Abstract
In soil, the determination of total concentration using an exhaustive extraction method has little relevance to evaluate the exposure of an organism to a chemical, because of sorption processes. This study aims to propose a mild extraction method to evaluate the bioavailability of the fungicide epoxiconazole to the earthworm Aporrectodea icterica. Experiments were conducted in soils presenting various textures and organic carbon contents, spiked with formulated epoxiconazole 7 to 56 days prior to their extraction. In parallel, the epoxiconazole concentration was determined in exposed earthworms and the fungicide’s effects were evaluated by measuring weight gain, enzymatic activities and total protein contents. Among the various mild chemical solvents tested to evaluate the environmental availability of the fungicide, the 50 mM hydroxypropyl-β-cyclodextrin solution allowed to extract around 30 % of epoxiconazole. This percentage corresponded to the ratio determined in exposed A. icterica under similar soil conditions. Furthermore, this mild method was demonstrated to be sensitive to soil sorption capacities and to ageing. The mild extraction method was then applied to explore the relationship between total and (bio)available concentrations in soil and in A. icterica, over 7- or 28-day exposure time. This demonstrated the proportionality between epoxiconazole concentration in earthworm and available in soil (up to 96 %, with regression coefficient R 2 = 0.98). Sublethal effects on earthworm remained not significant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Determining the bioavailability of pesticides in soil in order to evaluate their ecotoxicity constitutes a major issue. After entering the soil, pesticides may be subjected to volatilization, leaching, sorption to soil particles and chemical and biological degradation. Among these processes, adsorption/desorption is highly involved in the regulation of the contaminant’s concentrations in soil solution and hence exerts a large influence on contaminant transfer to natural waters, degradation and bioavailability (Yu et al. 2006). The sorption of pesticides in soil is governed by many different factors including soil characteristics and compound properties (Chaplain et al. 2011). The most influential soil characteristics are generally total organic matter and clay mineral content: the higher are those parameters, the greater is the sorption (Spark and Swift 2002; Ahangar et al. 2008; Wauchope et al. 2002). Furthermore, the residues become more strongly associated to the soil over time through a process called ageing (Alexander 2000; Reid et al. 2000). Sorption can also be greatly affected by pesticide’s properties including water solubility, vapour pressure, molecular size and hydrophobicity as evaluated through the octanol–water partition coefficient (log K ow). Classically, in the case of non-ionized compounds, as log K ow increases, solubility and bioavailability decrease whereas sorption increases (Yu et al. 2006; Chaplain et al. 2011).
The bioavailable fraction of a compound in soil can be considered as the amount that is freely available and able to cross an organism’s cellular membrane from the soil where the organism inhabits at a given time (Semple et al. 2004). This fraction is thus affected by the above-mentioned processes and the organism of concern and by changes occurring over time (Harmsen 2007). Bioavailability can be described using three steps: the environmental availability (or soil offer) related to the potential of a compound to engage interactions with an organism, the environmental bioavailability corresponding to the uptake of the compound by the organism and, finally, the toxicological bioavailability corresponding to the effect of the compound on the organism (Lanno et al. 2004; Harmsen 2007; ISO 17402 2008). For earthworms, the environmental bioavailability is classically evaluated by measuring the concentration in tissues (Lanno et al. 2004). Toxicological bioavailability studies may consider end-points such as mortality, genotoxicity, reproduction and/or symptoms of physiological disorder (for example, a reduced growth rate or modified total protein content) (Mosleh et al. 2003). Several methods have been proposed to quantify the environmental availability of pesticides for earthworms in soil matrix (Katayama et al. 2010). These methods include trapping or partitioning on a solid-phase extraction (SPE) support exposed in wet soil or soil suspension, the solid support being, for instance, a C18 membrane (Tang et al. 1999), a polymeric film (Andrade et al. 2014) or a SPME fibre (Van der Wal et al. 2004; Bielska et al. 2014). Methods can also be based on analysis of pore water or other aqueous extract (Folberth et al. 2009) as well as on organic or hydro-organic extracts obtained through supercritic fluid extraction, pressurized liquid extraction (Hawthorne et al. 2000) or agitation. The shared characteristic of the various methods is to present a mild extractive power in order to preserve selectivity of the extraction, addressing only non-highly bounded or non-trapped residues.
