Abstract
Tomato (Lycopersicon esculentum Mill.) becomes one of the world’s foremost vegetables, and its world production and consumption have increased fairly quickly. The capacity to induce oxidative stress in tomato plant, exposed to three xenobiotics such as alpha-cypermethrin, chlorpyriphos, and pirimicarb, was investigated by the evaluation of lipid peroxidation by measuring malondialdehyde (MDA) rate; also, we studied the response of tomato to this stress by assessing the response of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), glutathione-s-transferase (GST), and glutathione reductase (GR). The effect of the insecticides was observed using four concentrations (25, 50, 75, and 100 %) for germinating seeds and only the recommended concentration in agriculture (100 %) for growing plants. Our results show an important accumulation of MDA, demonstrating the increase of lipid peroxidation in consequence of the excessive reactive oxygen species (ROS) production due to insecticide treatment. In response to this oxidative stress in tomato seedlings and plants, the activities of antioxidant-enzyme system were generally enhanced. The electrophoretic analysis showed also the apparition of new isoenzymes as the case for CAT and POD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Application of pesticides is an important practice for control of plant growth in modern agriculture. However, the use of pesticides obtained by chemical synthesis represents the major cause of the contamination occurring in agriculture. Even if they are correctly applied, pesticides may present important risks because of their persistence, bioavailability, and mobility (Arias-Estévez et al. 2008).
Moreover, there is growing evidence indicating that pollutants such as pesticides and other organic toxic substances in the environment are able to induce the intracellular overproduction of reactive oxygen species (ROS), damaging then the plant cells (Peixoto et al. 2006; Song et al. 2006; Wang and Zhou 2006).
Plants have evolved various protective mechanisms to eliminate or reduce ROS caused by damages and stresses. The enzymatic antioxidant system is a protective mechanism, which operates with the sequential and simultaneous actions of enzymes including superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT). Some studies were caring out with some herbicides such as isoproturon, which induce a delay of growth on wheat plants, and affect also many physiological process. Isoproturon induce oxidative stress, and wheat plants activated a variety of antioxidative enzymes, such as SOD, CAT, APX, and POD, to diminish the ROS. Additionally, the activity of glutathione-s-transferase (GST), one of the typical detoxifying enzymes, was increased (Yin et al. 2008). In other hand, Wu et al. (2010) demonstrated that the herbicide fluroxypyr affected the growth of rice. Fluroxypyr-inhibited growth was closely linked to the generation of ROS in leaves. To deal with fluroxypyr-induced oxidative stress, several antioxidative enzymes such as SOD, CAT, APX, and POD were increased at low concentration of herbicide and decreased at higher concentrations. These biochemical results can be interpreted as an internal tolerant mechanism. In another study, Zhang et al. 2014 demonstrate that the accumulation of atrazine in rice plants led to toxic responses such as over-generation of ROS, which activate the plant defense system. This was best presented by the enhanced activities of several antioxidant enzymes such as SOD, CAT, and POD, and expression of genes responsible for the tolerance to atrazine toxicity.
In this study, we evaluate the response of the enzymatic antioxidant system in tomato plant (Lycopersicon esculentum Mill.) to the application of the most used insecticides in the northern of Morocco, such as alpha-cypermethrin (pyrethroid insecticide), chlorpyriphos (organophosphorus insecticide), and pirimicarb (carbamate insecticide), by using the SOD, CAT, POD, APX, glutathione reductase (GR), and GST parameters.
Materials and methods
Plant material and germination process
Tomato (L. esculentum Mill.) seeds were surface-sterilized in 10 % commercial bleach with stirring for 5 min, followed by extensive washing in sterile-distilled water. Batches of 50 seeds of tomato were germinated in Petri dishes (diameter of 9 cm) on top of two layers of filter paper moistened with 6 ml of either distilled water or insecticide solutions at the concentrations of 25, 50, 75, and 100 % (100 % represents the normal concentration used in agriculture) and maintained in a growth chamber in darkness at 25 °C for 6 days. At various stages of tomato seed germination for 3, 4, and 5 days, seeds of each replicate were collected for the measurement. Three replicates were performed. Germination time corresponds to the time of rupture of the seed coats and the emergence of the radical through the seed coat; seedlings were grown for up to a maximum of 6 days.
