant levels in biota from the selected study sites was scarce (Greek coast—Saronikos Gulf, Cyprus southeast coast, and Romanian coast—Constanta), additional samples of animals (mussels M. galloprovincialis and B. pharaonis) were collected for metals, PAHs, and PCBs analyses. The whole soft tissues of the mussels were stored at −20 °C until chemical analyses. Chemical analyses were performed at HCMR (M. galloprovincialis samples from Greek coast—Saronikos Gulf; PAHs and PCBs—pooled sample of 20 individuals per site; metals—6 pooled samples of 20 individuals per site) and at National Institute for Marine Research and Development (NIMRID), Romania (B. pharaonis samples from Cyprus southeast coast and M. galloprovincialis samples from Romanian coast—Constanta; PAHs, PCBs, and metals—pooled sample of ten individuals per each site).
Physiological status of the organisms
Condition index (CI) was determined as an indicator of the physiological status of the molluscs. CI is an ecophysiological measure of the health status of the animals that summarizes their physiological activity (e.g., growth, reproduction, secretion) under given environmental conditions (Lucas and Beninger 1985). The whole soft tissues of 10 to 15 individuals were dissected and lyophilized; shells were dried at 60 °C for 48 h and then weighed. The ratio of dry flesh weight to dry shell weight (FW/SW × 100) was used to determine CI for each sample (Davenport and Chen 1987).
Condition factor (CF) was determined as an indicator of the physiological status of the fish. CF is influenced by factors such as the nutritional and reproductive status, thus leading to weight variations (Rätz and Lloret 2003). CF was calculated as CF = 100 × total weight/total length3 (Nash et al. 2006).
Biochemical biomarker analyses
Catalase and glutathione S-transferase activity
Digestive glands (molluscs) and liver (fish) were homogenized using a Potter-Elvehjem homogenizer (Heidolph Electro GmbH, Kelheim, Germany) in 1:5 (w/v) 100 mM KH2PO4/K2HPO4, pH 7.4. Homogenates were centrifuged at 10,000×g for 30 min. All preparation procedures were carried out at 4 °C. CAT activity was measured through the loss of H2O2 that was measured colorimetrically with ferrous ions and thiocyanate on a microplate reader (Assys Digiscan reader 340) (Cohen et al. 1996). CAT activity was determined by the difference in the absorbance at 490 nm per unit of time. CAT activity results are expressed in terms of the first-order reaction rate constant (k) and protein content as follows: U/mg proteins = k/mg proteins = [ln (A 1/A 2)/(t 2 − t 1)]/mg proteins where U represents units, ln is the natural log, and A 1 and A 2 are the observed mean absorbance at 490 nm at two time points, t 1 = 1 min and t 2 = 4 min. GST was measured by the method of Habig and Jacoby (1981) with 1-chloro-2,4-dinitrobenzene (CDNB) as a conjugation substrate, adapted to microplate reading by McFarland et al. (1999). Activity was expressed as nanomoles of conjugate per minute per milligram of proteins. Total protein content in the tissue extracts was measured using bovine serum albumin (BSA) as a standard (Bradford 1976).
Acetylcholinesterase activity
Gill (molluscs), muscle (M. surmuletus), and liver (D. sargus sargus) tissues were homogenized using a Potter-Elvehjem homogenizer in 1:2 (w/v) 0.1 M Tris–HCl buffer containing 0.1 % TRITON X 100, pH 7. Homogenates were centrifuged at 10,000×g for 20 min. All preparation procedures were carried out at 4 °C. AChE activity was assayed by the method of Ellman et al. (1961) adapted to microplate reading by Bocquené et al. (1993) on an Assys Digiscan reader 340. Enzyme activity was expressed as nanomoles of acetylthiocholine hydrolyzed per minute per milligram of proteins.
Metallothioneins content
MTs concentration was measured in digestive glands (molluscs) and liver (fish) tissues according to Viarengo et al. (1997) on a Perkin Elmer UV/VIS spectrophotometer Lamda 20. The method is based on the estimation of the sulfhydryl content of MTs proteins by spectrophotometric determination of the –SH groups using Ellman’s reagent. MTs concentration was calculated utilizing reduced glutathione (GSH) as a reference standard and expressed as micrograms of MTs per gram of wet weight of tissue.
Chemical analyses in mussel tissues
Metal analyses were performed according to UNEP (1984) and IAEA-MEL (1999). The following metals were analyzed: Cd, Cu, Pb, Cr, and Zn. The accuracy and precision of the analytical methodology were verified with the standard reference material SRM 2976 which was provided by the National Institute of Standards and Technology—USA (NIST). The methodology used for PAH and PCB analysis at HCMR was described in Tsangaris et al. (2011a) PAH and PCB concentrations at NIMRD were determined according to IAEA-MEL (1995). The accuracy and precision of the analytical methodology was tested using certified reference material provided by IAEA (IAEA—432, mussel homogenate) (HCMR) and NIST (SRM—2977, mussel homogenate) (NIMRD). ΣPAHs: acenaphthene, acenaphthylene, anthracene, benzo[a]anthracene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo(g,h,i)perylene, crysene, dibenzo(a,h)anthracene, fluoranthene, fluorine, indeno(1,2,3-c,d)pyrene, naphthalene, phenanthrene, and pyrene, and ΣPCBs: 28-CB, 52-CB, 101-CB, 118-CB, 138-CB, 153-CB, and 180-CB, were determined.
