Abstract
Peanut (Arachis hypogaea L.) genotypes may differ greatly with regard to cadmium (Cd) accumulation, but the underlying mechanisms remain unclear. To determine the key factors that may contribute to Cd re-distribution and accumulation in peanut genotypes with different Cd accumulating patterns, a split-pot soil experiment was conducted with three common Chinese peanut cultivars (Fenghua-6, Huayu-20, and Huayu-23). The growth medium was separated into pod and root zones with varied Cd concentrations in each zone to determine the re-distribution of Cd after it is taken up via different routes. The peanut cultivars were divided into two groups based on Cd translocation efficiency as follows: (1) high internal Cd translocation efficiency cultivar (Fenghua-6) and (2) low internal Cd translocation efficiency cultivars (Huayu-20 and Huayu-23). Compared with Fenghua-6, low Cd translocation cultivars Huayu-20 and Huayu-23 showed higher biomass production, especially in stems and leaves, leading to dilution of metal concentrations. Results also showed that Cd concentration in roots increased significantly with increasing Cd concentrations in soils when Cd was applied in the root zone. However, there were no significant differences in the root Cd concentrations between different pod zone Cd treatments and the control, suggesting that root uptake, rather than pod uptake, is responsible for Cd accumulation in the roots of peanuts. Significant differences of Cd distribution were observed between pod and root zone Cd exposure treatments. The three peanut cultivars revealed higher kernel over total Cd fractions for pod than for root zone Cd exposure if only extra applied Cd was considered. This suggests that uptake through peg and pod shell might, at least partially, be responsible for the variation in Cd re-distribution and accumulation among peanut cultivars. Cd uptake by plants via two routes (i.e., via roots and via pegs and pods, respectively) and internal Cd translocation appear to be important mechanisms in determining Cd accumulation in the kernels of peanuts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peanut (Arachis hypogaea L.) is one of the most important oil crops in the world and represents about 10 % of the world production of oilseeds after soybeans, cottonseed, and rapeseed (Krishna et al. 2015). For a long time, aflatoxin contamination and pesticide residues (mainly daminozide) were the major challenges for safe peanut consumption in many developing counties (Waliyar et al. 2009). More recently, evidence has confirmed that peanut plants are also very susceptible to contamination by cadmium (Cd), a toxic heavy metal that threatens food quality and human health (Pal et al. 2006; Shi et al. 2014). Peanuts prove to be very effective in extracting Cd from soil and in transferring Cd from their roots to aboveground parts and kernels, compared with other oil crops, such as soybean, oil-seed rape, sesame, and sunflower (Wang 2002; Chaudhuri et al. 2003; Angelova et al. 2004). Liu et al. (2005) found that in the polluted area near a lead-zinc mine, Cd concentrations of peanut kernels were also significantly higher than for soybean, red bean, green bean, sorghum, and maize. According to a general survey of peanut production in 2003 for the north of China, the average Cd concentration of peanut kernels was in the range of 0.2–0.3 μg/g (Wan et al. 2005). Mostly, these concentrations could meet the national standard of China, which limits the concentration to 0.5 μg/g in the kernel (GB2762-2005). However, the national non-pollution food standard (NY5303-2005) that limits concentrations to less than 0.05 μg/g was exceeded, and partly, this was also the case for the 0.4 μg/g limit of the national green food standard (NY/T420-2009). Analysis of Cd in peanut kernels revealed that it is predominantly present in proteins and carbohydrates, and that little Cd is found in oil, regardless of whether the peanuts originate from clean or contaminated areas (Carrin and Carelli 2010; Wang and Li 2014). Therefore, if peanuts are used only as a source of cooking oil, Cd contamination is not likely to be hazardous. However, if peanuts are directly consumed, Cd accumulation in the kernels could be a health risk that warrants extra care. Since direct peanut consumption is increasing worldwide, Cd accumulation in peanut kernels is an appropriate concern (Wang and Zhang 2008).