For plant study, the seeds were sown in plastic pots for germination and growth. Each pot was filled with prepared soils. Seedlings were grown at 24 °C/20 °C (day/night) temperature, and 16 h of light, and watered each day. After growth for 30 days, the plants were treated by the insecticides at the concentration of 100 %, representing the normal concentration used in agriculture. The tests were realized in the 2nd, 5th, 8th, 11th, and 14th day after treatment.
Determination of lipid peroxidation
Lipid peroxidation was determined by calculating the rate of malondialdehyde (MDA) expressed in μmol of MDA formed/mg of proteins, according to Health and Packer (1981) who used the thiobarbituric acid (TBA) as substrate. Of the supernatant, 100 μl is added with 1 ml of a trichloroacetic acid solution (TCA) to 0.1 % (p/v). The homogenate was centrifuged at 10,000g during 5 min, and 200 μl of supernatant is mixed with 800 μl of 0.5 % TBA, prepared in 20 % TCA. The mixture was heated at 95 °C for 30 min, chilled on ice, and centrifuged at 10,000g throughout 5 min. The absorbance of the supernatant was measured at 532 nm. The value for non-specific absorbance at 600 nm was subtracted. The amount rate of MDA formed was calculated by using the extinction coefficient of 155 mM−1 cm−1.
Chlorophyll analyses
Chlorophyll was measured following Arnon (1949). The extraction and analysis of chlorophyll were performed by homogenization of 0.1 g fresh leaves in 8 ml of 80 % acetone (pH 7.8 adjusted with sodium phosphate buffer), followed by centrifugation at 500g for 10 min. The supernatant was used for spectrophotometric determination, and the rates of chlorophyll A and chlorophyll B were calculated.
Assay of antioxidant enzymes
Of the germinating seeds, 100 mg were homogenized in 100 mM phosphate buffer (pH 7.0) containing 1 mM EDTA, 1 mM PMSF, and 0.5 % PVP. The mixture was centrifuged at 9000g for 20 min. The supernatant was used as enzyme extract to determine enzyme activity.
SOD activity (EC 1.15.1.1) was assayed by measurement of its capacity of inhibiting the photochemical reduction of nitro-blue tetrazolium (NBT) according to Beauchamp and Fridovich (1971). Three milliliters of reaction mixture contained 50 mM phosphate buffer (pH 7.8), 10 mM methionine, 1.17 mM riboflavin, 56 mM NBT, and the enzyme extract. The absorbance of this solution was measured at 560 nm. One unit of SOD was defined as the enzyme activity that inhibited the photo-reduction of nitro-blue tetrazolium to blue formazan by 50 %.
CAT (EC 1.11.1.6) activity was determined by the consumption of H2O2 (extinction coefficient 39.4 mM × cm−1) at 240 nm for 30 s (Aeobi 1974). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 1 M H2O2, and the enzymatic extract.
POD (EC 1.11.1.7) activity was measured following the change of absorbance at 436 nm due to guaiacol oxidation (ε 436 = 6.39 mM−1 × cm−1). The activity was assayed for 1 min in a reaction solution composed of 100 mM potassium phosphate buffer (pH 7.0), 6 mM guaiacol, 10 mM H2O2, and the enzymatic extract (Putter 1978).
APX activity was measured by the decrease in absorbance at 290 nm. The reaction mixture contained 50 mM Hepes-NaOH (pH 7.6), 0.25 mM ascorbate, 0.1 mM H2O2, and the enzymatic extract (Hossain and Asada 1984).
GST activity was measured in accordance with Habig et al. (1974), using 1-chloro-2,4-dinitrobenzène (CDNB) as substrate. The reaction mixture contained 0.1 M potassium phosphate buffer (pH 6.8), 20 mM CDNB, 10 mM GSH, and the enzymatic extract. GST activity was measured by the increase in absorbance at 340 nm due to the reaction of GSH and CDNB.
