Abstract
This study focuses on spatiotemporal variations in the type of dissolved organic matter (DOM) and copper binding ability both upstream and downstream of Paris. It also compares the relative influence of both natural DOM upstream of Paris and effluent dissolved organic matter (EfDOM) output from a wastewater treatment plant (WWTP) on trace metal speciation and bioavailability in aquatic systems. In addition to the typical high- and low-affinity binding sites, a third family of very high-affinity binding sites has been highlighted for EfDOM. In receiving waters downstream of Paris during low-flow periods, the percentage of high- and very high-affinity sites originating from EfDOM reaches nearly 60 %. According to the speciation computation, the free copper concentration upstream of Paris exceeds the downstream Paris concentration by a factor of 2 to 4. As regards copper bioavailability, the highest EC50tot values were observed for EfDOM and downstream DOM, with a very low aromaticity and low UV absorbance. This finding suggests that specific ultraviolet absorbance (SUVA) is unlikely to be useful in assessing metal speciation and toxicity in aquatic systems subject to strong urban pressures. These results also highlight that the copper speciation computation for surface water exposed to considerable human pressures should include not only the humic and/or fulvic part of dissolved organic carbon but more hydrophilic fractions as well, originating for example from EfDOM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In receiving waters, the value of total metal concentrations does not provide an assessment of metal bioavailability and only partially reflects the environmental impact of trace metals (Campbell 1995). Metal bioavailability is very closely correlated with contaminant speciation (Tessier and Turner 1995) and, consequently, requires evaluating environmental factors that affect metal speciation, in particular dissolved organic matter (DOM), which is a key component of both trace metal speciation (Buffle 1988; Pernet-Coudrier et al. 2011b; Baken et al. 2011) and bioavailability (Campbell 1995; Pernet-Coudrier et al. 2008) in aquatic systems. Many studies have highlighted the trace metal binding ability of DOM in surface waters (Buffle 1988; Tessier and Turner 1995; Town and Filella 2000), yet the vast majority of published information generally concerns either the DOM collected from surface waters not affected by urbanization or humic substances (HS), as isolated from natural waters. HS are heterogeneous polyelectrolyte organic materials (Stevenson 1994) and represent the hydrophobic acid fraction of DOM (Thurman and Malcolm 1981); they typically constitute 40 to 60 % of dissolved organic carbon (DOC) in most natural surface waters ( Martin-Mousset et al. 1997; Perdue and Ritchie 2003; McDonald et al. 2004). However, in rivers under strong pressure from urban sources, the proportion of the hydrophobic fraction of DOM decreases as a result of various urban discharges and the high primary productivity induced by such discharges (Imai et al. 2002; Pernet-Coudrier 2008).
Recent studies have shown that non-humic substances (Sarathy and Allen 2005; Pernet-Coudrier 2008; Baken et al. 2011; Muresan et al. 2011), and especially the hydrophilic fraction of organic matter (Muresan et al. 2011; Pernet-Coudrier et al. 2011a; Louis et al. 2014), can play an important role in the metal complexation in some aquatic systems. Pernet-Coudrier et al. (2011b) demonstrated that the hydrophilic fraction of effluent organic matter (EfDOM) stemming from Wastewater Treatment Plant (WWTP) discharges actually controls, to a large extent, the level of lead speciation downstream of the Paris conurbation. The high binding capacity of this DOM fraction could therefore strongly influence the presence and transport of trace metals in aquatic systems.
This work focuses on the role of EfDOM from WWTP in copper speciation and bioavailability in receiving waters across the Paris conurbation. As opposed to other studies, which are often limited spatially and/or temporally, this study is dedicated to the spatiotemporal variation of the DOM composition and trace metal binding ability both upstream and downstream of Paris, as well as to the comparative role of natural DOM upstream of Paris and EfDOM relative to both trace metal speciation and bioavailability in aquatic systems.
Materials and methods
Sampling points
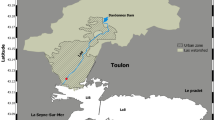
To collect EfDOM, seven campaigns were conducted at the Seine-Aval WWTP (see Fig. 1), which is responsible for collecting over 70 % of dry weather flows from the Paris conurbation (eight million inhabitants). These effluents account for more than 80 % of the EfDOM discharged into the Seine River from throughout the conurbation, thus offering highly representative samples. Treatment consists of primary settling for suspended solid removal, aerobic activated sludge for carbon removal, and biofilters for nitrogen removal (nitrification and denitrification).
In order to characterize the spatial evolution of DOM complexation parameters in receiving waters throughout the Paris conurbation, three sampling sites were selected (Fig. 1):
-
Ussy-sur-Marne (labeled “upstream I”) and Fontaine-le-Port (labeled “upstream II”) are located upstream of the conurbation on the Marne and Seine Rivers, respectively. Due to the low population density in their catchment, these two sites were chosen as “reference sites” for being exposed to minimal impact from urban discharges.
-
Andresy (labeled “downstream”) is located downstream of Paris approximately 9 km from the Seine-Aval WWTP outlet, ensuring a good level of mixture in Seine River water. This site was thus selected as being under high anthropogenic pressure.