Compared with other cereal crops, knowledge regarding the mechanism of Cd accumulation and re-distribution in peanut, especially into kernels, is limited (Shi et al. 2014; Cheng et al. 2015). An additional complication may be that accumulation of Cd in peanuts differs significantly between different genotypes (Shi et al. 2014). Popelka et al. (1996) found that Cd was mainly extracted by the main roots of peanut plants, while 11 % of Cd in the kernels came from the extraction of pod shells. However, McLaughlin et al. (2000) suggested that only 1–3 % of Cd in the kernels was directly absorbed by pods. For cereal crops, such as wheat and rice, it has been demonstrated that xylem loading and transport (internal translocation), rather than uptake by the roots, was one of the rate-controlling steps for Cd accumulation in the kernels (Stolt et al. 2003; Liu et al. 2007; Uraguchi et al. 2009). Therefore, internal translocation may be a major mechanism for differences in Cd accumulation in peanut kernels. The cause and mechanism of genotypic variation of Cd accumulation and distribution in peanut kernels have not been clearly identified. Bell et al. (1997) proposed that the ability of peanut roots in absorbing Cd from soil was the major mechanism for the genotypic variation of Cd concentration in the kernels, since there was a strong relationship between Cd concentration in kernels and the amount of Cd absorbed by peanut plants in their field trials. Their earlier suggestion was not supported by McLaughlin et al. (2000), who found significant differences between several peanut genotypes in the allocation of Cd to various plant parts but not in the extracted quantity of Cd.
In this paper, three genotypes of peanut widely cultivated in China were investigated to assess the re-distribution and accumulation of Cd, in particular regarding Cd accumulation in the kernels. Our hypothesis is that indeed, root uptake is important for Cd accumulation in the plants, in view of the large root density and large root zone volume and the specific absorption mechanism. Cd accumulation in the kernels, though, may be strongly affected both by direct Cd uptake by the pod shell and by specific characteristics in cadmium re-distribution of peanut cultivars.
Materials and methods
The used soil for the field experiment is a fluvo-aquic moist sandy loam according to the Chinese soil taxonomy system (GSCC), and an aquic ustochrepts soil according to the USDA soil taxonomy (ST). Major soil properties are given in Table 1, which also lists the analytical procedures.
Three peanut genotypes were considered, i.e., Huayu-20, Huayu-23, and Fenghua-6, and these were chosen because they are the major cultivars for commercial production in Shandong province, China. Fenghua-6 is a cultivar with high-protein concentration and middle kernel size which is favored for direct consumption. Huayu-20 is a large kernel size cultivar which is mainly used for export to overseas markets. Huayu-23 is a cultivar with a high-oil concentration and small kernel size. Some properties of these genotypes are quantified in Table 2.
The experiment involved five treatments of Cd exposure, in triplicate. These treatments differ both regarding the concentration of Cd in soil (which was 0, 0.5, or 1.0 μg/g) and whether Cd was applied to the upper soil zone (pod zone) or to the lower soil zone (root zone). Treatments are therefore coded for the control as P0-R0, for the pod zone exposure with P0.5-R0 (with 0.5 μg Cd g−1) and P1.0-R0 (1.0 μg Cd g−1), and for the root zone exposure treatments as P0-R0.5 and P0-R1.0. The choice to limit the concentration to 1.0 μg Cd g−1 for soil was motivated by our intention to avoid phytotoxic Cd concentrations for most contaminated arable soils and focus at Cd accumulation if yields as such are unaffected by Cd.