Data and statistical analysis
To compare the total metal content on both sediment and biological matrices across the study areas, the metal pollution index (MPI) (Usero et al. 1996) was calculated for both sediments and organisms:
where M n is the concentration of metal n expressed in micrograms per gram of dry weight.To assess for significant changes in the response of the different biomarkers and physiological indices (condition index and condition factor), univariate analysis of variances (ANOVA) followed by Fisher’s LSD multiple comparison test was applied comparing the values recorded at the reference site with those of the impacted sites in each geographical area. Prior to the analysis, data were checked for normality (Shapiro-Wilk’s test) and homogeneity of variances (Levene’s test). The variability of each biomarker was graphically expressed as percent alteration with respect to the reference site in each area, calculated according to the following formula:
Principal component analysis (PCA) was performed using two different data matrices: (1) percent alteration of biomarker and CI or CF values with respect to the reference site in each area for a better comparison of the variability obtained in each geographical area and sentinel species, and (2) biomarker and CI data obtained in mussel M. galloprovincialis, for a comparison of variability of levels and responses to pollution in this widely used sentinel species among geographical areas. STATISTICA 6.0 software (StatSoft) was used for all statistical processing.
The “Integrated Biological Response version 2” (IBRv2) index was applied to integrate biomarker data into a value representing the environmental stress level at the impacted sites in the various geographical areas (Sanchez et al. 2013). It is a modification of the “Integrated Biomarker Response” (IBR) index (Beliaeff and Burgeot 2002) based on the reference deviation concept, i.e., the deviation between a disturbed and an undisturbed state. The four biochemical biomarkers measured (CAT, AChE, and GST activities and MTs content) were introduced in the IBRv2 calculation. For the calculation of IBRv2, in each geographical area, individual biomarker data (X i) were compared to reference data (X 0) and a log transformation was applied to reduce variance: Y i = log X i/X 0. For each biomarker, the general mean (μ) and standard deviation (s) of Y i for all sites were computed and Y i was standardized as Z i = (Y i − μ)/s. Τhe mean of standardized biomarker response (Z i) and mean of reference biomarker data (Z 0) were used to define a biomarker deviation index (A), A i = Z i − Z 0 for each biomarker in each site. Then, to obtain the IBRv2, the absolute value of A parameters calculated for each biomarker in each site were summed as IBRv2 = Σ│Α│
Results
Contaminants in sediments and in sentinel species
Levels of metals, PAHs, and PCBs in sediments and in tissues of mussel and/or fish species at all sampling sites are presented in Tables 2 and 3, respectively. ERL values (effect range-low) proposed by Long et al. (1995) for chemicals in sediments are also reported in Table 2. In some cases, due to shortage of recent data in the literature, old data was used in an attempt to provide an indicative picture of the type of pollution at the sampling sites even if an in-depth chemical characterization was not possible. Overall, comparison of contaminant levels in sediments and in organisms between sites within each geographical area confirmed higher contamination at the impacted sites compared to the reference ones. Three reference sites were particular as regards contaminant levels in organisms, where even if several contaminants showed markedly lower levels than the impacted sites, this was not the case for all the contaminants analyzed, i.e., Cu, Pb, and Cr in mussels from Ca’ Roman (IT_Ref1) in the Italian coast, PCBs in mussels from East Constanta (RO_Ref) in the Romanian coast, and Cd, Pb, and Cr in mussels from Ayia Napa (CY_Ref1) in the Cyprus measured southeast coast. These sites are regarded as local reference sites (Moschino et al. 2011, 2012; Coatu et al. 2013) and were thus selected as such in the present study.
The most contaminated sediments, particularly by metals, were found at the Bay of Koper (where Cu, Zn, and Hg exceeded the ERL values), Bay of Strunjan, and Bay of Piran (where Hg and Cr exceeded the ERL values) on the Slovenian coast; at Perama Bay and Marina Zeas on the Greek coast (where Cu, Pb, Cr, and Zn exceeded the ERL values); and at South Constanta on the Romanian coast (where Cd, Cu, Pb, and Cr exceeded the ERL values). Higher metal concentrations were detected in mussels from Perama Bay and Marina Zeas, Greece, and from Blue Bay, Russia. These observations are also confirmed by the MPI values calculated for both sediments and organisms (Tables 2 and 3).
Biological responses
Mean values (±SE) of the biochemical biomarkers measured in different sentinel species are listed in Table 4. CAT activity showed low variability in the mussel M. galloprovincialis (ranging from 1.6 to 5.0 U/mg proteins), as well as in the veined whelk R. venosa (2.4–3.7 U/mg proteins). Higher values were observed in the fish species M. surmuletus and D. sargus sargus (10.2–11.2 U/mg proteins). The highest CAT activity was observed in the mussel B. pharaonis, with values ranging from 31.2 to 38.8 U/mg proteins. AChE activity in mussels M. galloprovincialis across areas ranged from 13 to 88 nmol/min/mg proteins. The highest AChE activity was detected in R. venosa (from 286.2 to 289.8 nmol/min/mg proteins) and in both fish species (from 171.1 to 295.3 nmol/min/mg proteins). B. pharaonis showed the lowest AChE activities (9.0–10.6 nmol/min/mg proteins). Particularly low GST activity was detected in M. galloprovincialis collected in the Slovenian coast (3.7–5.3 nmol/min/mg proteins), whereas mussels from the other studied areas showed higher values (34.1–83.4 nmol/min/mg proteins). The highest GST activity was exhibited by D. sargus sargus (265–314 nmol/min/mg proteins) and R. venosa (320–389 nmol/min/mg proteins). MTs content values were particularly low in M. galloprovincialis from the Lagoon of Venice (73–78 μg/g tissue) in comparison with those observed in mussels from the other geographical areas (130–251 μg/g tissue). The highest MTs content values were detected in D. sargus sargus (328 and 428 μg/g tissue in reference and impacted sites, respectively).
Comparisons between impacted and reference sites within each geographical area showed significantly lower CAT activities in M. galloprovincialis and R. venosa from the Slovenian, Greek, and Russian impacted sites (Fig. 2a). Significantly lower AChE activities at the impacted sites with respect to the reference sites were observed in mussels from the Slovenian, Greek, Italian, and Romanian coasts (Fig. 2b). On the contrary, fish from the Italian coast showed significantly higher AChE activity at the impacted compared to the reference site (Fig. 2b). Significant variations in GST between impacted and reference sites were found in mussels from the Slovenian, Greek, and Italian coasts, with higher activities at the three Greek impacted sites and lower activities at the Slovenian and Italian impacted sites (Fig. 2c). Significantly higher GST activity with respect to the reference site was also observed in fish from the impacted site in the Cyprus coast. MTs content was less variable in comparison with enzymatic activities in mussels (Fig. 2d). Significant differences in MTs content of mussels in comparison to the reference sites were observed only at SL_S2 and EL_S2 in the Slovenian and Greek coast, respectively. A markedly higher MTs content at the impacted site with respect to the reference (+231 %) was detected in R. venosa from the Russian coast.