GR activity was measured by the decrease in absorbance at 340 nm, due to the NADPH oxidation. The reaction mixture contained 50 mM Tris buffer (pH 7.8), 1 mM NADPH, 5 mM GSSG, and the enzymatic extract (Foyer and Halliwell 1976).
Polyacrylamide gel electrophoresis
The isoenzymes of SOD, CAT, and POD were separated on the discontinuous polyacrylamide gels (stacking gel 4 % and separating gel 12 %) under the non-denaturing conditions. Proteins were electrophoresed at 4 °C and under 80 V for 4 h.
SOD activity was determined on the gel as described by Beauchamp and Fridovich (1971). The gels were rinsed in water and incubated in darkness for 30 min at room temperature, in the assay mixture containing 50 mM potassium phosphate buffer (pH 7.8), 28 mM riboflavin, 28 μM tetramethylethylenediamine (TEMED), and 0.1 mM nitro-blue tetrazolium. Next, the gels were rinsed with water and exposed in a light box for 10 min at room temperature until the development of colorless bands of SOD activity; a purple- stained gel was visible.
For POD isoforms, the gels were stained for 20 min in 0.2 M acetate buffer (pH 5.5), 5 mM benzidine, and 5 mM H2O2 (Zhou et al. 2007).
Concerning the detection of CAT isoenzyme activity, the gel was soaked in deionized water during 15 min. Subsequently, the gel was incubated in 0.03 % H2O2 for 25 min and then carefully washed with deionized water to remove the residual H2O2. Next, the gel was stained in the solution of 1 % (w/v) potassium ferricyanide and 1 % (w/v) ferric chloride (Woodbury et al. 1971). This allows the gel to turn blue except at positions exhibiting CAT activity. When maximum contrast was achieved, the reaction was stopped by rinsing the gel with deionized water.
Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA) using Statistica Software (Statistica 1997). Post hoc testing was carried out using the Tukey test. A significance level of 0.05 was used for all statistical tests.
Results
Evaluation of lipid peroxidation
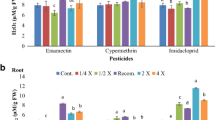
Table 1 shows a significant increase of MDA rate in treated seedlings since the 3rd day registered 0.89 μmol of MDA formed/mg of FW in response to the treatment with alpha-cypermethrin at the concentration of 100 % for example, compared to the control which was rounding 0.49 μmol of MDA formed/mg of FW. In the same way, Table 2 shows an increase in MDA rate in leafs and roots since the 2nd day after treatment, registered values fluctuating between 0.73 and 1.52 μmol/mg FW for leafs, and between 0.86 and 2.36 μmol/mg FW for roots. These concentrations are significantly higher than control’s rates which are rounding between 0.24 and 0.52 μmol/mg FW for leafs, and between 0.35 and 0.55 μmol/mg FW for roots.
Evaluation of chlorophyll rate
The application of insecticides influences negatively the rate of chlorophyll. A decrease in chlorophyll A and chlorophyll B concentrations has been observed after the treatment by both insecticides (Table 3).
Response of superoxide dismutase
Table 1 shows a significant increase of SOD rate in treated seedling since the 3rd day recorded values reaching 5.1 U/min/μg of proteins in response to the treatment with alpha-cypermethrin at the concentration of 100 % for example, compared to the control which was rounding 1.79 U/min/μg of proteins.
In the same way, Table 2 shows a stimulation of SOD activity in leaves and roots especially since the 5th day after treatment registered values fluctuating between 3.65 and 11.96 U/min/μg of proteins for the leaves, and from 2.72 to 4.55 U/min/μg of proteins for roots. These activities are significantly higher than control which activity was rounding between 2.25 and 2.9 U/min/μg of proteins for leafs, and between 1.39 and 2.01 U/min/μg of proteins for roots.