Five sampling campaigns were carried out at these three sites in 2010–2011, during periods of both high flow (Nov. 10 and Jan. 11) and low flow (Mar. 11, Jun. 11 and Sept. 11). For each sample, roughly 8 L of collected sample were filtered through glass microfiber (GF/F) filters (pore size = 0.7 μm; Whatman International Ltd., Maidstone, UK). To simplify determination of the copper complexation parameters, samples were pre-concentrated by means of rotary evaporation under vacuum (Buchi© Rotavapor Lab R-210/215), with a low heating bath temperature (40 °C) in order to avoid OM degradation. This pre-concentration step however was kept mild enough to maintain a DOC concentration in the sample (between 10 and 20 mg C L−1) close to the DOC concentration range in receiving water, thus preventing modification to the 3D DOM structure.
A standard fulvic acid (Suwannee River Fulvic Acid, SRFA 2S101F), purchased from the International Humic Substances Society, was used as a “natural organic matter” reference for follow-up investigations relative to the DOM influence on trace metal bioavailability.
DOM characterization
Dissolved organic matter (DOM) has been characterized using various physicochemical tools (Matar 2012), although two were found to be particularly effective, namely UV-visible adsorption and fractionation according to polarity criteria.
UV-visible absorption
UV-visible absorption enables determining the aromaticity of a sample in correlation with the absorbance for a 280-nm wavelength (see Eq. 1).
where Abs280 is the absorbance at 280 nm and [DOC] is the concentration of DOC in mole per liter (Chin et al. 1994).
UV-visible absorption also allows determining specific UV absorbance (SUVA), defined as the UV absorbance of a given sample determined at 254 nm and divided by the organic carbon concentration of the solution. UV absorbance was obtained using a Lambda Perkin Elmer spectrophotometer with 1-cm-long quartz cells.
Fractionation of dissolved organic matter
DOM was fractionated according to its polarity into three distinct fractions: hydrophobic (HPO), transphilic (TPI), and hydrophilic (HPI). Details of the procedure employed are described in Pernet-Coudrier et al. (2008). In summary, after the filtration and pre-concentration steps, the sample was run through DAX-8 and XAD-4 resin columns to obtain the hydrophobic (retained by DAX-8 column) and transphilic (retained by XAD-4 column) fractions (Leenheer 1981). The hydrophilic fraction was not retained by either of these columns. DOC measurements were conducted in both the influent and effluent of the DAX-8 and XAD-4 resins in order to assess the proportion of each fraction.
Characterization of the copper binding ability of DOM
The copper binding site affinity constants (K) and their concentrations (L) in DOM were determined through copper titrations. All chemicals used in the titration process were of ultrapure quality.
A 50-ml portion of the pre-concentrated DOM sample (between 10 and 20 mg C L−1) and KNO3 (0.1 mol L−1) was adjusted to pH 8.0 (a typical Seine River pH). The pH was kept constant (8.00 ± 0.02) by use of a pH stat (Metrohm) that automatically delivered a strong acid (HNO3) or base (KOH) so as to prevent pH alteration during the titration process. pH was measured with a combined electrode (Metrohm). Solutions were continuously stirred and purged with N2 (Linde Gas, Grade 5.0) before and during the titration period to eliminate any carbonate interference. Before beginning titration, the DOM sample was adjusted and maintained at the desired pH for 1 h to ensure its equilibrium under these experimental conditions of both pH and ionic strength. The Cu2+ ions were gradually added manually to the DOM solutions using solutions of Cu (NO3)2 (10−4 and 6.10−3 mol L−1) that had been slightly acidified (pH ~ 3) by HNO3.
After each copper addition, Cu2+ activity in the solution was measured by a cupric ion-selective electrode (Cu-ISE Metrohm) along with a reference electrode Ag (s)/AgCl (s)/KCl (3 mol L−1) (Metrohm) isolated from the solution by a double saline bridge filled with a solution of KNO3 (0.1 mol kg−1). The Cu-ISE was calibrated with a buffered Cu-nitrilotriacetic solution. Values were recorded after the equilibration time required for copper speciation to reach an apparent equilibrium (i.e., less than a 0.1 mv/min change in the potential during 5 min).
The DOM-bounded Cu concentration was estimated as the difference between the total Cu concentration and the inorganic Cu concentration present as Cu2+ ions or as complexes with inorganic substances (as obtained from measured Cu2+ activity and subsequent speciation modeling with Visual MINTEQ, Version 2.40b (Gustafsson et al. 2003)). The concentrations of complexing anions (chlorides, nitrates, and sulfates) in titrated DOM samples were taken into consideration when computing inorganic Cu.
DOM binding properties towards copper were modeled using a discrete distribution of mono-dentate binding ligands (L i ), each of which was defined by two parameters, i.e.,: total site concentration (L imax) and conditional stability constant (K i ). These complexation parameters were then determined by adjusting the experimental curve (ML = f (M)) according to the Langmuir isotherm (Lu and Allen 2002). The following reaction was considered between the metal and the ligand present on the DOM:
The mass action law applied to this equilibrium when considering i ligands leads to Eq. 2 below:
with:
-
i denoting the types of ligands present (i = 1, 2, 3…)
-
ML the concentration of metal bound to an organic ligand
-
M the free copper concentration
-
L imax and K i , respectively, the total adsorption site concentration (mol L−1) and conditional stability constant of ligand i (L mol−1)
For the sake of simplicity, in bimodal distributions, modal distribution 1 corresponding to low-affinity ligands is labeled “carboxylic,” while modal distribution 2 corresponding to high-affinity ligands is labeled “phenolic,” even though the reality is much more complex (Eq. 2). The fit of experimental data (usually involving about 15 ML, in M pairs) to Eq. 2, with respect to the nonlinear regression, serves to determine complexation parameters L imax and K i . The adjustment step uses Sigma Plot 11 software (Jandel Scientific); moreover, the resultant complexation parameters are conditional and dependent on experimental conditions.