Catalase activity (a), acetylcholinesterase activity (b), glutathione S-transferase activity (c), and metallothioneins content (d), expressed as % alteration with respect to each reference site. SL Slovenian coast, EL Greece (Saronikos Gulf), IT Italy (Lagoon of Venice and Apulian coast), RO Romania coast, CY Cyprus southeast coast, RUS Russian coast, MG Mytilus galloprovincialis, BP Brachidontes pharaonis, RV Rapana venosa, MS Mullus surmuletus, DSS Diplodus sargus sargus. ANOVA: *p < 0.05; **p < 0.01; ***p < 0.001. Shapes denote type of expected response to pollution; bell: bell shaped, downward pointing arrow: inhibition, upward pointing arrow: induction
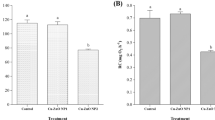
CI values of molluscs and CF values of fish (mean ± SE), and the statistical comparison between impacted and reference sites within each geographical area, are shown in Figs. 3 and 4, respectively. CI values in M. galloprovincialis showed high variability ranging from 4.5 at EL_Ref in the Greek coast to 23.7 at SL_S2 in the Slovenian coast. CI values of molluscs were significantly lower than at the reference sites at SL_S3, IT_S1, and RUS_S1, whereas at all the Greek sites and in CY_S1 they were significantly higher than at the reference sites. On the contrary, the comparison between CF values of fish did not exhibit statistically significant differences (Fig. 4).
Condition index (mean ± SE), calculated as (dry meat weight/dry shell weight) × 100, detected in molluscs (Mytilus galloprovincialis, Brachidontes pharaonis, and Rapana venosa) at the various sampling locations. SL Slovenian coast, EL Greece (Saronikos Gulf), IT Italy (Lagoon of Venice), RO Romania coast, CY Cyprus southeast coast, RUS Russian coast, Ref reference sites, S impacted sites. ANOVA: *p < 0.05; **p < 0.01; ***p < 0.001
The multivariate analysis performed on the dataset obtained from the percentage alteration of the biomarkers and CI or CF in the different geographical areas shows that factor 1 and factor 2 explain over 65 % of total variance in the data matrix (Fig. 5). Factor 1 explains 37.33 % of total variance and is characterized by negative loading of the variables CI/CF (−0.90). Factor 2 explains 27.86 % of total variance with CAT and AChE showing the higher loading values (0.81 and 0.79, respectively). The distribution of the biological alteration detected in the various sentinel organisms on the cases score plot highlights the separation of mussels M. galloprovincialis and B. pharaonis from the two fish species, M. surmuletus and D. sargus sargus, together with mussels collected along the Romanian coast in the upper right part of the plot, as well as from the gastropod R. venosa in the left part of the plot. In the multivariate analysis performed with the M. galloprovincialis biomarkers and CI dataset, factor 1 and factor 2 explain over 80 % of total variance in the data matrix (Fig. 6). Factor 1 explains 58.8 % of total variance and is characterized by positive loading of the variable CI (0.88) and negative loading of the variables CAT and GST (−0.90 and −0.88, respectively). Factor 2 explains 22.12 % of total variance and is characterized by negative loading of the variable MT (−0.88). The geographical variability in mussel biomarkers is higher than the variability between impacted and reference sites within each location, with the single exception of EL_S2 (Marina Zeas, Greece).
PCA performed with the data obtained from the % alteration with respect to each reference site of each biomarker (CAT, AChE, GST, MTs) and condition index/factor in the various geographical areas. SL Slovenian coast, EL Greece (Saronikos Gulf), IT Italy (Lagoon of Venice and Apulian coast), RO Romanian coast, CY Cyprus southeast coast, RUS Russian coast, S impacted sites
PCA performed with the data of mussel Mytilus galloprovincialis biomarkers (CAT, AChE, GST, MTs) and condition index (CI) from the sampling sites in the various geographical areas. SL Slovenian coast, EL Greece (Saronikos Gulf), IT Italy (Lagoon of Venice), RO Romanian coast, Ref reference sites, S impacted sites
The values of the IBRv2 index ranged between 1.8 and 7.0 (Fig. 7). Sites showing IBRv2 values of 3 or lower (indicating lower stress levels) were the CY_ S1, CY_S2, and IT-S2. Five sites showed values between 3.7 and 5.3: SI_S1, SL_S3, EL_S1, EL_S3, and IT_S1. The highest values (≥6) were observed at SL_2, EL_ S2, and RUS_S1.