The electrophoretic analysis of SOD in seedling (Fig. 1) showed no qualitative difference between control and treated samples. In both cases, the study revealed the presence of 3 isoenzymes of SOD, with a quantitative difference between them; the treated sample bands are more marked. In leaves, Fig. 2 demonstrates an essentially quantitative difference between treated samples and the control, presenting both of them 3 isoenzymes of SOD. In roots, the study highlighted a predominance of a single isozyme in both treated and control samples, and more marked in the treated ones.
Response of catalase
Table 1 illustrates a significant stimulation of the catalase activity in treated seedlings with the application of all insecticides from the 3rd day of monitoring. This stimulation was 2- to 7-fold greater when compared with control, reaching for example in the 3rd day 0.075 μmol/min/μg of proteins with α-cypermethrin (100 %) vs. about 0.011 μmol/min/μg of proteins for control.
Table 2 demonstrates an increase in catalase activity in treated leaves from the first week of test period. Catalase activity was generally 2 to 3 times higher in comparison with control. For example, in the 5th day, the activity of catalase in leaves treated by chlorpyriphos was 0.094 μmol/min/μg of proteins vs. 0.033 μmol/min/μg of proteins for control.
Concerning the roots (Table 2), a significant response of catalase was obtained on the 5th day after treatment, recording values between 0.046 and 0.056 μmol/min/μg of proteins vs. control (0.021 μmol/min/μg of proteins).
The electrophoretic analysis of catalase in treated seedlings (Fig. 1) highlighted more important intensity of bands compared to control, and proportional to the concentration of in insecticide used. We also noted the emergence of a second minority isoenzyme in treated samples, especially with the insecticide concentration of 50, 75, and 100 %.
Figure 2 shows an appearance of two new isozymes in treated leaves, less important than the 3rd isoenzyme more marked in both control and treated samples.
Regarding roots, Fig. 2 reveals the presence of a single isozyme of catalase in both treated samples and control, with a higher density in the treated roots.
Response of peroxidase
Table 1 shows a significant increase of the POD activity in treated seedlings with the application of all insecticides from the 3rd days of test period. This stimulation was 2- to 5-fold higher by referring to control, reaching for example in the 4th day 0.07 μmol/min/μg of proteins with α-cypermethrin at 50 % against 0.015 μmol/min/μg of proteins for control.
Table 2 illustrates an increase of POD activity in treated leaves from the 5th day of test period, marking a maximum on the 8th day, with values between 0.095 and 0.106 μmol/min/μg of proteins; the control registered only 0.041 μmol/min/μg of proteins. Otherwise, an increase of the POD activity was recorded in roots from the 5th day of monitoring, with a maximum of 0.049 μmol/min/μg of proteins in roots treated with pirimicarb, vs. 0.016 μmol/min/μg of proteins for control.
Figure 1 emphasizes from 5 to 6 isoenzymes in treated seedling vs. one important isoenzyme in control. Moreover, the intensity of the bands in treated samples was more important compared to the control.
Electrophoretic study of SOD (Fig. 2) showed the apparition of 5 new isoenzymes in treated leaves, and a new isoenzyme in treated roots, non present in control essay. Moreover, the intensity of the bands in treated samples was generally more important compared to the control.
Response of ascorbate peroxidase
A very important increase in APX activity in treated seedlings was underlined (Table 1). This stimulation was recorded from the 1st day of monitoring, and it was generally 1.5- to 5-fold higher when compared to control. For example, in the seedlings treated with chlorpyriphos (100 %) on the 4th day of test period, the APX activity was of 0.199 μmol/min/μg of proteins vs. control (0.38 μmol/min/μg of proteins).
Table 2 shows that the activity APX increased in treated leaves from the beginning of the test period, reaching the maximum on the 8th day (from 0.223 to 0.233 μmol/min/μg of proteins) compared to control (about 0.083 μmol/min/μg of proteins).
Concerning the roots (Table 2), a significant response of APX was obtained on the 5th day after treatment; recording values between 0.08 and 0.09 μmol/min/μg of proteins, vs. 0.029 μmol/min/μg of proteins for control.