Characterization of DOM influence on copper bioavailability
Bioassays of acute toxicity with Daphnia magna were performed on samples from receiving water collected in September 2011 (low-flow period) and an EfDOM sample collected in June 2011 at the Seine-Aval WWTP. The clone used for these tests, D. magna Straus, was kept in Volvic mineral water. D. magna were cultivated in a climate-controlled room (21° ± 1 °C), under lighting controlled at 600 cd sr m−2 (Philips TLD-84 white cool), with a 16 h/8 h day/night cycle and in being fed algae (Pseudokirchneriella subcapitata). Each month, a new culture was started with neonates. The weakly mineralized Volvic water was chosen for the experimental media in order to minimize the influence of the hardness cations on the metal-organism interaction and to avoid precipitation of the metal with carbonates. Two types of media were produced: (i) an inorganic medium with Volvic water and (ii) an organic medium using the DOM sample diluted with Volvic water to obtain a concentration of dissolved organic carbon at 2 mg C L−1. For each medium, five solutions of copper and one control solution were generated, with dissolved copper concentrations ranging from 0 to 1000 μg L−1.
Five D. magna juveniles (<24 h old) were exposed in triplicate within 15 mL of various media inside a thermostatically controlled chamber at 21 °C. All solutions were prepared the day before the experiment to allow for overnight equilibration (one night at 22 °C). The number of immobilized D. magna was counted after 24 h of exposure. A pH = 8 was chosen to replicate environmental conditions as closely as possible.
Total copper concentrations were determined by means of flame atomic absorption spectroscopy (Spectraa 220 F, Varian). EC50tot with a total dissolved copper concentration was calculated from the nonlinear fit of experimental data using the REGTOX logistics model (based on Monte Carlo simulations, E. Vindimian, available at http://eric.vindimian.9online.fr), with a 95 % confidence interval (Isnard et al. 2001). The effect displayed by the percentage of immobilized Daphnia is expressed as a function of total dissolved copper concentration. Control testing (in a medium without additional copper) revealed no toxicity of DOM towards Daphnia. These tests led to determining EC50 after 24 h, in accordance with the NF EN ISO 6341 Standard.
Furthermore, in all cases, copper speciation has been computed at the EC50tot dissolved copper concentration. For samples collected in the framework of the present study, the binding parameters used were those experimentally determined during this protocol. The competition of copper with other trace metals or major elements for DOM complexation has not been taken into account. The mineral composition of Volvic water was included for computation purposes. In addition to expressing EC50 in total dissolved copper (EC50tot), these computations derived an expression for each media EC50FREE in free cupric ion concentration.
Results and discussion
DOM characterization
The spatiotemporal variation of the DOM composition in receiving waters, and in particular its polarity, will be studied in this section.
During the low-water period, the hydrophobic fraction represented approx. 32 % of the DOC for upstream sites. This proportion increased to about 50 % of DOC over the high-water period (see Table 1). In contrast, from the low-water to high-water period, the hydrophilic fraction decreased roughly from 40 to 30 %: this drop was probably due to allochthonous organic matter inputs from terrestrial organic matter, as derived from land plants during the high-water period on the one hand, and on the other, to the decrease of autochthonous organic matter production during the high-flow period compared to low-flow period. Downstream of Paris, the DOM was more hydrophilic than upstream of Paris, particularly during the low-flow period, most likely as a result of the low dilution of EfDOM discharges. In EfDOM, the proportion of non-humic substances (TPI + HPI) equaled approx. 80 % of DOC. Furthermore, 3D spectrofluorescence highlighted the functional richness, notably the presence of nitrogenated groups in these EfDOM samples (Matar 2012). These urban discharges exerted little impact during high-flow periods due to their strong dilution in receiving waters.
As regards aromaticity (Table 1), upstream sites showed lower percentages during the low-water period than at high-water periods, thus confirming the humic characteristic of DOM when water level is high. Aromaticity percentages downstream of Paris exhibited very limited temporal variability. A significant decrease in aromaticity could be noted across the Paris conurbation during the high-water period; this finding cannot be due to urban discharges given their high dilution factor (ranging from 22 to 35 during high flow) in receiving waters.
Complexation parameters for various types of DOM
To yield the overall results of this investigation, the experimental data were appropriately adjusted by the model with two or three ligands (R 2 varies between 0.8 and 0.95), with a 95 % confidence interval. The complexation parameter averages of all samples are shown in Table 2 and Fig. 2. As previously explained in “Characterization of the copper binding ability of DOM,” the DOM-bounded Cu concentration was estimated as the difference between the total and the inorganic Cu concentrations. Under our experimental conditions, the inorganic copper mainly appeared as Cu2+ and CuOH+ cations. Copper complexation by sulfates made up less than 10 % of the total inorganic copper. Complexation by chlorides and nitrates remained very low, making up less than 2 % of the total inorganic copper. No copper complexation by carbonates could be ascribed to the N2 purge of each solution before copper titration.