Epoxiconazole (EPX) is a triazole fungicide widely used from 1993 to protect crops such as cereals (wheat, barley, oat, rye and maize) and sugar beet against septoria and rusts. It is generally spread at a recommended dose (RD) of 125 g ha−1, i.e. 0.17 mg kg−1, if a penetration within a 5-cm soil layer is considered. According to PPDB database (2015), this compound is hydrophobic (log K ow = 3.3), poorly soluble in water (7.1 mg L−1) and rather sorbed according to its Freundlich adsorption constant (K f 12.18 L kg−1, 1/n 0.836) and its K foc (1073 L kg−1). Thus, it mostly remains at the soil surface. EPX is considered as non-volatile (vapour pressure 0.01 mPa and Henry’s low constant 0.47 mPa m3 mol−1, at 25 °C). Poorly sensitive to photolysis and hydrolysis, this compound is also hardly biodegraded and, thus, persistent in soil (field half-life 120–354 days). Its acute toxicity against earthworms Eisenia fetida is low (lethal concentration for 50 % of the tested population LC50 >500 mg kg−1). On the contrary, its chronic toxicity is of concern, as its no observable effect concentration (NOEC) is 0.087 mg kg−1 on E. fetida, i.e. half of the RD (PPDB 2015). Furthermore, this species is known to be generally less sensitive to pesticides than other species found in cultivated fields (Pelosi et al. 2013). Moreover, this species of earthworm is rarely found in mineral soils and is thus very uncommon in the cultivated fields where pesticides are applied (Lowe and Butt 2007). Thus, Aporrectodea icterica, an endogeic species of earthworm leaving in cultivated fields and potentially more sensitive (Pelosi et al. 2013, 2015), was chosen as a model to be exposed to EPX in order to evaluate the fungicide’s effects on this organism. The aim of this study was to establish a link between environmental availability, environmental bioavailability and toxicological bioavailability, using a commercial formulation of EPX and the earthworm A. icterica. This implied (1) to find a method to evaluate environmental availability and then (2) to perform the three “bioavailability” evaluations on a same experiment.
Materials and methods
Chemicals
Soil spiking was performed using EPX (2RS,3SR)-1-[3-(2-chlorophenyl)-2,3-epoxy-2-(4-fluorophenyl)propyl]-1H-1,2,4-triazole (EPX) formulated as Opus® (EPX concentration of 125 g L−1; BASF). The EPX used as analytical standard (Pestanal grade), hydroxypropyl-β-cyclodextrin (CD), Na2SO4, NaCl, reduced glutathione (GSH), 1-chloro-2,4-dinitrobenzene (CDNB) and bicinchoninic acid kit for protein determination were purchased from Sigma-Aldrich. All organic solvents of analytical grade were purchased from Carlo-Erba, and LC-grade water was obtained through a Milli-Q system (Millipore).
Soil and earthworms
Two soils were sampled at 5–20-cm depth, air-dried and passed through a 2-mm sieve. The loamy soil was sampled from an uncontaminated fallow in Versailles, 15 km southwest of Paris (48° 47′ N, 2° 04′ E), that had received no chemicals for more than 20 years. Its main physical and chemical properties were as follows: pH 5.7, organic matter 29.9 g kg−1, C/N 21.1, 45 % sand, 36 % silt and 18 % clay. The sandy soil was collected in an agricultural field where cereals are grown (under wheat crop when collected) in Pierrelaye, 30 km northwest of Paris (49° 03′ N, 2° 04′ E). Its main physical and chemical properties were as follows: pH 8.2, organic matter 13.6 g kg−1, C/N 10.4, 84 % sand, 7 % silt and 9 % clay. Only traces of EPX were detected in both soils (<2 μg kg−1 dry soil). According to the experiments, each soil was mixed or not with 10 % (dry weight) of horse dung beforehand frozen and defrosted twice and then milled (<1 mm). This addition increased the organic matter content in soils, allowing both to test the effect of this parameter on availability and to feed the earthworms.
Juveniles of A. icterica were collected by hand sorting from the same place as the loamy soil. Even though they were juveniles, earthworms were easily identifiable due to their coloration and their welts. This species is commonly found in pastures and cultivated fields (Bouché 1972; Blakemore 2000; Pelosi et al. 2009). Stock earthworms were cultured for 1 month in the laboratory at 15 °C ± 2 °C in the Versailles loamy soil mixed with 10 % horse dung. The moisture content was adjusted to approximately 90 % of the water holding capacity (WHC) for earthworm welfare, because horse dung absorbed a lot of water (Lowe and Butt 2007). Prior to use, the earthworms were removed from the cultures, rinsed in tap water and weighed without voiding their gut contents. Earthworms were randomly allocated to microcosms.
Preparation of incubations and microcosms
To choose and test the mild extraction procedure, soils were eventually mixed with horse dung (10 %), wetted to 30 % of WHC, spiked with EPX and then adjusted to 70 % of WHC and mixed. Spiking was performed using aqueous dilutions of formulated EPX at 17 or 1.7 mg EPX L−1 in order to obtain 10- or 1-fold the RD, i.e. 1.7 or 0.17 μg EPX g−1 dry soil (or dry soil + horse dung). Experiments were conducted using the equivalent of 3-g dry soil (or dry soil + horse dung) per trial, in duplicate during the selection procedure and triplicate during the testing procedure. Ageing was performed during 7, 28 or 56 days in the dark at 15 ± 2 °C.