IBRv2 calculated for biomarkers (CAT, AChE, GST, and MTs) measured in molluscs (Mytilus galloprovincialis, Brachidontes pharaonis, and Rapana venosa) and fish (Mullus surmuletus, Diplodus sargus sargus) from the various sites in Eastern Mediterranean and Black Sea coastal areas. SL Slovenian coast, EL Greece (Saronikos Gulf), IT Italy (Lagoon of Venice and Apulian coast), RO Romania coast, CY Cyprus southeast coast, RUS Russian coast, MG M. galloprovincialis, BP B. Pharaonis, RV R. Venosa, MS M. surmuletus, DSS D. sargus sargus
Discussion
The suite of biochemical biomarkers in the well-established sentinel species M. galloprovincialis revealed responses of CAT, AChE, and GST activities at the impacted sites across the different geographical areas and MTs responses only at two sites exceeding ERL guidelines values for metals. CAT and AChE responses were consistent across areas showing lower enzymatic activities at the impacted sites, whereas GST showed either lower or higher activities at the impacted sites compared to the reference ones. CAT is an antioxidant enzyme that detoxifies hydrogen peroxide (H2O2), the main cellular precursor of the hydroxyl radical (HO•), a highly reactive and toxic form of reactive oxygen species (ROS) involved in oxidative stress, which may cause lipid, protein, and DNA damage (Kehrer 2000). CAT is widely applied as a biomarker of oxidative stress that can be induced by exposure to organic xenobiotics and metals (Livingstone 2001). The enzyme response to pollutants shows a bell-shaped trend, with an initial increase in activity due to the activation of enzyme synthesis followed by a decrease in enzymatic activity, due to the enhanced catabolic rate and/or a direct inhibitory action of toxic chemicals on the enzyme molecules (Viarengo et al. 2007). Thus, high CAT activities found in mussels and fish at polluted sites are considered an adaptive response to ROS-inducing contaminants (Roméo et al. 2003; Cappello et al. 2013; Jebali et al. 2013) whereas low CAT activities at polluted sites are linked with increased susceptibility to oxidative stress (Regoli et al. 2004; Pampanin et al. 2005a; Tsangaris et al. 2011b; Oliva et al. 2012). Accordingly, the low CAT activities observed in this study in mussels collected from the impacted sites suggest oxidative stress experienced by these animals.
AChE is an enzyme involved in nerve impulse transmission, and its inhibition is an established biomarker of neurotoxicity (Fulton and Key 2001). Although organophosphate and carbamate pesticides are the two main classes of compounds ascribed for AChE inhibition (Fulton and Key 2001), it has also been shown that other chemicals can interact with AChE activity, such as metals, detergents, and PAHs (Lionetto et al. 2013). Thus, low AChE activities are frequently found in mussels and fish at impacted sites in various regions under different types of pollution (Lehtonen et al. 2006; Jebali et al. 2013; Bellas et al. 2014) which is in agreement with the present study.
GST response to toxic chemicals follows a bell-shaped profile (Viarengo et al. 2007) and consequently increased and/or decreased GST activities are reported in specimens from impacted areas (Roméo et al. 2003; Regoli et al. 2004; Bebianno et al. 2007; Turja et al. 2014). GST is induced by organic contaminants as part of the phase II biotransformation pathway whereas GST inhibition has been reported as a more non-specific response to chemicals (Regoli et al. 2003). Thus, the GST induction observed in mussels from the Greek impacted sites may actually be due to higher concentrations of organic pollutants, as highlighted by chemical data both in the sediments and biota (Tables 2 and 3), whereas the inhibition detected in the Lagoon of Venice and Slovenian coasts might be associated with higher levels of metals, particularly Cd, Cu, and Zn.
MTs are low molecular weight, cysteine-rich proteins that play a primary role in the homeostasis of essential metals such as Cu and Zn, and in metal detoxification, as they act as chelating agents for intracellular excesses of nonessential metals, such as Ag, Cd, and Hg (Amiard et al. 2006). Their induction is therefore considered as a biomarker of metal contamination and is widely used as a tool in biomonitoring programs (Viarengo et al. 2007; Thain et al. 2008). In the present study, the lack of response of MTs in most areas can be attributed to low metal concentrations, even distribution of metals among sites or natural confounding factors (Marigómez et al. 2013; Zorita et al. 2007) and interactions with other chemicals such as PAHs (Benedetti et al. 2015) which could reduce the effect of metals on MT induction.
The fish sentinel species used in this study (D. sargus sargus and M. surmulletus) showed higher enzymatic activities compared to mussels in accordance with previous studies (Lionetto et al. 2003; Kopecka et al. 2006); however, they did not generally exhibit differences in biomarker levels between the impacted and reference sites. This lack of response, which contradicts the general expectation that fish are more sensitive than molluscs, particularly as regards AChE inhibition (Monserrat et al. 2007; Viarengo et al. 2007), is not easily explained, as it has not been possible to undertake an in-depth characterization of the chemicals present in the areas in which the fish were collected. However, our results on D. sargus sargus biomarkers from the Apulian coast (Italy) are in agreement with a previous study in this area by Lionetto et al. (2003) that failed to reveal differences in AChE and CAT activities of fish Mullus barbatus from the same sites. These authors attributed the lack of AChE response to the fact that fish were sampled offshore where the distribution of chemicals can be different compared to inshore sites, and this can also be the case in the present study. With regard to the results on M. surmulletus from the coast of Cyprus, the absence of biomarker responses possibly reflects no significant pollution levels in the area (DFMR 2012) highlighted by the similarly low MPI in fish from both sites (MPI = 0.1, Table 3).
R. venosa whelks were used in the Black Sea since mussel populations in its northern extent have been decreasing over the last 20 years (Gudimov 2008), whereas whelks are widespread and present even in localities where no mussels are remaining. To our knowledge, this is the first time these biochemical biomarkers have been measured in R. venosa. This species has been proposed as a promising indicator for monitoring metal contamination as it has shown high bioaccumulation capacity of Cd and Ni (Liang et al. 2004). Although AChE and GST enzyme activities were relatively high in the whelks and comparable to those observed in fish, only CAT activity showed a significant response at the impacted site. Interestingly, R. venosa is the only species used in the present study showing a strong induction in MTs content in impacted compared to the reference site. This MTs induction is consistent with the metal bioaccumulation information reported from the Blue Bay and Tuzla Spit and the large differences between the two sites in MPI values (MPI = 27.3 and 3.8, respectively).