As is the case for most studies on this topic, one difficulty involved taking into account the DOM binding sites, which were already occupied by trace metals. In this study, however, we attempted to “decontaminate” the pre-concentrated DOM samples by equilibrium (24 h) with a Chelex-100 resin. It cannot be excluded that very high-affinity binding sites remained occupied by trace metals, although no significant differences in Cu-complexation parameters were observed between the decontaminated and non-decontaminated samples.
Cu-DOM stability constants
For DOM in receiving waters, the trace metal complexation was modeled with a bimodal distribution, in accordance with the literature (Milne et al. 2001). For EfDOM, however, in order to adjust the experimental titration curve using the Langmuir isotherm, a trimodal binding site distribution proved to be more suitable with a very high-affinity site type, in addition to the typical low- and high-affinity sites. For both DOM in receiving waters and EfDOM, the conditional stability constants ranged from 107.3 to 107.9 M−1 for low-affinity sites (L1) and from 1010.2 to 1011.2 M−1 for high-affinity sites. These values are consistent with previous studies (Sarathy and Allen 2005). For low- and high-affinity sites, no significant difference was found across the various sampling points and moreover no spatial or temporal variations in stability constants (log K) were observed. In the case of EfDOM, the stability constants of very high-affinity (L3) sites ranged from 1012.2 to 1013.5 M−1. Previously, Croue et al. (2003) and Pernet-Coudrier et al. (2011b) had also used a trimodal distribution to describe the behavior of nitrogen-enriched organic materials. More specifically, Pernet-Coudrier et al. (2011b) assumed that this third family of binding sites (L3) could be associated with proteinic structures, amines, and amide groups present in the hydrophilic fraction of EfDOM originating from the same WWTP. This expanded functionality, and especially the presence of nitrogenated groups, was highlighted by 3D spectrofluorescence in these samples, as presented elsewhere (Matar 2012). Some of these binding sites (L3) might also be associated with amino polycarboxylates, organophosphonates, and hydroxycarboxylates (Knepper 2003).
Total binding site densities and concentrations for various types of DOM
figure 2 shows the binding site density (moles of sites per kilogram of carbon) as well as the binding site concentration (moles of sites per liter) for low-affinity sites (L1) and high-affinity sites (L2). For very high-affinity sites (L3), results are presented in Table 2.
Low-affinity (L1) binding sites
A low seasonal variation in L1 binding site densities was observed for all upstream and downstream sites, with a very slight increase during low-water periods (Fig. 2). Intraseasonal variability was quite high for both the upstream II and downstream sites. Intersample variability was also quite high for EfDOM. An unexplained L1 density increase was recorded across the conurbation, especially during the low-flow period (+63 %); this increase was probably not caused by EfDOM, which displays a lower L1 density than DOM in upstream receiving waters.
High-affinity (L2) binding sites
Concerning L2 binding site densities, a low seasonal variation was observed for upstream I, whereas the seasonal variation was very high for upstream II with greater densities during the high-water period (Fig. 2). The downstream site exhibited a high seasonal variation with greater site densities during the low-water period. Intraseasonal variability was fairly high for the upstream II site, while intersample variability was also rather high for EfDOM. Spatial variation across the Paris conurbation was low during the high-water period; for the low-water period, however, a significant increase in site densities could be observed at the downstream site. This increase was probably due to EfDOM, which displayed both high L2 densities and high L2 concentrations. Such a trend was not observed however during high-flow periods due to EfDOM dilution in receiving waters (the dilution factor varied from 22 to 35 during high flow and from 6 to 16 during low flow). Let us note that the binding site densities (L1 and L2) obtained in this study are consistent with the literature (Breault et al. 1996; Town and Filella 2000; Sarathy and Allen 2005).
Very high-affinity (L3) binding sites
These sites have only been highlighted for EfDOM. The L3 site density was less than L2 and L1 densities (see Table 2 and Fig. 2); this site density amounted to between 25 and 33 % of the L2 density in EfDOM, with a molar concentration just below 1 μmol L−1 in treated effluent. Even though the molar concentration of the L3 site was less than that of other sites, it still remains environmentally relevant given that trace metal concentrations in receiving waters were considerably lower, ranging from 10−10 to 10−7 mol L−1. The absence of this third type of ligand downstream of Paris might have been due to the dilution of EfDOM, yielding an L3 concentration under the analytical detection limit. It might also have been due to the strong trace metal complexation by these very strong ligands occurring in receiving waters, in which case these previously occupied sites had not been quantified according to the copper titration method.