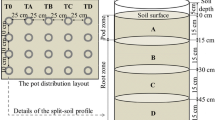
Soil was air-dried, sieved, and then divided into three portions. For the control, no Cd was added, and for the other treatments, analysis grade CdSO4.8/3H2O reagent was added to two portions of the soil, resulting in soil Cd concentrations of 0.5 or 1.0 μg Cd g−1, respectively. Tap water was then applied to the soils until the moisture content was about 70 % of field capacity, and this moisture content was maintained during a 60-day incubation period. The soils were then again air-dried and homogenized. The homogenized soils were then transferred to the experimental pottery pot with a height and inner diameter (ID) of 30 cm. Each pot was filled with 21.0 kg soils, giving a soil layer thickness in the pot of approximately 18 cm (Fig. 1). This soil formed the “root zone.” Analysis grade urea, potassium dihydrogen phosphate, and potassium sulfate were applied to the root zone soil of each pot at a rate of 0.13 g/kg N, 0.05 g/kg P, and 0.14 g/kg K. Tap water was applied to the pots to establish a soil moisture content of 70 % of field capacity.
Seeds of peanuts were soaked in saturated CaSO4 solution for 24 h, after which they were thoroughly rinsed with deionized water and germinated on filter paper moistened with deionized water at 20 °C in the dark. When the radical emerged (approximately 2 cm in length), two seedlings were sown on the soil in the center of each pot. The seeds were covered by 6 cm of soil using the surrounding soil in the pot. This practice was designed to promote the elongation of the hypocotyl to an acceptable length before cotyledon exposure (larger than 4 cm) for setting its pod zone. To avoid a negative impact of pot warming from direct solar radiation during the summer season, the pottery pots were buried in the farm soil, so that a 5-cm rim was exposed above the ground. The pots were arranged in a randomized complete block design. After emergence of the seedlings (7 days after sowing), the 6 cm of soil previously covering the seeds was removed to its original place in the pot. One seedling was left in each pot and the other was cut using stainless steel scissors. The chips were left inside the pot.
An aluminum pan was designed to split the belowground part into “pod” and “root” compartments according to Wright (1989). The pan was 12 cm in height and 29 cm in outer diameter (OD), with a growth hole of 1.5 cm in diameter in the center of its bottom plate (Fig. 1). The aluminum pan (that forms the pod zone) was thus positioned on top of the pottery pot (root zone), letting the seedling just pass through the growth hole. With skimmed cotton, the growth hole was blocked around the seedling, both to prevent soil from falling from the pod into the root zones, and roots to grow from the root zone into the pod zone.
A quantity of 6.5 kg of pre-incubated air-dried soil was applied to each of the aluminum pans (which resulted in approximately 8-cm soil layer in the pan). NPK nutrients were applied at equal rates as to the root zone soil. Tap water was applied to establish a moisture content at 70 % of field capacity. A space of 1 cm was left between the outer side of the aluminum pan and the inner side of the pottery pot to enable water application to the root zone soil. During the dry season, 300-ml tap water was added every 7 days to each pot (including the pod and root zones). During rainy days, standing water in the pot (pod zone) was immediately removed and stored separately. When the pot soil (pod zone) became dry, earlier collected water was returned to the pot where the water was collected from. The peanut plants were harvested after 127 days.
After harvesting, the plants were separated into leaves, stems, roots, pegs (gynophores), pod shells, and kernels. Plant samples were rinsed with deionized water, dried at 100 °C, weighted, and then pulverized with a stainless steel grinder. Plant samples were digested in a (5:1) mixed concentrated solution of HNO3/HClO4, after which the Cd concentration in the solution was determined in a graphite furnace with atomic absorption spectrophotometry. Analysis of a national standard reference material (GBW07605) resulted in Cd concentrations not significantly different from certified values.
Data were analyzed with PASW statistics 18 (SPSS Inc.). Differences between treatments and cultivars were analyzed by one-way ANOVA (p < 0.05) according to the Tukey’s multiple range test. All results were expressed on a dry weight basis.