The preparation procedure was identical when the relationship between the three bioavailability evaluations was tested, except soil humidity which was adjusted to 90 % WHC. The loamy soil enriched with organic matter (horse dung) was spiked with formulated EPX at doses corresponding to 1, 3 and 10 RD. A control treatment receiving only water was also prepared. Four replicates of 500-g wet soil in 1-L plastic box were used for each treatment and received or not three earthworms. After 7 or 28 days, earthworms were weighed and frozen at −80 °C, either directly for biochemical measurement or after 48-h starvation for EPX uptake evaluation (to empty the gut’s content, in Petri dishes on damp filter paper in the dark at 15 ± 2 °C). Soil was homogenized and used for evaluation of total EPX, available EPX and dry weight (by weighing after 48 h at 105 °C).
EPX extraction
To perform EPX measurement in earthworm tissues, a new sample preparation method was proposed, according mostly to Azzouz et al. (2011), Anastassiades et al. (2003) and Hong et al. (2004), respectively, for extraction, liquid phase partitioning and purification steps. Frozen earthworms were individually homogenized using a FastPrep®-24 (MP Biomedicals) in 2 mL of water in a 15-mL polypropylene tube (Falcon BD) with ceramic spheres. EPX extraction was then performed by adding 2 mL acetonitrile in the tube, shaking on an orbital shaker (Ika KS 501, at 300 rpm for 10 min) and sonicating for 15 min. Then, 0.8 g Na2SO4 and 0.2 g NaCl were added. The tube was vigorously shaken and centrifuged for 5 min at 2500g and 4 °C (Beckman Coulter Allegra X-15R). The upper organic layer was collected, placed at least 2 h at −40 °C and centrifuged for 10 min at 10,000g and 4 °C. The supernatant was again dried through 1-g Na2SO4 addition and centrifugation (5 min, 2500g, 4 °C). A 0.5 mL aliquot of supernatant was then evaporated to dryness under a N2 stream and redissolved in chloroform. This extract was further purified on 500-mg Florisil cartridges (preconditioned successively by 2 mL of methanol, acetone and chloroform). Elution was performed by 5 mL of a 9:1 (v/v) chloroform/acetone mixture. After evaporation under a N2 stream, the residue was redissolved in 1 mL of 8:2 (v/v) water/acetonitrile and analyzed by liquid chromatography-mass spectrometry. According to non-exposed earthworms spiked with EPX standard solution, recovery was estimated as 97.2 ± 5.6 %.
To perform EPX exhaustive extraction from soil, the latter was first frozen and lyophilized. Then, triplicate soil subsamples of 5 g were placed in 50-mL polypropylene tube (Falcon BD) and 15 mL of a 6:2:2 mixture of methanol/66.7 mM EDTA, Na2 aqueous solution/McIlvaine buffer pH 8 (v/v/v) was added to each tube. The tubes were shaken on orbital shaker (10 min, 300 rpm) and sonicated for 30 min, before being centrifuged for 10 min at 1300g and 4 °C. After collecting 10 mL of supernatant, the soil was again extracted by 10 mL of the same mixture and shaken, sonicated and centrifuged as previously. Then, 10 mL of supernatant was collected and mixed with the first extract. An aliquot was diluted using a water/acetonitrile mixture (80:20, v/v) by a factor 10 for 1 RD dose and by a factor 20 for 3 RD and 10 RD doses. This dilution allowed both solutions to be within the calibration curve range and matrix effect to be limited (evaluated as less than 20 %). Samples were then stocked at −20 °C prior to analysis, performed within 2 weeks. Recovery was quantitative in both soils, even after 7-day ageing.
To evaluate the environmental availability of EPX in soil, a wet soil mass equivalent to 3 g of dry soil was placed in a 50-mL polypropylene tube (Falcon BD) and either 6 mL of hydro-organic mixture or 9 mL of aqueous solution was added during the selection step. Solution compositions are presented in the results and discussion part. During the test procedure and the study of relationships between (bio)availability evaluations, only 50 mM (70 g L−1) of hydroxypropyl-β-cyclodextrin in aqueous solution was used. Tubes were shaken for 17.5 h on a reciprocating shaker at 50 rpm at ambient temperature and then centrifuged at 10,000g (10 min, 4 °C). The supernatant was diluted by 20 in a mixture of water–acetonitrile 85:15 prior to analysis, in order to be within the calibration curve range and to limit the matrix effect (evaluated at less than 15 %). Analyses were performed within 24 h after preparation.