The condition index in molluscs and the condition factor in fish were used as supporting parameters indicative of the trophic status and reproductive condition of the organisms. The condition index is considered as a useful tool to assess the nutritive status of bivalves and has been widely used to characterize “fitness” of cultured stocks (Lucas and Beninger 1985). Similarly, the condition factor is used to assess the condition and well-being in fish (Rätz and Lloret 2003) reflecting feeding intensity, age, and growth rates. The high condition indices found in mussels at the more contaminated sites in the Greek and Levantine coasts could be explained by the observation that polluted sites might be characterized by high nutrient loads and consequently might be highly productive environments (Meneghetti et al. 2004). Higher condition index in mussels at polluted sites can be also due to the presence of increased organic matter in the environment (Benali et al. 2015). On the other hand, both condition index and condition factor can be negatively affected by exposure to pollutants (Pampanin et al. 2005b; Kopecka et al. 2006). Thus, low condition index at the impacted sites in the Slovenian, Italian, and Russian coasts could be due to contaminant exposure.
Multivariate analysis on percent alteration of biomarker and CI/CF data with respect to reference sites highlighted similar distribution patterns on the score plot for M. galloprovincialis and B. pharaonis indicating similar biomarker response patterns. R. venosa showed a clear spatial separation from mussels mostly due to a strong influence of the condition index and also of the MTs response. Similar responses were observed in the two fish species, D. sargus sargus and M. surmuletus, and in the mussels from the Romanian coast.
The PCA analysis on M. galloprovincialis biomarker and CI data distinguished sites by geographical area, reflecting the variability in biomarker values among the different areas. It is widely acknowledged that biomarkers are influenced by natural environmental factors such as temperature, salinity, oxygen tension, trophic status as well as size, age, and reproductive condition of the sentinel organisms (Hagger et al. 2006; Holmstrup et al. 2010). Thus, in line with our results, large-scale studies show differences in biomarker ranges and baseline biomarker values among geographical areas that are mainly attributed to difference in temperature, salinity, and trophic status (Lehtonen et al. 2006; Gagné et al. 2008). This pattern complicates the establishment of baseline levels and consequently the definition of assessment criteria for several biomarkers has to be set at the level of regions (Bellas et al. 2014). Currently, background assessment criteria (BAC) and environmental assessment criteria (EAC) have been proposed for only a few biomarkers and regional areas, for example AChE in M. galloprovincialis for West Mediterranean Sea and Atlantic Ocean areas (Davies and Vethaak 2012). To our knowledge, baseline levels and assessment criteria for the biomarkers applied in the present study are not available for the Eastern Mediterranean Sea and Black Sea.
The use of indices to summarize biomarker responses for the evaluation of contaminant-induced stress has been increasingly employed, and this approach is useful from an environmental management perspective (Beliaeff and Burgeot 2002; Broeg and Lehtonen 2006; Hagger et al. 2009). In this study, the IBRv2 index based on the reference deviation concept was applied to integrate biomarker responses into a stress index (Sanchez et al. 2013). Preferably, calculation of the IBRv2 index should use established baseline levels of the individual biomarkers (Sanchez et al. 2013); however, an alternative approach is the use of values measured at the reference sites (Olivares-Rubio et al. 2013), and this approach was applied to assess the individual biomarker responses in this study. In this case, the suitability of the reference site is a key factor for the evaluation of biomarker responses and the IBRv2 index. Despite well-recognized uncertainties related with comparisons between impacted and reference sites, there are currently no intention/initiatives towards the standardization of reference sites at the regional level. The selection of investigated sites depends on the expert knowledge of researchers and is based on previous data. The use of common standardized reference sites, preferably several sites in a region, could minimize uncertainties, increase the robustness of the index, and allow comparisons at large spatial scales. In the present study, although IBRv2 index results did not fully correspond to the characterization of the sites with regard to contaminant levels, the highest stress levels were found at three of the sites characterized as most contaminated, i.e., SL_2 in the Bay of Strunjan, EL_S2 in Marina Zeas, and RUS_S1. Furthermore, chemical characterization of the sites in the present study was indicative and based on certain classes of chemicals, while the presence of additional contaminants, which could influence the stress response, cannot be excluded.
In conclusion, among the biomarkers applied, the present study showed responses of AChE, CAT, and GST activities at sites described as impacted in different geographical areas in the mussels M. galloprovincialis, suggesting usefulness for assessing pollution effects in large-scale monitoring. B. pharaonis mussels seemed to follow a similar biomarker response pattern as M. galloprovincialis. Among the alternative sentinel species used, only R. venosa showed marked responses of CAT activities and MTs content. In the absence of established baseline levels for the applied biomarkers in the study regions, the approach based on the reference deviation concept was useful for the interpretation of biomarker results. Results contribute to the assessment of pollution effects in the study areas and are expected to be useful in future biomonitoring programs as well as environmental risk assessments in these regions.