Response of glutathione-s-transferase
A very important increase of GST activity in treated seedlings was emphasized (Table 1). This stimulation was registered from the 1st day of monitoring and was generally 2- to 8-fold higher in comparison with control. For instance, in seedlings treated with chlorpyriphos (100 %) on the 4th day of test period, the GST activity was of 0.016 μmol/min/μg of proteins (vs. 0.002 μmol/min/μg of proteins for control).
GST activity increased in treated leaves from the 5th day of the test period (Table 2), recording values between a maximum of 0.03 and 0.07 μmol/min/μg of proteins, compared to control (from 0.013 to 0.026 μmol/min/μg of proteins).
Regarding roots (Table 2), a significant response of GST was obtained on the 5th day after treatment, registering values between 0.0015 and 0.0018 μmol/min/μg of proteins (vs. 0.0008 μmol/min/μg of proteins in control).
Response of glutathione reductase
Table 1 illustrates a significant stimulation of the GR activity in treated seedlings from the 3rd day of monitoring, when applying all insecticides. This stimulation was 2- to 7-fold greater when referring to control, and reaching for example on the 3rd day 0.087 μmol/min/μg of proteins with α-cypermethrin (100 %); in control, this activity was rounding 0.012 μmol/min/μg of proteins.
Table 2 shows an increase in GR activity in treated leaves from the 5th day of test period. GR activity was generally 2 to 3 times higher by considering the control and recording values between 0.08 and 0.092 μmol/min/μg of proteins, compared to control (about 0.026 μmol/min/μg of proteins).
In other side, a significant response of GR in treated roots was obtained from the 5th day after treatment (Table 2), recording values between 0.029 and 0.052 μmol/min/μg of proteins (vs. 0.017 μmol/min/μg of proteins in control).
Discussion
To evaluate lipid peroxidation, we measured changes in MDA rate as a result of lipid peroxidation reaction. Our results showed a significant increase in MDA concentration in treated samples compared to the control ones. Generally, under stress condition, there is an increase in the rate of the lipid peroxidation, result of an accumulation increase of ROS. In this way, some compounds such as singlet oxygen, hydroxyl radical, anion superoxide, or hydrogen peroxides attack the unsaturated lipids, especially fatty-acids, and cause their peroxidation, leading to the liberation of an important rate of the MDA (Elstner 1990). The superoxide radicals are converted into hydroperoxyl radicals that penetrate the cellular membrane and interact with the internal lipid membrane, being unattainable for the radical superoxide (Bartosz 2003). Mishra et al. (2008) signaled elsewhere a significant increase in MDA rate in Momordica charantia L. leafs after treatment by dimethoate. In the same way, Song et al. (2007) demonstrated an important increase in lipid peroxidation in Triticum aestivum following the treatment by chlorsulfuron.
To evaluate a degree of toxicology of the three insecticides on tomato, chlorophyll rate was evaluated. Our results showed a significant decrease in both chlorophyll A and chlorophyll B rates in treated samples, compared to the control ones. Moreover, these results are also supported by the delay of growth and a perturbation of reserve substances observed after treatment by alpha-cypermethrin, chlorpyriphos, and pirimicarb in our previous studies (Chahid et al. 2013a, b). These results reflect the serious damages reaching plants after treatment by insecticides.
SOD is the first enzyme of the detoxification process, operating by catalyzing the conversion of free radicals on molecular oxygen and peroxide of hydrogen (McCord and Fridovich 1969). Our study reveals an important stimulation of SOD activity in treated seedling and plants of tomato. This stimulation may be a response to the accumulation of ROS and especially superoxide anion after insecticide use. In concordance with our results, Bashir et al. (2007) showed a similar increase of SOD activity after application of deltamethrin to Glycine max. Moreover, SOD activity increased in M. charantia after treatment by dimethoate (Mishra et al. 2009). In other side, the electrophoretic analysis revealed the presence of 3 isoenzymes of SOD in tomato seedlings and leaves and just one isoenzyme in roots; generally, the isoenzymes in treated samples were more active compared to control. These 3 isoenzymes correspond to Cu/Zn-SOD, Mn-SOD, and Fe-SOD which are different by their metallic cofactor (Beyer and Fridovich 1987).