Analysis by ultra-high performance liquid chromatography–tandem mass spectrometry
Analyses were performed on an ultra-high performance liquid chromatograph (Acquity UPLC, Waters) coupled through an electrospray interface to a tandem mass spectrometer (TQD, Waters). Chromatographic separation was carried out on a Acquity BEH C18 column (2.1 × 100 mm, 1.7-μm particle size, Waters) at 0.3 mL min−1 flow rate using a 80/20 to 0/100 gradient in 7.5 min of water/acetonitrile, each containing 0.1 % acetic acid. Injection volume was 10 μL. The electrospray source operated in positive mode with the following settings: capillary 3 kV, cone voltage 30 V, source and desolvation gas temperature 120 and 300 °C, respectively, and flow of desolvation gas and cone gas (nitrogen) 800 and 20 L h−1, respectively. EPX quantification was performed in MRM mode at 22 eV collision energy and 3.4 × 10−3 mbar argon in the collision cell, using 330 > 121 and 332 > 121 as quantitation and confirmation transitions, respectively. Concentrations were determined by external calibration up to 150 ng mL−1 (weighting 1/x, residues below 20 %) using QuanLynx (Waters). The limit of quantification was estimated at 0.03 ng mL−1, corresponding, for instance, for a 600-mg earthworm to 0.05 ng g−1 (EPX/fresh earthworm weight) or for exhaustive quantification in soil to 0.135 ng g−1 (EPX/dry soil weight).
Biological and biochemical measurements on earthworms
The weight gains were determined using the following equation, from Martin (1986):
Frozen earthworms were individually homogenized using an Ultra-Turrax (IKA T10, at 15,000 rpm) in 1 mL of ice-cold phosphate buffer 100 mM at pH 7.2 and 1 mM EDTA per 100 mg of earthworm. A portion of the homogenate was centrifuged at 9000g, 30 min, 4 °C. Glutathione-S-transferase (GST) activities were measured in the fresh supernatant, immediately after homogenization since a decrease of activity had been observed when supernatants were stored at −80 °C. Measures of energy reserves were done on the homogenate frozen at −20 °C. All biochemical measurements were carried out in triplicate for each individual.
GST activity was assayed as described by Habig et al. (1974). The substrate used was 1-chloro-2,4-dinitrobenzene (CDNB). Earthworm supernatants were diluted 1:2 (v/v) with the phosphate buffer. A 4 μL aliquot of each dilute sample was mixed with the assay buffer to achieve the following final conditions: 100 mM phosphate buffer, 2 mM GSH and 1 mM CDNB in a final volume of 200 μL. The formation of reduced glutathione (GSH)–CDNB complex was monitored at 340 nm (ε = 9.6 mM−1 cm−1) for 5 min at 25 °C, after an initial 1-min incubation step. Blanks (without sample) and negative controls (without GSH) were included to ensure measurement quality. The final GST activity was corrected for non-specific reaction and was expressed as micromole of GSH–CDNB complex produced per min per milligram of protein.
Protein contents were determined as described by Smith et al. (1985), in the supernatant and in the total homogenate after freezing at −20 °C, using the bicinchoninic acid method and bovine serum albumin as a standard.
Data treatment
Data were represented by the mean ± the standard deviation. The normality and homogeneity of variance were checked with Kolmogorov–Smirnov and Levene tests with a significance level of 5 %. If parametric conditions were satisfied, ANOVA tests were used to analyze the results and ascertain differences between systems. When the data did not satisfy the conditions, non-parametric tests were used, i.e. Kruskal–Wallis tests. The correlations between toxicological bioavailability and EPX availability (environmental availability or environmental bioavailability) were tested using Spearman coefficient. All statistical analyses were performed using R software (R Development Core Team 2011) or with Microsoft Excel Software.
Results and discussion
Choice of a mild extractant to evaluate environmental availability
In a preliminary study (Pelosi et al. 2015), earthworms A. icterica were exposed to EPX at 1 or 10 RD in the loamy soil during 7 or 28 days. EPX was then evaluated in their tissues and in the soil (five replicates for each of the four conditions). For all the spiking levels and exposure times, the EPX concentration evaluated in earthworm corresponded to around 33 % of the EPX concentration present in the soil (Table 1). Thus, to be able to evaluate the EPX availability in the loamy soil, it was necessary to find a method that allowed extracting around 33 % of the EPX present in this soil. One can notice that a longer time of exposure induced a small (non-significant) decrease of EPX concentration in soil, which is in accordance to its known persistency.
To choose the mild solvent to be used to evaluate the environmental availability, experiments were conducted in duplicate on sandy and loamy soil enriched or not with organic matter, with a spiking level of 10-fold the RD and 7 days of contact time. Since most of the differences between duplicates were inferior to 5 %, their means are presented in Fig. 1. The aqueous solution of 10 mM CaCl2, considered as mimicking rain water, is classically used to perform sorption and desorption tests to determine the K d or K f coefficients (Chaplain et al. 2011). It has also been successful to evaluate the (bio)availability of various organic contaminants (Barriuso et al. 2004; Förster et al. 2009). However, in the present study, extraction yields obtained with 10 mM CaCl2 were in the 2.3–3.3 % range except on sandy soil (without OM enrichment) where it reached 8.9 %. These very low yields, close to those obtained for EPX in sediments or forest soil, may be related to EPX hydrophobicity and low solubility in water (Passeport et al. 2011).