Site distribution for various types of DOM
Low-affinity to high-affinity site (L1/L2) distributions were calculated for all samples (Table 2). By far, EfDOM presents the lowest ratio, with values ranging from 1.3 to 4.6, in comparison with the DOM from receiving waters, whose average ratio varied between 4.9 and 10. Furthermore, EfDOM has a third type of ligand displaying a very high copper affinity. The high binding ability of EfDOM could be explained by their nitrogen and sulfur group contents (Muresan et al. 2011; Pernet-Coudrier et al. 2011a). It could be noticed that EfDOM revealed a high trace metal binding ability despite its low aromaticity, as indicated by its low SUVA (Table 1). These results therefore are also consistent with studies demonstrating the strong binding capacity of EfDOM (Sarathy and Allen 2005; Pernet-Coudrier 2008).
Binding site molar concentrations across the Paris conurbation
During the low-water period, the binding site molar concentrations evolved significantly from upstream to downstream, with an increase of 173 % for L1 sites and 157 % for L2 sites (Fig. 2). This increase in binding site concentrations was probably due to EfDOM discharges in the receiving waters. Indeed, L1 and L2 site concentrations in EfDOM were respectively three times and ten times higher than in upstream waters.
During the high-water period, as a likely consequence of higher urban discharge dilution, the L2 site concentrations did not evolve to any significant extent from upstream to downstream. On the other hand, L1 site concentrations increased by about 50 %; this increase was left unexplained and could not be correlated with EfDOM discharges due to their dilution during high flows (the dilution factor ranged from 22 to 35).
Seine-Aval WWTP contribution to the binding site fluxes in receiving waters
The ratio of binding sites originating from EfDOM to total binding sites measured downstream of Paris was calculated as follows:
with:
L XEfDOM flux = [LX]EfDOM * EfDOM flow: binding site flux originating from EfDOM
L XDown flux = [L X ]Down * down flow: binding site flux downstream of Paris
x: type of binding site: 1, 2, or 3 for low, high, and very high-affinity sites, respectively
[L X ]: binding site concentration, expressed in mole per liter
Down flow: average daily flow measured downstream of Paris, expressed in liter per second
EfDOM flow: average monthly flow, expressed in liter per second
This flux ratio has been calculated for all sampling dates (Fig. 3). Nevertheless, for each date, only one binding site concentration was determined, thus making this result an instantaneous flux ratio of sites and not an average flux ratio. Moreover, this ratio could not be calculated for the third type of ligand of highest affinity (L3), since only two ligand types were found downstream of Paris. However, the percentage of the sum of sites (L2 + L3) derived from the Seine-Aval WWTP, compared to the number of sites (L2) present downstream, was actually calculated.
During high-flow periods, the averaged values of this ratio were around 5 and 23 %, respectively, for low-affinity (L1) and high-affinity (L2 + L3) sites. Due to the lower EfDOM dilution during the low-flow period, these averaged values were two to five times higher, i.e., reaching 19 and 58 %, respectively. For downstream Paris at times of low water, the major portion of high-affinity binding sites stems from EfDOM; the other minor portion originates from upstream Paris. Downstream of Paris, the percentages of high-affinity sites (L2 + L3) originating from EfDOM are much greater than those of low-affinity sites (L1), which underscores the strong impact, especially during low-flow periods, of urban discharges on the high ligand concentration downstream of Paris.
Modeling of copper speciation
To assess the environmental implications relative to the strong copper binding ability of EfDOM, copper speciation was computed in the Seine-Aval WWTP effluent and in receiving waters both upstream and downstream of the WWTP discharge point. Only those binding parameters experimentally determined in this study (Table 2) were used to conduct the computation. The competition of copper with other trace metals or major elements for DOM complexation was not taken into account. For both cases studied, pH was set at 8, which is the known pH value for the Seine River. The carbonate and chloride concentrations were set, respectively, at 4 and 0.5 mmol L−1.
Modeling of copper speciation in Seine-Aval WWTP effluent
Copper speciation was computed for three different realistic copper concentrations (Table 3). The binding site concentrations and EfDOM affinity constants used for this computation were averaged (seven campaigns, see Table 2).
At low copper concentrations (1 and 10 μg L−1), over 99 % of the total dissolved copper was bound to dissolved organic matter, which therefore was able to control Cu speciation in the effluent. Very high-affinity binding sites (L3) bound more than 98 % of total copper, whereas the high- and low-affinity sites (L2 and L1) were barely involved in copper binding. The free copper proportion was extremely low, i.e., less than 10−4 % of total dissolved copper.
At a higher copper concentration (100 μg L−1), the L3 sites became saturated, which in the present case induced Cu binding with both L3 and L2 sites in very similar proportions (≈50 % for each). This situation also induced a sharp increase in the inorganic, especially the free, copper fractions, whose proportions (despite remaining quite low) increased by a factor of 100 when compared with a 10 μg L−1 copper concentration. This comparison was not attempted in the present study, but it is likely that only considerably higher Cu concentrations are capable of causing significant Cu binding by low-affinity L1 sites.
Modeling of copper speciation in receiving water both downstream and upstream of the Paris conurbation
Copper speciation was computed upstream and downstream of Paris during the low-water period, when as a result of low dilution the impacts of urban discharges (in particular those of Seine-Aval WWTP) were potentially at their highest in receiving waters. Binding site concentrations and affinity constants of the upstream (I and II) and downstream sites used in this computation were averaged (three campaigns during the low-water period). The upstream site complexation parameters were obtained by averaging upstream I and upstream II weighted by their flows.