In the second episode of the detoxification chain, CAT catalyzes the decomposition of H2O2, generated after the conversion of O2 − by SOD into H2O2 and O2 (Aeobi 1974). Our results showed a high increase of CAT activity in treated seedling and plants of tomato, may be due to H2O2 accumulation, because catalase is one of the major antioxidant enzymes responsible for its elimination (Song et al. 2006). Literature reported that in consequence to exposition to xenobiotics, catalase was generally stimulated to eliminate the H2O2 generated, as underlined for example in T. aestivum, which displayed an important increase in CAT activity after exposure to pentachlorophenol et 2,4-dichlorophenol (Michałowicz et al. 2009). The electrophoretic study of catalase activity revealed the apparition of a new isoenzyme in treated seedlings of tomato when applying a higher concentration of insecticides and two new isoenzymes in treated leaves; whereas, in roots, there is no qualitative difference between treated samples and control. Generally, the isoenzymes in treated samples were more active compared to control. A study made in Nicotiana plumbaginifolia has brought out three types of catalase, very similar in the sequence of their genes, but involved in distinct cellular processes (Willekens et al. 1994). Cat1 is highly expressed in photosynthetic cells, where it can play the role of scavengers of H2O2 produced during the photo-respiration. Cat2 is distributed uniformly in the plan, with some preference for the vascular tissue; it would play a role in the elimination of H2O2 during stress conditions. Cat3 is more abundant in the seed, and therefore, it is probably associated to glyoxysome function (Willekens et al. 1994).
POD is implicated in the process of hydrogen peroxide detoxification, and its activity increased in treated seedling and plants of tomato. This stimulation may be a response of H2O2 accumulation. POD catalyzes the oxidation of many substrates in the presence of hydrogen peroxide, thus allowing the transformation of a H2O2 molecule into two molecules of water (Putter 1978). The electrophoretic study of POD activity showed an apparition of many new isoenzymes in treated seedlings and plants of tomato. In literature, several studies support this defensive role of the POD against xenobiotics. Song et al. (2007) demonstrated that treatment with chlorotoluron induced a significant stimulation of the POD in T. aestivum; this stimulation affected the majority of isoenzymes both in leaves and roots. The stimulation of POD activity, due to exposition to chlorimuron-ethyl, was also reported in T. aestivum (Wang and Zhou 2006).
APX is part of the ascorbate-glutathione cycle and is also responsible for the elimination of hydrogen peroxide. The stimulation of the APX activity is reported in literature as a response of plants to stress conditions; APX activity is induced after treatment of Solenostemon rotundifolius by the triazole (Kishorekumar et al. 2008). Chagas et al. (2008) reported likewise that the treatment by Parquat (2 mM) induced stimulation of the APX in Saccharum officinarum. Our results are in concordance with literature data since a high increase of APX activity was observed in both treated seedling and plants of tomato. We suggest that this stimulation may be a response of H2O2 accumulation and/or related to the activation of the ascorbate-glutathione cycle.
GST is a multifunctional enzyme playing a very important part in the detoxification process in plants. Our study illustrated a high increase of GST activity in treated seedling and plants of tomato; the GST stimulation supports the activation of the ascorbate-glutathione cycle as a response to oxidative stress. GST is responsible of the catalysis of the conjugation of several substrates including glutathione-pesticides to form a non-toxic peptide derivative (Dixon et al. 1998). Stimulation of GST activity is reported in literature in plants as a response to xenobiotics. Thus, exposure of Arachis hypogaea to glyphosate induced stimulation of GST activity and raised the glutathione rate (Mukesh and Bhalla-Sarin 2001). In other side, the GST stimulation was also observed T. aestivum following the treatment by the prometryn (Jiang and Yang 2009). In plants, there are six classes of GST. The phi and tau GST are plant-specific classes and the most abundant; their role consists on the conjugation of various types of xenobiotics including pesticides. The theta GST operates as a glutathione peroxidase and its role is apparently associated to the reduction of organic hydroperoxide produced during oxidative stress. The zeta GST plays the role of glutathione isomerase catalyzing the conversion of maleylacetoacetate in fumarylacetoacetate. The other two classes are dehydroascorbate reductase (DHARS) and lambda GST; they play the role of thioltransferase (Edwards and Dixon 2005).