A large range of organic solvents are cited in literature to perform mild extraction of organic pollutants in order to evaluate their availability. Among them, methanol (or methanol/water mixture) is the most popular, even if many others, water-miscible or not, have been proposed such as hexane, n-butanol, ethanol, acetone or acetonitrile (Kelsey et al. 1997; Barriuso et al. 2004; Yu et al. 2005; Wu et al. 2011). We thus chose to test methanol/water and acetonitrile/water mixtures, using compositions previously shown as efficient (9:1, 1:1, v/v). Apart from the suitable solvents, biomimetic extraction using an excess of hydroxypropyl-β-cyclodextrin (CD) is also considered as a way of choice to evaluate bioavailability (Reid et al. 2000; Hartnik et al. 2008). Three CD concentrations were used, according to Hartnik et al. (2008): 20, 50 and 120 mM. As shown on Fig. 1, all extraction yields obtained with organic solvents were superior to the targeted yield of 33 %, ranking between 51 and 92 %. On the opposite, hydroxypropyl-β-cyclodextrin (CD) solutions showed lower extraction yields, between 14 and 29 %. In accordance with previous studies, the CD concentration had an effect on EPX extraction. Indeed, a lower extraction yield was obtained when a 20-mM CD solution was used, whereas higher and similar yields were achieved for CD solutions of 50 and 120 mM. This behaviour is consistent with the formation of a 1:1 CD–EPX inclusion complex. From these experiments, it was concluded that extraction using a 50-mM CD solution could be appropriate to perform the extraction of the environmentally available EPX from soil. This condition was thus chosen and its sensitivity tested with various parameters known to have an effect on sorption.
Test of sensitivity of the mild extraction method to soil parameters
The effects of organic matter content, soil texture and dose of EPX on mild extraction yields (presumably environmental availability) have been tested after 56 days of contact time without earthworms. Enrichment in organic matter content decreased the mild extraction yields in all cases, and the change was significant for three out of the four conditions (Table 2). EPX was also less extracted (less available) in the loamy than in the sandy soil in all conditions, this difference being significant three out of four times. These decreasing effects are consistent with the increased sorption of organic compounds described in the literature (Ahangar et al. 2008; Wauchope et al. 2002). Thus, the proposed mild extraction method by agitation in 50 mM CD allowed discriminating the EPX availability between soil’s composition while changing the organic matter content or the texture. On the opposite, the dose effect was not clear: when comparing the mild extraction yields obtained at 1 RD and 10 RD, no influence was observed without organic matter enrichment, and the classifications of the yields were inconsistent when organic matter was added (being once 1 RD > 10 RD and once 1 RD < 10 RD). The absence of a clear dose effect is contradictory to the higher availability observed for another triazole (triticonazole) at a higher dose (Beigel et al. 1999). However, the two doses in this study differed only by a 10-fold factor instead of 400-fold in the other study, which implies the influence of the formulating agents.
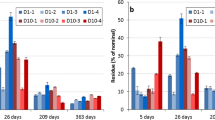
The effect of ageing clearly appeared during the 56-day period, as shown on Fig. 2. Mild extraction yields (environmental availability) determined after 56 days of exposure were two to four times lower after 7 days. Indeed, yields were evaluated at 65 to 75 and 16 to 35 % at 7 and 56 days, respectively. Differences were significant in all cases between those two time points. The yields obtained after 28 days were either at the midpoint of the 7- and 56-day extraction yields or very close to the 56-day yields, depending on the experimental conditions (Fig. 2). Yields presented, in some cases, a large heterogeneity between the three samples. However, in the loamy soil enriched with organic matter, a clear difference between 7 and 28 days was observed both at the 1 RD and 10 RD doses. The evaluation of the three (bio)availability types was thus presumed to be possible in this soil over a maximum exposure time of 28 days.
Evolution with contact time of the extraction yields obtained by the mild extraction method (50 mM hydroxypropyl-β-cyclodextrin, mean of triplicate; error bars correspond to standard deviations). Letters correspond to the differences (p < 0.05) within each serial according to ANOVA test (except loamy soil, 1 RD where Kruskal-Wallis test was performed). RD recommended dose, OM organic matter (10 % weight of horse dung)
Relationship between the three bioavailability evaluations
The relationship between environmental availability, environmental bioavailability and toxicological bioavailability was studied in a dedicated experiment where those three aspects were studied concomitantly in the loamy soil enriched with organic matter, after 7 or 28 days of exposure.