This computation was performed for copper concentrations ranging from 0.1 to 10 μg L−1, which is consistent with total dissolved copper concentrations observed in aquatic systems (Table 4).
In both cases and for all copper concentrations, over 98 % of copper was bound to dissolved organic matter, especially to high-affinity sites. At all copper concentration levels, the inorganic copper and free copper were both more abundant upstream of Paris, where free copper exceeded the downstream value by a factor of 2 to 4. This result was probably due to the higher complexing affinity of DOM downstream of Paris, as previously highlighted in “Complexation parameters for various types of DOM.”
Although these computations are not fully representative of the actual media, they nonetheless demonstrate, for the first time, that EfDOM can exert a greater impact on copper speciation than “natural” hydrophobic DOM sampled upstream of Paris in an aquatic system not significantly affected by urban discharges. These results also highlight that the Cu speciation computation in surface water subjected to strong human pressures should include not only the humic and/or fulvic part of DOC but also other more hydrophilic fractions stemming, for example, from EfDOM.
Evaluation of the role of DOM on copper bioavailability
EC50tot values, expressed in total dissolved copper concentration for each studied DOM and for the inorganic matrix, are presented in Fig. 4a. First of all, these results indicate a low variability for all three replicates performed in the mineral matrix, thus suggesting good repeatability of the bioassay and a non-evolution of Daphnia breeding clones.
The EC50tot values were systematically higher in the presence of DOM, in comparison with tests conducted in the inorganic matrix. By binding copper, DOM actually reduced its bioavailability and hence required higher copper concentrations to achieve the same effect, resulting in the EC50 increasing to higher concentrations. This outcome was very consistent with the literature, which portrays the role of organic matter in reducing the toxicity of metals (Markich et al. 2003; Schwartz et al. 2004; De Schamphelaere et al. 2004; DePalma et al. 2011; Trenfield et al. 2011).
Statistically significant differences (by a factor of 2) in EC50tot, depending on the type of DOM, have been observed. DOM originating from upstream Paris displayed significant differences with the lower EC50tot value for upstream II when compared to upstream I. The upstream I value was comparable to that obtained for the reference hydrophobic organic matter (SRFA). For the downstream site, a higher EC50tot was recorded, in comparison with upstream sites as well as with SRFA. The highest EC50tot was found for EfDOM, which confirms the influence of EfDOM in reducing metal toxicity, as demonstrated in a previous study ( Pernet-Coudrier et al. 2008). As opposed to what has been observed in natural waters (De Schamphelaere et al. 2004), no positive correlation could be derived either between EC50tot and SUVA, which acts as a good proxy for DOM aromaticity, or between EC50tot and the aromaticity percentage (Table 1). The highest EC50tot values however were observed for EfDOM and DOM from downstream with very low aromaticity and a low UV absorbance. Compared to DOM from upstream I and II, EfDOM and downstream DOM presented higher densities of high-affinity binding sites (L2) and even very high-affinity binding sites (L3) for EfDOM, which may be responsible for the greater protective role played by these DOM samples. This finding demonstrates the key protective role of EfDOM as regards copper toxicity on D. magna.
EC50FREE values, expressed in free cupric ion concentration for each studied DOM and for the inorganic matrix, are presented in Fig. 4b. For SRFA and upstream II DOM, the free copper concentrations obtained from the copper speciation computation at EC50tot were quite similar around 5 μg L−1 and not statistically different from the value in inorganic media, which is in good agreement with the biotic ligand model (BLM) prediction. For EfDOM, upstream I, and downstream DOM, however, the free copper concentrations ranged from 13.6 to 28.3 μg L−1, which was significantly higher than in inorganic media; this finding was in disagreement with the BLM prediction. To explain such disagreement, the hypothesis could be forwarded that the DOM protective effect is not solely due to copper binding. The biotic ligand might be occupied or coated by organic molecules that prevent copper binding by the biotic ligand and thereby decrease free copper toxicity.
Environmental impacts
These results may have major environmental implications, notably for trace metal toxicity modeling in an aquatic system under high anthropogenic pressure. According to the widely used BLM, which predicts metal toxicity, the DOC is actually considered to offer an “active” fraction for binding metal ions along with another fraction that remains inert relative to metal binding. Consequently, the proportion of active DOC has a significant effect on the modeled metal speciation and toxicity. This active fraction has been found to vary between 40 and 80 % as a fulvic acid (Dwane and Tipping 1998). A default active fraction value of 50 % however is often applied (De Schamphelaere et al. 2002, 2004). Furthermore, the use of specific UV absorbance to estimate the active fraction actually improves the accuracy of BLM toxicity forecasts by a factor ranging between 1.3 and 2 ( Al-Reasi et al. 2012; De Schamphelaere et al. 2004; Kramer et al. 2004; Sanchez-Marin et al. 2010). Yet these studies merely focused on the most hydrophobic fraction of the DOM (humic and fulvic substances) and/or on natural DOM not affected by wastewater discharges, which mainly displayed trace metal binding groups (e.g., carboxylic and phenolic) with strong SUVA. A strong correlation between specific UV absorbance and this active DOC fraction might therefore have been reasonably expected in such cases.