GR, acting in the process of detoxification, catalyzes the reduction of oxidized glutathione to reduced glutathione. Our results showed a high increase of GR activity in treated seedling and plants of tomato. This stimulation of GR activity could be due to (i) high activity of the cycle glutathione-ascorbate to ensure detoxification of the high rate of ROS due to oxidative stress or (ii) an important activity of GR necessary to maintain a high rate of Glutathione reduced, not limiting then synthesis of the phytochelatins which are oligomers of glutathione involved in the kidnapping of metals at the level of the vacuole, and the inactivation of some pesticides (Bashir et al. 2007). Similar results were reported in literature since Diuron application induced GR activity stimulation in Lemna minor (Teisseire and vernet 2000) and the exposure of Arabidopsis thaliana to copper at concentrations higher than 50 μM increased significantly GR activity (Drazkiewicz et al. 2003).
In conclusion, the application of insecticides to tomato plant induces an important accumulation of MDA, demonstrating an increase of lipid peroxidation in consequence of the excessive ROS production following the plant treatment by insecticides. In response to this oxidative stress created in tomato seedlings and plants, the activities of antioxidant enzymes such as SOD, POD, CAT, APX, GST, and GR were generally enhanced. The electrophoretic analysis showed the apparition of same new isoenzymes such CAT and POD.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GR:
-
Glutathione reductase
- GST:
-
Glutathione-s-transferase
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- SOD:
-
Superoxide dismutase
References
Aeobi H (1974) Catalase: H.U methods of enzymatic analysis. Bergmayer, vol 2, Academic Press, pp 673–684
Arias-Estévez M, Lopez-Periago E, Martinez-Carballo E, Simal-Gandara J, Mejuto JC, Garcia-Rio L (2008) The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric Ecosyst Environ 123:247–260
Arnon DI (1949) Copper enzymes in isolated chloroplasts, P-polyphénoloxidase in Beta vulgaris. Plant Physiol 24:1–13
Bartosz G (2003) The second face of oxygen. Warsaw: PWN. In: Song NH, Yin XL, Chen GF, Yang H (2007) Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 68:1779–1787
Bashir F, Uzzafar M, Siddiqi TO, Iqbal M (2007) The antioxidative response system in Glycine max (L.) Merr. Exposed to deltamethrin, a synthetic pyrethroid insecticide. Environ Pollut 147:94–100
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Chagas RM, Silveira JAG, Ribeiro RV, Vitorello VA, Carrer H (2008) Photochemical damage and comparative performance of superoxide dismutase and ascorbate peroxidase in sugarcane leaves exposed to paraquat-induced oxidative stress. Pestic Biochem Physiol 90(3):181–188
Chahid K, Laglaoui A, Zantar S, Ennabili A (2013a) Effect of three insecticides on tomato (Solanum lycopersicum) seedling germination and early plants growth. Biol Divers Conserv 6/1:57–61
Chahid K, Laglaoui A, Zantar S, Ennabili A (2013b) Changes evaluation of reserve substances and degradation enzymes after exposure of tomato plants (Lycopersicon esculentum Mill.) to alpha-cypermethrin, chlorpyriphos and pirimicarb. ProEnvironment 6:33–41
Dixon DP, Cole DJ, Edwards R (1998) Purification regulation and cloning of a glutathione transferase (GST) from maize resembling the auxin-inducible type III GSTs. Plant Mol Biol 36(1):75–87
Drazkiewicz M, Skorzynska-Polit E, Krupa Z (2003) Response of the ascorbate/glutathione cycle to excess copper in Arabidopsis thaliana (L.). Plant Sci 164:195–202
Edwards R, Dixon DP (2005) Plant glutathione transferases. Methods Enzymol 401:169–186