Results
Plant biomass (dry weight)
As shown in Table 3, peanut cultivars differed with regard to biomass on a dry weight basis for all parts (roots, pegs, stems, leaves, shells, and kernels) as well as for the entire plant. There were no obvious differences in dry weight, either of the different parts or of the entire plant, for each peanut cultivar as a function of soil Cd concentration, regardless of whether Cd was added to the root or pod zone. However, statistically significant difference was observed in dry weight of different parts and the total plant among the cultivars in reference to the average biomass of all treatments, with Fenghua-6 having the smallest biomass and Huayu-23 the highest biomass.
Plant Cd concentrations
Table 4 showed the Cd concentrations in different tissues of the three peanut cultivars under different treatments. The Cd concentrations increased significantly as the Cd concentration in the soil increased, particularly for plants where Cd was applied to soil in the root zone. For each peanut cultivar, regardless of the Cd concentration or whether the Cd was applied to pod or root zones, significant differences in Cd concentrations were found between the different plant tissues. Concentrations increased in the order of leaves ≥ stems ≥ roots > pegs ≥ testas > pod ≥ shells ≥ kernels. Considerable differences were found between peanut cultivars for Cd concentrations in kernels, pod shells, and pegs, with the high-protein cultivar Fenghua-6 having the largest Cd concentrations. When 0.5 and 1.0 μg/g of Cd were added to the root zone, the Cd concentrations in kernels of Fenghua-6 were 7 and 10 %, and 5 and 30 % larger than for the Huayu-20 and Huayu-23 cultivars, respectively.
Plant Cd accumulation and distribution
Total Cd uptake and its distribution in three peanut cultivars were shown in Table 5. For the same Cd treatment, significant differences in Cd uptake were observed between the peanut cultivars, in increasing order of Cd uptake: Huayu-23 > Huayu-20 > Fenghua-6. It is apparent that Cd applied to the root zone increased Cd accumulation in peanut plants more than Cd applied to the pod zone.
Table 5 also showed that most Cd was allocated to the leaves (36.7–52.5 %) and stems (24.9–28.3 %), and whereas a still appreciable percentage was found in the kernels (11.4–20.7 %), a remarkably small percentage was found in the roots (2.70–3.58 %). The proportion of the amount of Cd in kernels to the total Cd uptake by plants differed significantly between the three peanut varieties. Considering the treatments, the proportions of kernel Cd to total plant Cd were 20.7, 11.4, and 12.4 % for Fenghua-6, Huayu-20, and Huayu-23, respectively (Table 5).
We found that the fraction of Cd accumulation in kernels to total plant Cd ranged from 10.0 to 20.4 and 10.3 to 23.6 % for treatments where Cd was applied to the root and pod zones, respectively, if we take into account both the indigenous and extra applied Cd. If only the extra applied Cd is considered, the proportion of kernel Cd to total plant Cd increased significantly (ranging from 14.7 to 49.1 %) if Cd was applied to the pod zone, whereas the ratio changed only slightly (ranging from 9.7 to 21.5 %) if Cd was applied to the root zone. This suggested that the path of Cd transportation from the pod shells and pegs to kernels is more efficient than from the main roots to kernels. By subtracting plant Cd of the control from plant Cd in pod zone Cd treatment, the contribution of applied Cd to the proportion of Cd uptake by pegs and pods to the total plant Cd (extracted via roots, pegs and pods) was calculated. These proportions were 19 and 14, 21 and 10, and 11 and 9 % for the Fenghua-6, Huayu-20, and Huayu-23 cultivars, where 0.5 and 1.0 μg/g Cd were applied, respectively. This indicates that Cd uptake in peanuts via pegs and pod shells cannot be ignored.
Relationships between Cd concentration in kernels and total Cd uptake by plants
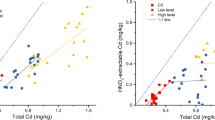
As shown in Fig. 2, cadmium concentrations in the kernel were highly related to total Cd uptake by any peanut cultivar in our experiment. The fitted lines were Y = 0.67ln(X)-2.23 (R 2 = 0.906); Y = 0.48ln(X)-1.81 (R 2 = 0.885); Y = 0.42ln(X)-1.66 (R 2 = 0.927) for Fenghua-6, Huayu-20, and Huayu-23, respectively. However, the significance of the relationship between kernel Cd and total plant Cd decreased if the different genotypes are combined, because of the large differences in internal translocation of Cd by the different genotypes.