The environmentally available EPX, determined using mild extraction, was directly correlated to its total concentration in soil (R 2 = 0.99) and the overall yield was 58.1 ± 0.5 % (Fig. 3a). This yield was at the midpoint of those obtained during the sensitivity test at 7 and 28 days: 69 ± 5 and 30 ± 3 %, respectively. Neither the contact time nor the presence/absence of earthworms exerted an observable effect on this linear relationship. The absence of ageing effect was not consistent with the result obtained during the previous tests, which showed a significant difference between 7 and 28 days (in the absence of earthworms). This could be due to the higher water content, i.e. 90 % WHC instead of 70 %, as this factor is known to increase EPX bioavailability (Bromilow et al. 1999). Because of the absence of an ageing effect, it was not possible to determine if the presence of earthworms in the soil increased the contaminant residues’ availability, as previously demonstrated for apolar pesticides such as DDT and HCH (Verma and Pillai 1991). Independently of doses and earthworm presence/absence, the total EPX concentration remained identical between 0 and 7 days but decreased significantly between 7 and 28 days (reaching globally 75 ± 9 % of initial spiking). This decrease could be due both to the EPX degradation and/or the formation of residues which were non-extractable by the “exhaustive” procedure performed in this study (Bromilow et al. 1999; Passeport et al. 2011).
Concerning the environmental bioavailability, contrary to preliminary results, exposure time influences EPX concentration measured in the earthworm tissues (Fig. 3b). After 28 days of exposure, concentrations in earthworms corresponded to 33 % of total EPX concentration, as previously obtained. However, the concentrations were higher after 7 days of exposure and corresponded to 51 % of total EPX concentration, perhaps suggesting a difference in earthworms’ behaviour during the first days of the new set of experiments. The comparison of Fig. 3b, c, presenting EPX concentration in earthworm tissues versus concentration of EPX in soil, showed that the mild extraction method used to evaluate available EPX allowed increasing the slopes from 51 to 84 and 33 to 58 % at 7 and 28 days, respectively, thus approaching but not reaching the “ideal” 100 % goal. However, as differences between 7 and 28 days slopes appeared to be mainly due to two experimental points (28 days, 10 RD), data were transformed to their logarithmic value (Gaw et al. 2012; Hu et al. 2005) (Fig. 3d). This transformation allowed increasing the slope up to 96 % with no distinction between the two exposure times. Furthermore, the regression coefficient obtained was satisfying (R 2 = 0.98), the dispersion being mainly due to EPX traces in the controls, which corresponded to the higher −Log C values.
Finally considering toxicological bioavailability, non-significant effects of EPX on earthworms were found for weight gain (p = 0.28 and 0.61, respectively, at 7 and 28 days according to ANOVA test) (Fig. 4), GST activity (p = 0.83 and 0.37, respectively, at 7 and 28 days) and total protein content (p = 0.67 and 0.24, respectively, at 7 and 28 days). The results on weight gain were not in accordance with those obtained in another experiment done by the same research team using the same species and pesticide (Pelosi et al. 2015). They found a significant decrease in weight gain as the EPX concentration increases. The differences in experimental conditions (particularly, the WHC which was 70 % in the other study) and the lower number of replicates (four versus ten in the other experiment) could explain these non-significant results. Results for GST activity were similar to those obtained in the other experiment. As the response of GST activity to contaminants is time-dependent (Velki and Hackenberger 2012) and because this enzymatic activity was not monitored between 7 and 28 days, it is possible that some GST activity changes were missed. GST activity and total protein content slightly decreased with increasing EPX concentrations, but the differences were not significant (Fig. 4b, c). Finally, no significant correlation was found between earthworm parameters representing toxicological bioavailability and the EPX availability (environmental availability or environmental bioavailability). Again, this could be due to the low number of replicates that did not emphasize the significant differences.
Conclusions
Several solvent systems were assessed in order to establish a chemical extraction procedure able to represent the bioavailability of EPX in soil to the earthworm A. icterica. A solution of 50 mM of hydroxypropyl-β-cyclodextrin fulfils the requirements of the ISO 17402 norm. This medium mimics the earthworm’s uptake and is able to take into account sorption-related aspects that control the biological uptake. These points were clearly demonstrated by evidencing the effects of sorption parameters on the (bio)availability and by plotting linear regressions. However, a limited number of soil characteristics were addressed in this study. Other soil textures, organic matter level or nature and other variables such as water content may affect sorption. Thus, more soils and organisms with different characteristics and a longer ageing time should be considered to determine the limits of this evaluation method of the EPX bioavailability. This study could also be expanded to different organic contaminants with a wide range of water solubility and log K ow, in order to tend toward a reliable predictability of the ecotoxicological risks which would take bioavailability in consideration.