References
Amiard J-C, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76:160–202
ARSO (2012) Common database on monitoring of water quality data. Environmental Agency of the Republic Slovenia http://kazalci.arso.gov.si/?data=indicator&ind_id=69
ARSO (2013) Common database on monitoring of water quality data. Environmental Agency of the Republic Slovenia http://kazalci.arso.gov.si/?data=indicator&ind_id=69
ARSO (2015) Common database on monitoring of water quality data. Environmental Agency of the Republic Slovenia http://kazalci.arso.gov.si/?data=indicator&ind_id=69
Bajt O (2012) Aliphatic and polycyclic aromatic hydrocarbons in sediments of the Slovenian coastal area (Gulf of Trieste, northern Adriatic). Environ Monit Assess 184:7439–7452
Bebianno MJ, Lopes B, Guerra L, Hoarau P, Ferreira AM (2007) Glutathione S-transferases and cytochrome P450 activities in Mytilus galloprovincialis from the South coast of Portugal: effect of abiotic factors. Environ Int 33:550–558
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21:1316–1322
Bellas J, Albentosa M, Vidal-Liñán L, Besada V, Franco MA, Fumega J, González-Quijano A, Viñas L, Beiras R (2014) Combined use of chemical, biochemical and physiological variables in mussels for the assessment of marine pollution along the N-NW Spanish coast. Mar Environ Res 96:105–117
Benali I, Boutiba Z, Merabet A, Chèvre N (2015) Integrated use of biomarkers and condition indices in mussels (Mytilus galloprovincialis) for monitoring pollution and development of biomarker index to assess the potential toxic of coastal sites. Mar Pollut Bull doi:10.1016/j.marpolbul.2015.03.041
Benedetti M, Giuliani ME, Regoli F (2015) Oxidative metabolism of chemical pollutants in marine organisms: molecular and biochemical biomarkers in environmental toxicology. Ann NY Acad Sci 1340:8–19
Bocquené G, Galgani F, Burgeot T, Le Dean L, Truquet P (1993) Acetylcholinesterase levels in marine organisms along French coasts. Mar Pollut Bull 26:101–106
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 772:248–264
Broeg K, Lehtonen KK (2006) Indices for the assessment of environmental pollution of the Baltic Sea coasts: integrated assessment of a multi-biomarker approach. Mar Pollut Bull 53:508–522
BSC (2008). State of the Environment of the Black Sea (2001–2006/7). Edited by Temel Oguz. Publications of the Commission on the Protection of the Black Sea Against Pollution (BSC) 2008-3, Istanbul, Turkey, 448 pp
Cappello T, Maisano M, D’Agata A, Natalotto A, Mauceri A, Fasulo S (2013) Effects of environmental pollution in caged mussels (Mytilus galloprovincialis). Mar Environ Res 91:52–60
Coatu V, Oros A, Tiganuş D (2013) Contamination indicators in Report on the state of the marine and coastal environment in 2012. Cercet Mar 43:46–75, ISSN 0250–3069
Cohen G, Kim M, Ogwu V (1996) A modified catalase assay suitable for a plate reader and for the analysis of brain cell cultures. J Neurosci Methods 67:53–56
Corsi I, Mariottini M, Menchi V, Sensini C, Balocchi C, Focardi S (2002) Monitoring a marine coastal area: use of Mytilus galloprovincialis and Mullus barbatus as bioindicators. Mar Ecol 23(Supplement 1):138–153
Database of the State Oceanographic Institute http://esimo.oceanography.ru/esp2/index/index/esp_id/10/section_id/8/menu_id/4426
Davenport J, Chen X (1987) A comparison of methods for the assessment of condition in the mussel (Mytilus edulis L.). J Molluscan Stud 53:293–297
Davies IM, Vethaak AD (2012) Integrated marine environmental monitoring of chemicals and their effects. ICES Coop Res Rep No 315, 277 pp
Dell’Anno A, Mei ML, Pusceddu A, Danovaro R (2002) Assessing the trophic state and eutrophication of coastal marine systems: a new approach based on the biochemical composition of sediment organic matter. Mar Pollut Bull 44:611–622
DFMR (2012) Initial assessment of the marine environment of Cyprus—part I—characteristics. Implementation of article 8 of the marine strategy framework-directive (2008/56/EC). Department of Fisheries and Marine Research, Republic of Cyprus, pp 38
Ellman G, Courtney KD, Jr Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fulton MH, Key PB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 20:37–45
Gagné F, Burgeot T, Hellou J, St-Jean S, Farcy É, Blaise C (2008) Spatial variations in biomarkers of Mytilus edulis mussels at four polluted regions spanning the Northern Hemisphere. Environ Res 107:201–217
Galloway TS, Brown RJ, Browne MA, Dissanayake A, Lowe D, Jones MB, Depledge HM (2004) Ecosystem management bioindicators: the ECOMAN project—a multibiomarker approach to ecosystem management. Mar Environ Res 58:233–237
Giltrap M, Ronan J, Hardenberg S, Parkes G, McHugh B, McGovern E, Wilson JG (2013) Assessment of biomarkers in Mytilus edulis to determine good environmental status for implementation of MSFD in Ireland. Mar Pollut Bull 71:240–249
Gudimov AV (2008) Marine Mussels of the Karadag (Black Sea): population decay, ecology, and physiological adaptations. Dokl Biol Sci 422:330–332
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405
Hagger JA, Jones MB, Leonard DRP, Owen R, Galloway TS (2006) Biomarkers and integrated environmental risk assessment: are there more questions than answers? Integr Environ Assess Manage 2:312–329
Hagger JA, Galloway TS, Langston W, Jones MB (2009) Application of biomarkers to assess the condition of European Marine Sites. Environ Pollut 157:2003–2010
HCMR (2003) In: Zeri C (ed) Record and assessment of the state of the marine environment in marina Zeas. Final report. Hellenic Center for Marine Research, Anavyssos, p 19
Holmstrup M, Bindesbøl A-M, Janneke Oostingh G, Dusch A, Scheil V, Köhler HR, Loureiro S, Soares AMVM, Ferreira ALG, Kienle C, Gerhardt A, Laskowski R, Kramarz PE, Bayley M, Svendsen C, Spurgeon DJ (2010) Interactions between effects of environmental chemicals and natural stressors: a review. Sci Total Environ 408:3746–3762
Hyne RV, Maher WA (2003) Invertebrate biomarkers: links to toxicosis that predict population decline. Ecotox Environ Saf 54:366–374
IAEA-MEL (1999) Standard operating procedures for trace metals determination
IAEA-MEL/Marine Environmental Studies Laboratory (1995) Training manual on the measurement of organochlorine and petroleum hydrocarbons in environmental samples
Ianni C, Ruggieri N, Frache R (2003) Distribution and speciation of heavy metals in Apulian coastal sediments. Toxicol Environ Chem 85:169–182
Jebali J, Sabbagh M, Banni M, Kamel N, Ben-Khedher S, M’hamdi N, Boussetta H (2013) Multiple biomarkers of pollution effects in Solea solea fish on the Tunisia coastline. Environ Sci Pollut Res 20:3812–3821
Kaberi H, Zeri C (2004) Heavy metal distribution in the inner Saronikos Gulf: an area affected by the Athens WWTP outfall—Greece, 9th FECS Conference—Behaviour of Chemicals in the Environment, Abstracts, p.430.