Discussion
In previous studies, genotypic variation in Cd accumulation by peanuts has been explicitly addressed (McLaughlin et al. 2000; Su et al. 2013; Wang and Li 2014). However, the mechanism of genotypic variation of Cd accumulation and distribution in peanuts, especially in kernels, is still not clear and formed the major incentive for the present investigation. This study investigated the response of three peanut cultivars with different Cd accumulating patterns. They are different with regard to the importance of various uptake routes of Cd and its influence on Cd accumulation and distribution within the plants. Our results confirmed that peanuts both had a high capability for Cd uptake and exhibited large genotypic variation of Cd accumulation and within plant Cd allocation. The proportion of kernel Cd to total plant Cd was 20.7, 11.4, and 12.4 %, for the genotypes Fenghua-6, Huayu-20, and Huayu-23, respectively (Table 5). Our results also showed that compared with the high Cd accumulation cultivar (Fenghua-6), low Cd accumulation cultivars (Huayu-20 and Huayu-23) showed higher biomass production, especially in stems and leaves (Table 3). This was in agreement with Shi et al. (2014), who observed a negative correlation between Cd concentration in peanut seeds and the biomass of vegetative tissues. We also found that Cd accumulation in seeds was not related to those in stems and leaves (Table 5). These results indicated that high biomass cultivars could accumulate more Cd in their vegetative organs while maintaining a lower Cd concentration due to the dilution effect; thereby decreasing Cd accumulation in seeds by limiting Cd transport from vegetative organs to seed in peanut plants.
The cultivars used in the present study could therefore be classified into two groups, the high efficient internal Cd translocation cultivar (Fenghua-6) and the low efficient internal Cd translocation cultivars (Huayu-20 and Huayu-23). The high Cd translocation cultivar has been observed to be more sensitive to Cd contamination. Fenghua-6 is a high-protein peanut cultivar, which had the highest Cd concentration and proportion in kernels of the three peanut cultivars considered in the present study, suggesting that protein concentration is related to kernel Cd levels. That seed composition may be related to differences in Cd accumulation and partitioning into kernels for peanut cultivars is plausible from previous studies that have demonstrated that Cd in seeds is mainly bound to proteins (Lei et al. 2003; Vogel-Mikus et al. 2010). Thus, Lei et al. (2003) observed that over 80 % of Cd in flaxseed was extracted with proteins in a Tris buffer. Furthermore, Shi et al. (2014) indicated that 74.2 and 67.8 % of peanut seed Cd is generally contained in protein extracts both for the controls and the Cd treatments. Genotypic variations, particularly in kernel Cd accumulation, therefore enable the screening of high or low efficient internal Cd translocation cultivars, and thus select cultivars that are more appropriate to grow in Cd-contaminated soils. The growth medium was separated into pod and root zones with varied Cd concentrations in each zone to determine the key factor which affects the Cd accumulation of different tissues of peanuts. Our result showed that Cd concentration in roots was increased significantly with increasing Cd concentrations in soils when Cd was applied in the root zone. However, there were no significant differences in the root Cd concentrations between different pod zone Cd treatments (1.31–1.89 μg/g) and the control (1.03–1.41 μg/g), except for the Huayu-20 cultivar in the treatment of P1.0-R0 This observation is in agreement with those of McLaughlin et al. (2000), who found that there was less effect of pod zone Cd on the root Cd concentration of two peanut cultivars than root zone Cd (cv. NC7 and Srreeton). Lu et al. (2013) suggested that root morphology, particularly of the fine roots, plays a crucial role in determining Cd accumulation in peanuts. Accordingly, Cd applied to the root zone, rather than to the pod zone, is responsible for Cd accumulation in the roots of peanuts.