Elstner EF (1990) Der Sauerstoff- Biochemie Biologie Medizin. BI-Wissenschaftsverlag, Mannheim. In: Mishra V, Srivastava G, Prasad SM, Abraham G (2008) Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate. Pesticide Biochemistry and Physiology 92:30–37
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 246:7130–7139
Health RL, Packer L (1981) Photoperoxidation in isolated chloroplastes. I. Kinetics and stoichiometry of fatty acid and peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Asada K (1984) Inactivation of ascorbate peroxidase in spinach chloroplast on dark addition of hydrogen peroxide: its protection by ascorbate. Plant Cell Physiol 25:1285–1295
Jiang L, Yang H (2009) Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol Environ Saf 72(6):1687–1693
Kishorekumar A, Abdul Jaleel C, Manivannan P, Sankar B, Sridharan R, Murali PV, Panneerselvam R (2008) Comparative effects of different triazole compounds on antioxidant metabolism of Solenostemon rotundifoliu. Colloids Surf B: Biointerfaces 62:307–311
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein. J Biol Chem 224:6049–6055
Michałowicz J, Posmyk M, Duda W (2009) Chlorophenols induce lipid peroxidation and change antioxidant parameters in the leaves of wheat (Triticum aestivumL.). J Plant Physiol 166:559–568
Mishra V, Srivastava G, Prasad SM, Abraham G (2008) Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate. Physiology 92:30–37
Mishra V, Srivastava G, Prasad SM (2009) Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci Hortic 120:373–378
Mukesh J, Bhalla-Sarin N (2001) Glyphosate-induced increase in glutathione S-transferase activity and glutathione content in groundnut (Arachis hypogaea L.). Pestic Biochem Physiol 69:143–152
Peixoto F, Alves-Fernandes D, Santos D, Fontainhas-Fernandes A (2006) Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pestic Biochem Physiol 85:91–96
Putter J (1978) peroxydase. In: Bergmayer HU (ed) Methods of enzymatic analysis. vol 2, academic press, pp 685–690
Song NH, Yang ZM, Zhou LX, Wu X, Yang H (2006) Effect of dissolved organic matter on the toxicity of chlorotoluron to Triticum aestivum. Science 17:101–108
Song NH, Yin XL, Chen GF, Yang H (2007) Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 68:1779–1787
Statistica (1997) Statistica statsoft Inc. release 5.1. Tulsa, OK, USA
Teisseire H, Vernet G (2000) Is the “Diuron effect” due to a herbicide strengthening of antioxidative defenses of Lemna minor? Pestic Biochem Physiol 66:153–160
Wang ME, Zhou QX (2006) Effect of herbicide chlorimuron-ethyl on physiological mechanisms in wheat (Triticum aestivum). Ecotoxicol Environ Saf 64:190–197
Willekens H, Langebartels C, Tiré C, Van Montagu M, Inzé D, Van Camp W (1994) Differential expression of catalase gene Nicotiana plumbaginifolia (L). Proc Natl Acad Sci U S A 91:10450–10454
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305
Wu GL, Cui J, Tao L, Yang H (2010) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 19:124–132
Yin XL, Jiang L, Song NH, Yang H (2008) Toxic reactivity of wheat (Triticum aestivum) plants to herbicide isoproturon. J Agric Food Chem 56:4825–4831
Zhang JJ, Lu YC, Zhang JJ, Tan LR, Yang H (2014) Accumulation and toxicological response of atrazine in rice crops. Ecotoxicol Environ Saf 102:105–112
Zhou ZS, Huang SQ, Guo K, Mehta SK, Zhang PC, Yang ZM (2007) Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J Inorg Biochem 101:1–9
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Chahid, K., Laglaoui, A., Zantar, S. et al. Antioxidant-enzyme reaction to the oxidative stress due to alpha-cypermethrin, chlorpyriphos, and pirimicarb in tomato (Lycopersicon esculentum Mill.). Environ Sci Pollut Res 22, 18115–18126 (2015). https://doi.org/10.1007/s11356-015-5024-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5024-3