Kehrer JP (2000) The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149(1):43–50
Kopecka J, Lehtonen KK, Baršienė J, Broeg K, Vuorinen PJ, Gercken J, Pempkowiak J (2006) Measurements of biomarker levels in flounder (Platichthys flesus) and blue mussel (Mytilus trossulus) from the Gulf of Gdan’sk (southern Baltic). Mar Pollut Bull 53:406–421
Laboratory Network of the Environmental Quality monitoring of the Hellenic Seas, 2006. Environmental Quality Monitoring Program of the Hellenic Seas, Final Technical Report, Scoullos M. (ed) Athens
Lazar L, Boicenco L, Coatu V, Oros A, Tiganus D, Mihailov ME (2013) Nutrient levels and eutrophication of the Romanian Black Sea Waters (2006–2011)—assessment related to the marine strategy framework directive implementation. Cercet Mar 43:148–162, ISSN 0250–3069
Lehtonen KK, Schiedek D, Köhler A, Lang T, Vuorinen PJ, Förlin L, Barŝiené J, Pempkowiak J, Gercken J (2006) The BEEP project in the Baltic Sea: overview of results and outline for a regional biological effects monitoring strategy. Mar Pollut Bull 53:523–5377
Liang LN, He B, Jiang GB, Chen DY, Yao ZW (2004) Evaluation of mollusks as biomonitors to investigate heavy metal contaminations along the Chinese Bohai Sea. Sci Total Environ 324:105–113
Lionetto MG, Caricato R, Giordano ME, Pascariello MF, Marinosc L, Schettino T (2003) Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymes activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46:324–330
Lionetto MG, Caricato R, Calisi A. Giordano M.E, Schettino T (2013). Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. BioMed Res Int 2013: art. no. 321213
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:656–666
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Lucas A, Beninger G (1985) The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 187:67–85
Lyons BP, Thain JE, Stentiford GD, Hylland K, Davies IM, Vethaak AD (2010) Using biological effects tools to define Good Environmental Status under the European Union Marine Strategy Framework Directive. Mar Pollut Bull 60:1647–1651
Marigómez I, Zorita I, Izagirre U, Ortiz-Zarragoitia M, Navarro P, Etxebarria N, Orbea A, Soto M, Cajaraville MP (2013) Combined use of native and caged mussels to assess biological effects of pollution through the integrative biomarker approach. Aquat Toxicol 136–137:32–48
McFarland VA, Inouye LS, Lutz C, Jarvis AS, Clarke JU, McCant DD (1999) Biomarkers of oxidative stress and genotoxicity in livers off field-collected brown bullhead, Ameiurus nebulosus. Arch Enviro Contam Toxicol 37:236–241
Meneghetti F, Moschino V, Da Ros L (2004) Gametogenic cycle and variations in oocyte size of Tapes philippinarum from the Lagoon of Venice. Aquaculture 240:473–488
Monserrat JM, Martínez PE, Geracitano LA, Amado LL, Martins MC, Pinho CL, Chaves IS, Ferreira-Cravo M, Ventura-Lima J, Bianchini A (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol C 146:221–234
Moore M, Depledge M, Readman JW, Leonard DRP (2004) An integrated biomarker-based strategy for ecotoxicological evaluation of risk in environmental management. Mutat Res 552:247–268
Moschino V, Delaney E, Meneghetti F, Ros L (2011) Biomonitoring approach with mussel Mytilus galloprovincialis (Lmk) and clam Ruditapes philippinarum (Adams and Reeve, 1850) in the Lagoon of Venice. Environ Monit Assess 177:649–663
Moschino V, Delaney E, Da Ros L (2012) Assessing the significance of Ruditapes philippinarum as a sentinel for sediment pollution: bioaccumulation and biomarker responses. Environ Pollut 171:52–60
Mozetič P, Solidoro C, Cossarini G, Socal G, Precali R, Francé J, Bianchi F, De Vittor C, Smodlaka N, Fonda Umani S (2010) Recent trends towards oligotrophication of the Northern Adriatic: evidence from chlorophyll a time series. Estuar Coast 33:362–375
Nash RD, Valencia AH, Geffen AJ (2006) The origin of Fulton’s condition factor—setting the record straight. Fisheries 31(5):236–238
Oliva M, Vicente JJ, Gravato C, Guilhermino L, Galindo-Riaño MD (2012) Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): seasonal and spatial variation. Ecotox Environ Saf 75:151–162
Olivares-Rubio HF, Martínez-Torres ML, Domínguez-López ML, García-Latorre E, Vega-López A (2013) Pro-oxidant and antioxidant responses in the liver and kidney of wild Goodea gracilis and their relation with halomethanes bioactivation. Fish Physiol Biochem 39:1603–1617
Pampanin DM, Camus L, Gomiero A, Marangon I, Volpato E, Nasci C (2005a) Susceptibility to oxidative stress of mussels (Mytilus galloprovincialis) in the Venice Lagoon (Italy). Mar Pollut Bull 50:1548–1557
Pampanin DM, Volpato E, Marangon I, Nasci C (2005b) Physiological measurements from native and transplanted mussel (Mytilus galloprovincialis) in the canals of Venice. Survival in air and condition index. Comp Biochem Physiol A 140:41–52
Pavlidou A, Kontoyiannis H, Zarokanelos N, Hatzianestis I, Assimakopoulou G, Psyllidou-Giouranovits R (2014) Seasonal and spatial nutrient dynamics in Saronikos Gulf: the impact of sewage effluents from Athens sewage treatment plant. In: A.A. Ansari and S.S. Gill (Eds.) Eutrophication: causes, consequences and control Vol. 2, Springer, 598p.