References
Ahangar AG, Smernik RJ, Kookana RS, Chittleborough DJ (2008) Clear effects of soil organic matter chemistry, as determined by NMR spectroscopy, on the sorption of diuron. Chemosphere 70:1153–1160
Alexander M (2000) Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ Sci Technol 34:4259–4265
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Andrade NA, Centofanti T, McConnell LL, Hapeman CJ, Torrents A, Nguyen A, Beyer WN, Chaney RL, Novak JM, Anderson MO, Cantrell KB (2014) Utilizing thin-film solid-phase extraction to assess the effect of organic carbon amendments on the bioavailability of DDT and dieldrin to earthworms. Environ Pollut 185:307–313
Azzouz A, Souhail B, Ballesteros E (2011) Determination of residual pharmaceuticals in edible animal tissues by continuous solid-phase extraction and gas chromatography-mass spectrometry. Talanta 84:820–828
Barriuso E, Koskinen WC, Sadowsky MJ (2004) Solvent extraction characterization of bioavailability of atrazine residues in soils. J Agric Food Chem 52:6552–6556
Beigel C, Charnay MP, Barriuso E (1999) Degradation of formulated and unformulated triticonazole fungicide in soil: effect of application rate. Soil Biol Biochem 31:525–534
Bielska L, Smidova K, Hofman J (2014) Solid phase microextraction of organic pollutants from natural and artificial soils and comparison with bioaccumulation in earthworms. Ecotoxicol Environ Saf 100:44–52
Blakemore RJ (2000) Ecology of earthworms under the ‘Haughley experiment’ of organic and conventional management regimes. Biol Agric Hortic 18:141–159
Bouché MB (1972) Lombriciens de France: Ecologie et Systématique. INRA Ann. Zool. Ecol. Anim. Publication, France
Bromilow RH, Evans AA, Nicholls PH (1999) Factors affecting degradation rates of five triazole fungicides in two soil types: 1. Laboratory incubations. Pestic Sci 55:1129–1134
Chaplain V, Mamy L, Vieublé-Gonod L, Mougin C, Benoit P, Barriuso E, Nélieu S (2011) Fate of pesticides in soils: toward an integrated approach of influential factors. In: Stoytcheva M (ed) Pesticides in the modern world. Intech, pp 535–560
Folberth C, Scherb H, Suhadolc M, Munch JC, Schroll R (2009) In situ mass distribution quotient (iMDQ)—a new factor to compare bioavailability of chemicals in soils? Chemosphere 75:707–713
Förster M, Laabs V, Lamshöft M, Groeneweg J, Zühlke S, Spiteller M, Krauss M, Kaupenjohann M, Amelung W (2009) Sequestration of manure-applied sulfadiazine residues in soils. Environ Sci Technol 43:1824–1830
Gaw S, Northcott G, Kim N, Wilkins A, Jensen J (2012) Comparison of earthworm and chemical assays of the bioavailability of aged 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene,1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane, and heavy metals in orchad soils. Environ Toxicol Chem 31:1306–1316
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Harmsen J (2007) Measuring bioavailability: from a scientific approach to standard methods. J Environ Qual 36:1420–1428
Hartnik T, Jensen J, Hermens JLM (2008) Nonexhaustive β-cyclodextrin extraction as a chemical tool to estimate bioavailability of hydrophobic pesticides for earthworms. Environ Sci Technol 42:8419–8425
Hawthorne SB, Grabanski CB, Martin E, Miller DJ (2000) Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: recovery, selectivity and effects on sample matrix. J Chromatogr A 892:421–433
Hong J, Kim HY, Kim DG, Seo J, Kim KJ (2004) Rapid determination of chlorinated pesticides in fish by freezing-lipid filtration, solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A 1038:27–35
Hu XY, Wen B, Zhang S, Shan XQ (2005) Bioavailability of phthalate congeners to earthworms (Eisenia fetida) in artificially contaminated soils. Ecotoxicol Environ Saf 62:26–34
ISO 17402 (2008) Soil quality—guidance for the selection and application of methods for the assessment of bioavailability of contaminants in soil and soil materials. ISO, Geneva
Katayama A, Bhula R, Burns GR, Carazo E, Felsot A, Hamilton D, Harris C, Kim Y-H, Kleter G, Koerdel W, Linders J, Peijnenburg JGMW, Sabljic A, Stephenson RG, Racke DK, Rubin B, Tanaka K, Unsworth J, Wauchope RD (2010) Bioavailability of xenobiotics in the soil environment. Rev Environ Contam Toxicol 203:1–86
Kelsey JW, Kottler BD, Alexander M (1997) Selective chemical extractants to predict bioavailability of soil-aged organic chemicals. Environ Sci Technol 31:214–217