After uptake, Cd becomes re-allocated into different parts of the peanut plant by internal translocation. Significant differences were observed between pod and root zones Cd exposure treatments in terms of Cd distribution. If only extra applied Cd was considered, Fenghua-6, Huayu-20, and Huayu-23 accounted for higher Cd distribution fractions under pod zone (ranging from 14.7 to 49.1 %) than root zone Cd exposure treatments (ranging from 9.7 to 21.5 %). This suggested that the path of Cd translocation from the pod shells and pegs to kernels is more efficient than from the main roots to kernels. Previous study established that direct pod uptake also play a key role in controlling Cd absorb and transport into kernels by peanut plants (McLaughlin et al. 2000). These researches suggested that peg and pod shell might, at least partially, be responsible for the variation in Cd accumulation and distribution among peanut cultivars.
Two routes, i.e., Cd uptake by plants (either via roots or through pegs and pods) and internal Cd translocation, are important mechanisms in determining Cd concentrations in the kernels of peanuts. In other words, the genetic variation in kernel Cd accumulation does not solely result from differences in Cd uptake by the roots or pods, but also from differences in the internal Cd translocation. The proportion of Cd in kernels to total plant Cd was significantly higher when Cd was extracted by the pods than by the roots. This suggests that the pathway of Cd translocation from the pod shells and pegs to kernels is more efficient than that from the main roots to kernels. Therefore, higher concentrations of cadmium in the top soil (the main layer for peg and pod shell development) may directly induce a higher concentration of Cd in the kernels.
Conclusions
In conclusion, peanut cultivars exhibit high genotypic variations in biomass, Cd re-distribution and accumulation in plant tissues, especially in kernels. Among the three peanut cultivars, Fenghua-6 exhibited a high and Huayu-20 and Huayu-23 a low internal Cd translocation efficiency. Compared with Fenghua-6, cultivars Huayu-20 and Huayu-23 showed higher biomass production, especially in stems and leaves, leading to a metal dilution phenomenon. Interestingly, we found that plant biomass and Cd uptake routes may be important factors that determine the differences in Cd re-distribution and accumulation in peanut seeds. Peanut cultivars with high biomass such as Huayu-20 and Huayu-23 enable plants to retain larger amounts of Cd within their vegetative tissues while maintaining a lower Cd concentration due to the dilution effect. This limits the shoot-to-seed Cd transport and results in lower Cd concentration in kernels. Cd uptake routes by plants (via roots, pegs, and pods) were also confirmed to be important mechanisms in determining Cd accumulation in the kernels of peanuts. The internal translocation of Cd from the developing peg and pod shell to the main roots was limited, however, a direct transport of Cd from peg and pod shell to the kernels was unimpeded. It appeared that pod to kernel Cd translocation was more efficient than for roots. This suggests that peg and pod shell might, at least partly, be responsible for the variation in Cd re-distribution and accumulation between peanut cultivars.