Pereira P, Carvalho S, Pereira F, de Pablo H, Gaspar MB, Pacheco M, Vale C (2012) Environmental quality assessment combining sediment metal levels, biomarkers and macrobenthic communities: application to the Óbidos coastal lagoon (Portugal). Environ Monit Assess 184:7141–7151
Ramšak A, Ščančar J, Horvat M (2012) Evaluation of metallothioneins in blue mussels (Mytilus galloprovincialis) as a biomarker of mercury and cadmium exposure in the Slovenian waters (Gulf of Trieste): a long-term field study. Acta Adriat 53:71–86
Rätz H-J, Lloret J (2003) Variation in fish condition between Atlantic cod (Gadus morhua) stocks, the effect on their productivity and management implications. Fish Res 60:369–380
Regoli F, Winston GW, Gorbi S, Frenzilli G, Nigro M, Corsi I, Focardi S (2003) Integrating enzymatic responses to organic chemical exposure with total oxyradical absorbing capacity and DNA damage in the European eel Anguilla anguilla. Environ Toxicol Chem 22:56–65
Regoli F, Frenzilli G, Bocchetti R, Annarumma F, Scarcelli V, Fattorini D, Nigro M (2004) Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a field translocation experiment. Aquat Toxicol 68:167–178
Richardson N, Gordon AK, Muller WJ, Whitfield AK (2011) A weight-of-evidence approach to determine estuarine fish health using indicators from multiple levels of biological organization. Aquat Conserv 21:423–432
Roméo M, Hoarau P, Garello G, Gnassia-Barelli M, Girard JP (2003) Mussel transplantation and biomarkers as useful tools for assessing water quality in the NW Mediterranean. Environ Pollut 122:369–378
Sanchez W, Porcher J-M (2009) Fish biomarkers for environmental monitoring within the Water Framework Directive of the European Union. Trac Trend Anal Chem 28:150–158
Sanchez W, Burgeot T, Porcher JM (2013) A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ Sci Pollut Res 20:2721–2725
Ščančar J, Zuliani T, Turk T, Milačič R (2007) Organotin compounds and selected metals in the marine environment of Northern Adriatic Sea. Environ Monit Assess 127:271–282
Seabra Pereira CD, Abessa DM, Choueri RB, Almagro-Pastor V, Cesa A, Maranho LA, Martín-Díaz ML, Torres RJ, Gusso-Choueri PK, Almeida JE, Cortez FS, Mozeto AA, Silbiger HL, Sousa EC, Del Valls TA, Bainy AC (2014) Ecological relevance of sentinels’ biomarker responses: a multi-level approach. Mar Environ Res 96:118–126
Sfriso A, Facca C (2013) Annual growth and environmental relationships of the invasive species Sargassum muticum and Undaria pinnatifida in the lagoon of Venice. Estuar Coast Shelf 129:162–172
Sklivagou E, Varnavas SP, Hatzianestis I, Kanias G (2008) Assessment of aliphatic and polycyclic aromatic hydrocarbons and trace elements in coastal sediments of the Saronikos Gulf, Greece (Eastern Mediterranean). Mar Georesour Geotechnol 26:372–393
Stathopoulou E, Dassenakis M, Skoullos M (2001) Levels of mercury concentration in sediments of the Saronikos Gulf. Proceedings 7th International Conference on Environmental Science and Technology, 3–6 September, Syros, Greece, p 854–860.
Thain JE, Vethaak AD, Hylland K (2008) Contaminants in marine ecosystems: developing an integrated indicator framework using biological-effect techniques. ICES J Mar Sci 65:1508–1514
Tsangaris C, Hatzianestis I, Catsiki VA, Kormas KA, Strogyloudi E, Neofitou C, Andral B, Galgani F (2011a) Active biomonitoring in Greek coastal waters: application of the integrated biomarker response index in relation to contaminant levels in caged mussels. Sci Total Environ 412(413):359–365
Tsangaris C, Vergolyas M, Fountoulaki E, Nizheradze K (2011b) Oxidative stress and genotoxicity biomarker responses in grey mullet (Mugil cephalus) from a polluted environment in Saronikos Gulf, Greece. Ach Environ Con Tox 61:482–490
Tsapakis M, Dakanali E, Stephanou EG, Karakassis I (2010) PAHs and n-alkanes in Mediterranean coastal marine sediments: aquaculture as a significant point source. J Environ Monit 12:958–963
Turja R, Höher N, Snoeijs P, Baršienė J, Butrimavičienė L, Kuznetsova T, Kholodkevich SV, Devier M-H, Budzinski H, Lehtonen KK (2014) A multibiomarker approach to the assessment of pollution impacts in two Baltic Sea coastal areas in Sweden using caged mussels (Mytilus trossulus). Sci Total Environ 473–474:398–409
UNEP (1984) Determination of total Cd, Zn, Pb and Cu in selected marine organisms by atomic absorption spectrophotometry. Reference Methods for Marine Pollution Studies, No 11, Rev 1;1984
Usero J, Gonzalez-Regalado E, Gracia I (1996) Trace metals in the bivalve mollusk Chamelea gallina from the Atlantic coast of Southern Spain. Mar Pollut Bull 32:305–310
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: application to Mediterranean and Antarctic molluscs. Mar Environ Res 44:69–84
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol C 146:281–300
Zorita I, Apraiz I, Ortiz-Zarragoitia M, Orbea A, Cancio I, Soto M, Marigómez I, Cajaraville MP (2007) Assessment of biological effects of environmental pollution along the NW Mediterranean Sea using mussels as sentinel organisms. Environ Pollut 148(1):236–250
Acknowledgments
This study was funded by the PERSEUS (Policy-oriented marine Environmental Research for the Southern EUropean Seas) FP7-OCEAN-2011-287600 Project. The authors greatly appreciate the contribution of E. Papathanassiou in the implementation of this study. We also thank D. Kaparou and L. Bordbar for assistance with biomarker analyses and N. Kouerinis for assistance in chemical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
An erratum to this article can be found at http://dx.doi.org/10.1007/s11356-016-7740-8.
Rights and permissions
About this article
Cite this article
Catherine, T., Vanessa, M., Evangelia, S. et al. Biochemical biomarker responses to pollution in selected sentinel organisms across the Eastern Mediterranean and the Black Sea. Environ Sci Pollut Res 23, 1789–1804 (2016). https://doi.org/10.1007/s11356-015-5410-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5410-x