Lanno R, Wells J, Conder J, Bradham K, Basta N (2004) The bioavailability of chemicals in soil for earthworms. Ecotoxicol Environ Saf 57:39–47
Lowe CN, Butt KR (2007) Earthworm culture, maintenance and species selection in chronic ecotoxicological studies: a critical review. Eur J Soil Biol 43:S281–S288
Martin NA (1986) Toxicity of pesticides to Allolobophora caliginosa (Oligochaeta, Lumbricidae). N Z J Agric Res 29:699–706
Mosleh YY, Paris-Plalcios S, Couderchet M, Vernet G (2003) Acute and sublethal effects of two insecticides on earthworms (Lumbricus terrestris L.) under laboratory conditions. Environ Toxicol 18:1–8
Passeport E, Benoit P, Bergheaud V, Coquet Y, Tournebize J (2011) Epoxiconazole degradation from artificial wetland and forest buffer substrates under flooded conditions. Chem Eng J 173:760–765
Pelosi C, Bertrand M, Capowiez Y, Boizard H, Roger-Estrade J (2009) Earthworm collection from agricultural fields: comparisons of selected expellants in presence/absence of hand-sorting. Eur J Soil Biol 45:176–183
Pelosi C, Joimel S, Makowski D (2013) Searching for a more sensitive earthworm species to be used in pesticide homologation tests—a meta-analysis. Chemosphere 90:895–900
Pelosi C, Lebrun M, Beaumelle L, Cheviron N, Delarue G, Nélieu S (2015) Sublethal effects of epoxiconazole on the earthworm Aporrectodea icterica. Environ Sci Pollut Res. doi:10.1007/s11356-015-4845-4
PPDB (2015) http://sitem.herts.ac.uk/aeru/ppdb/en/atoz.htm
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.Rproject.org/
Reid BJ, Stokes JD, Jones KC, Semple KT (2000) Nonexhaustive cyclodextrin-based extraction technique for the evaluation of PAH bioavailability. Environ Sci Technol 34:3174–3179
Semple KT, Doick KJ, Jones KC, Burauel P, Craven A, Harms H (2004) Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ Sci Technol 38:228A–231A
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Spark KM, Swift RS (2002) Effect of soil composition and dissolved organic matter on pesticide sorption. Sci Total Environ 298:147–161
Tang J, Robertson BK, Alexander M (1999) Chemical-extraction methods to estimate bioavailability of DDT, DDE, and DDD in soil. Environ Sci Technol 33:4346–4351
Van der Wal L, Jager T, Fleuren RHLJ, Barendregt A, Sinnige TL, Van Gestel CAM, Hermens JLM (2004) Solid-phase microextraction to predict bioavailability and accumulation of organic micropollutants in terrestrial organisms after exposure to a field-contaminated soil. Environ Sci Technol 38:4842–4848
Velki M, Hackenberger BK (2012) Species-specific differences in biomarker responses in two ecologically different earthworms exposed to the insecticide dimethoate. Comp Biochem Physiol C: Toxicol Pharmacol 156:104–112
Verma A, Pillai MKK (1991) Bioavailability of soil-bound residues of DDT and HCH to earthworms. Curr Sci 61:840–843
Wauchope RD, Yeh S, Linders JBHJ, Kloskowski R, Tanaka K, Rubin B, Katayama A, Kördel W, Gerstl Z, Lane M, Unsworth JB (2002) Pesticide soil sorption parameters: theory, measurement, uses, limitations and reliability. Pest Manag Sci 58:419–445
Wu XW, Yu YL, Li M, Long YH, Fang H, Li SN (2011) Prediction of bioavailability of chlorpyrifos residues in soil to earthworms. J Soil Sci Plant Nutr 11:44–57
Yu YL, Wu XM, Li SN, Fang H, Tan YJ, Yu JQ (2005) Bioavailability of butachlor and myclobutanil residues in soil to earthworms. Chemosphere 59:961–967
Yu YL, Wu XM, Li SN, Fang H, Zhan HY, Yu JQ (2006) An exploration of the relationship between adsorption and bioavailability of pesticides in soil to earthworm. Environ Pollut 141:428–433
Acknowledgments
This work was supported by INRA, Department of Environment and Agronomy (Pari Scientifique AdVerPe), and by grant from Région Ile-de-France for UHPLC-MS-MS analyses. The authors gratefully thank Olivier Crouzet and Jodie Thénard for their technical support. Biochem-Env is a service of the “Investment d’Avenir” infrastructure AnaEE France, overseen by the French National Research Agency (ANR) (ANR-11-INBS-0001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Nélieu, S., Delarue, G., Ollivier, E. et al. Evaluation of epoxiconazole bioavailability in soil to the earthworm Aporrectodea icterica . Environ Sci Pollut Res 23, 2977–2986 (2016). https://doi.org/10.1007/s11356-015-5270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5270-4