In this study, we have demonstrated the importance of EfDOM, which also exhibits strong copper binding properties in spite of a low SUVA and low aromaticity. This feature suggests that SUVA is unlikely to be useful for predicting metal speciation and toxicity in an aquatic system with major urban inputs, which is indeed consistent with other studies ( Pernet-Coudrier et al. 2008, 2011a; Muresan et al. 2011; Baken et al. 2011). If DOM with low aromaticity is not taken into account in the BLM, especially in receiving waters under high anthropogenic pressure, then both the bioavailability and metal toxicity may be overestimated.
Conclusion
Trace metal complexation by DOM in receiving waters has been modeled using a bimodal distribution, while for EfDOM, a trimodal binding site distribution has proven to be more suitable with a very high-affinity site type as well as with more typical low- and high-affinity sites. This third family of binding sites is probably correlated with the proteinic structures, amines, and amide groups present in the hydrophilic EfDOM fraction. Even though the molar concentration of these very high-affinity binding sites was less than that of other sites, i.e., just below 1 μmol L−1 in EfDOM, it remains environmentally relevant insofar as trace metal concentrations in receiving waters were significantly lower, ranging from 10−10 to 10−7 M. During low-water periods, binding site molar concentrations increased dramatically from Paris upstream to downstream. This increase was likely due to EfDOM discharges in receiving waters. For the low-flow period, the percentage of high-affinity sites in receiving waters downstream of Paris stemming from EfDOM reached nearly 60 %. According to the speciation computation, copper was almost entirely bound to very high-affinity binding sites in EfDOM. In the upstream receiving waters, the free copper concentration was higher by a factor of 2 to 4 than the downstream concentration, most likely as a result of the higher complexing affinity of downstream DOM. As regards copper bioavailability, the highest EC50tot values were observed for both EfDOM and downstream DOM, with very low aromaticity and low UV absorbance. This feature suggests that SUVA is unlikely to be useful in assessing metal speciation and toxicity in aquatic systems subjected to strong urban pressures. These results may considerably influence the environmental impact on trace metal toxicity modeling in aquatic systems under high anthropogenic pressure, in addition to highlighting the fact that the Cu speciation computation in surface waters exposed to strong human pressures should include not only the humic and/or fulvic part of DOC but more hydrophilic fractions as well, namely those stemming from EfDOM.
References
Al-Reasi HA, Scott Smith D, Wood CM (2012) Evaluating the ameliorative effect of natural dissolved organic matter (DOM) quality on copper toxicity to Daphnia magna: improving the BLM. Ecotoxicology 21:524–537
Baken S, Degryse F, Verheyen L, Merckx R, Smolders E (2011) Metal complexation properties of freshwater dissolved organic matter are explained by its aromaticity and by anthropogenic ligands. Environ Sci Technol 45:2584–2590
Breault RF, Colman JA, Aiken GR, McKnight D (1996) Copper speciation and binding by organic matter in copper-contaminated streamwater. Environ Sci Technol 30:3477–3486
Buffle J (1988) Complexation reactions in aquatic system: an analytical approach. Ellis Horwood, New York, p 692
Campbell PGC (1995) Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, Chichester, Grande-Bretagne, pp 45–102
Chin YP, Aiken G, Oloughlin E (1994) Molecular-weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ Sci Technol 28:1853–1858
Croue JP, Benedetti MF, Violleau D, Leenheer JA (2003) Characterization and copper binding of humic and nonhumic organic matter isolated from the South Platte River: evidence for the presence of nitrogenous binding site. Environ Sci Technol 37:328–336
De Schamphelaere KAC, Heijerick DG, Janssen CR (2002) Refinement and field validation of a biotic ligand model predicting acute copper toxicity to Daphnia magna. Comp Biochem Physiol C-Toxicol Pharmacol 133
De Schamphelaere KAC, Vasconcelos FM, Tack FMG, Allen HE, Janssen CR (2004) Effect of dissolved organic matter source on acute copper toxicity to Daphnia magna. Environ Toxicol Chem 23:1248–1255
DePalma SGS, Arnold WR, McGeer JC, Dixon DG, Smith DS (2011) Effects of dissolved organic matter and reduced sulphur on copper bioavailability in coastal marine environments. Ecotoxicol Environ Saf 74:230–237
Dwane GC, Tipping E (1998) Testing a humic speciation model by titration of copper-amended natural waters. Environ Int 24:609–616
Gustafsson JP, Pechova P, Berggren D (2003) Modeling metal binding to soils: the role of natural organic matter. Environ Sci Technol 37:2767–2774
Imai A, Fukushima T, Matsushige K, Kim YH, Choi K (2002) Characterization of dissolved organic matter in effluents from wastewater treatment plants. Water Res 36:859–870
Isnard P, Flammarion P, Roman G, Babut M, Bastien P, Bintein S, Essermeant L, Ferard JF, Gallotti-Schmitt S, Saouter E, Saroli M, Thiebaud H, Tomassone R, Vindimian E (2001) Statistical analysis of regulatory ecotoxicity tests. Chemosphere 45:659–669
Knepper TP (2003) Synthetic chelating agents and compounds exhibiting complexing properties in the aquatic environment. Trac-Trends Anal Chem 22:708–724
Kramer KJM, Jak RG, van Hattum B, Hooftman RN, Zwolsman JJG (2004) Copper toxicity in relation to surface water-dissolved organic matter: biological effects to Daphnia magna. Environ Toxicol Chem 23
Leenheer JA (1981) Comprehensive approach to preparative isolation and fractionation of dissolved organic-carbon from natural-waters and wastewaters. Environ Sci Technol 15:578–587
Louis Y, Pernet-Coudrier B, Varrault G (2014) Implications of effluent organic matter and its hydrophilic fraction on zinc(II) complexation in rivers under strong urban pressure: aromaticity as an inaccurate indicator of DOM-metal binding. Sci Total Environ 490:830–837
Lu YF, Allen HE (2002) Characterization of copper complexation with natural dissolved organic matter (DOM)—link to acidic moieties of DOM and competition by Ca and Mg. Water Res 36:5083–5101
Markich SJ, Brown PL, Jeffree RA, Lim RP (2003) The effects of pH and dissolved organic carbon on the toxicity of cadmium and copper to a freshwater bivalve: further support for the extended free ion activity model. Arch Environ Contam Toxicol 45:479–491
Martin-Mousset B, Croue JP, Lefebvre E, Legube B (1997) Distribution and characterization of dissolved organic matter of surface waters. Water Res 31:541–553
Matar Z (2012) Influence de la matière organique dissoute d'origine urbaine sur la spéciation et la biodisponibilité des métaux dans les milieux récepteurs anthropisés, Université Paris-Est, 258p.