References
Angelova V, Ivanova R, Ivanov K (2004) Heavy metal accumulation and distribution in oil crops. Comm Soil Sci Plant Anal 35:2551–2566
Bell MJ, McLaughlin MJ, Wright GC, Cruickshank A (1997) Inter- and intra-specific variation in accumulation of cadmium by peanut, soybean, and navybean. Aust J Agric Res 48:1151–1160
Carrin ME, Carelli AA (2010) Peanut oil: compositional data. Eur J Lipid Sci Tech 112:697–707
Chaudhuri D, Tripathy S, Veeresh H, Powell MA, Hart BR (2003) Mobility and bioavailability of selected heavy metals in coal ash- and sewage sludge-amended acid soil. Environ Geol 44:419–432
Cheng SF, Huang CY, Lin SC, Chen KL (2015) Feasibility of using peanut (Arachis hypogaea L.) for phytoattenuation on lead-contaminated agricultural land-an in situ study. Agr Ecosyst Environ 202:25–30
Krishna G, Singh BK, Kim E-K, Morya VK, Ramteke PW (2015) Progress in genetic engineering of peanut (Arachis hypogaea L). Plant Biotechnol J 13:147–162
Lei B, Li-Chan ECY, Oomah BD, Mazza G (2003) Distribution of cadmium- binding components in flax (Linum usitatissimum L) seed. J Agric Food Chem 51:814–821
Liu HY, Anne P, Liao BH (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166
Liu JG, Qian M, Cai GL, Yang JC, Zhu QS (2007) Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J Hazard Mater 143:443–447
Lu Z, Zhang Z, Su Y, Liu C, Shi G (2013) Cultivar variation in morphological response of peanut roots to cadmium stress and its relation to cadmium accumulation. Ecotoxicol Environ Saf 91:147–155
McLaughlin MJ, Bell MJ, Wright GC, Cozens GD (2000) Uptake and partitioning of cadmium by cultivars of peanut (Arachis hypogaea L). Plant Soil 222:51–58
Pal M, Horvath E, Janda T, Paldi E, Szalal G (2006) Physiological changes and defense mechanism induced by Cd stress in maize. J Plant Nutr Soil Sci 169:239–246
Popelka JC, Schubert S, Schulz R, Hansen AP (1996) Cadmium uptake and translocation during reproductive development of peanut (Arachis hypogaea L.). Angew Bot 70:140–143
Shi G, Su GQ, Lu ZW, Liu C, Wang XM (2014) Relationship between biomass, seed components and seed Cd concentration in various peanut (Arachis hypogaea L) cultivars grown on Cd-contaminated soils. Ecotoxicol Environ Saf 110:174–181
Stolt JP, Sneller FEC, Bryngelsson T, Lundborg T, Schat H (2003) Phytochelatin and cadmium accumulation in wheat. Environ Exp Bot 49:21–28
Su G, Li F, Lin J, Liu C, Shi G (2013) Peanut as a potential crop for bioenergy production via Cd-phytoextraction: a life-cycle pot experiment. Plant Soil 365:337–345
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Vogel-Mikuš K, Arčon I, Kodre A (2010) Complexation of cadmium in seeds and vegetative tissues of the cadmium hyperaccumulator Thlaspi praecox as studied by x-ray absorption spectroscopy. Plant Soil 331:439–451
Waliyar F, Reddy SV, Lava-Kumar P (2009) Review of immunological methods for the quantification of aflatoxins in peanut and other foods. Peanut Sci 36:54–59
Wan SB, Shan SH, Li CJ (2005) Safety status and development strategy of peanut in China. J Peanut Sci 34:1–4 (in Chinese)
Wang KR (2002) Tolerance of cultivated plants to cadmium and their utilization in polluted farmland soils. Acta Biotechnol 21:189–198
Wang S, Li G (2014) Assessment of cadmium bioaccumulation and distribution in the kernels of peanut widely cultivated in China. Ecotoxicol Environ Saf 108:23–28
Wang KR, Zhang L (2008) Research advances in cadmium pollution of peanut (Arachis hypogaea L): a review. Chinese J Appl Ecol 19:2757–2762 (in Chinese)
Wright GC (1989) Effect of pod zone moisture content on reproductive growth in three cultivars of peanut (Arachis hypogaea). Plant Soil 116:111–114
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 40871224) and the Research Foundation for Advanced Talents of Qingdao Agricultural University (no. 6631115029) as general projects. Part of this work was done during the first author’s sabbatical leave at Wageningen University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Wang, K., Song, N., Zhao, Q. et al. Cadmium re-distribution from pod and root zones and accumulation by peanut (Arachis hypogaea L.). Environ Sci Pollut Res 23, 1441–1448 (2016). https://doi.org/10.1007/s11356-015-5348-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5348-z