McDonald S, Bishop AG, Prenzler PD, Robards K (2004) Analytical chemistry of freshwater humic substances. Anal Chim Acta 527:105–124
Milne CJ, Kinniburgh DG, Tipping E (2001) Generic NICA-Donnan model parameters for proton binding by humic substances. Environ Sci Technol 35:2049–2059
Muresan B, Pernet-Coudrier B, Cossa D, Varrault G (2011) Measurement and modeling of mercury complexation by dissolved organic matter isolates from freshwater and effluents of a major wastewater treatment plant. Appl Geochem 26:2057–2063
Perdue EM, Ritchie JD (2003) Dissolved organic matter in freshwaters. Treatise on Geochem 5:273–318
Pernet-Coudrier B (2008) Influence de la matière organique dissoute sur la spéciation et la biodisponibilité des métaux: Cas de la Seine, un milieu sous forte pression urbaine. Université Paris-Est 292p.
Pernet-coudrier B, Clouzot L, Varrault G, Tusseau-vuillemin MH, Verger A, Mouchel JM (2008) Dissolved organic matter from treated effluent of a major wastewater treatment plant: characterization and influence on copper toxicity. Chemosphere 73:593–599
Pernet-Coudrier B, Varrault G, Saad M, Croue JP, Dignac MF, Mouchel JM (2011a) Characterisation of dissolved organic matter in Parisian urban aquatic systems: predominance of hydrophilic and proteinaceous structures. Biogeochemistry 106:89–106
Pernet-Coudrier B, Companys E, Galceran J, Morey M, Mouchel JM, Puy J, Ruiz N, Varrault G (2011b) Pb-binding to various dissolved organic matter in urban aquatic systems: key role of the most hydrophilic fraction. Geochimica Et Cosmochimica Acta 75:4005–4019
Sanchez-Marin P, Santos-Echeandia J, Nieto-Cid M, Anton Alvarez-Salgado X, Beiras R (2010) Effect of dissolved organic matter (DOM) of contrasting origins on Cu and Pb speciation and toxicity to Paracentrotus lividus larvae. Aquat Toxicol 96
Sarathy V, Allen HE (2005) Copper complexation by dissolved organic matter from surface water and wastewater effluent. Ecotoxicol Environ Saf 61:337–344
Schwartz ML, Curtis PJ, Playle RC (2004) Influence of natural organic matter source on acute copper, lead, and cadmium toxicity to rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 23:2889–2899
Stevenson FJ (1994) Humus chemistry: genesis, composition, reaction, 2nd edn. Wiley, New York
Tessier A, and Turner DR (1995) Metal speciation and bioavailability in aquatic systems, 679p., ed. B.J.a.V.L.H. P., John Wiley & sons: Chichester.
Thurman EM, Malcolm RL (1981) Preparative isolation of aquatic humic substances. Environ Sci Technol 15:463–466
Town RM, Filella M (2000) A comprehensive systematic compilation of complexation parameters reported for trace metals in natural waters. Aquat Sci 62:252–295
Trenfield MA, McDonald S, Kovacs K, Lesher EK, Pringle JM, Markich SJ, Ng JC, Noller B, Brown PL, van Dam RA (2011) Dissolved organic carbon reduces uranium bioavailability and toxicity. 1. Characterization of an aquatic fulvic acid and its complexation with uranium VI. Environ Sci Technol 45:3075–3081
Acknowledgments
This study was conducted within the framework of the OPUR and Piren-Seine research programme.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Matar, Z., Soares Pereira, C., Chebbo, G. et al. Influence of effluent organic matter on copper speciation and bioavailability in rivers under strong urban pressure. Environ Sci Pollut Res 22, 19461–19472 (2015). https://doi.org/10.1007/s11356-015-5110-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5110-6