Abstract
The response of phytoplankton assemblages to the closure of urban sewage outfalls (USOs) was examined for the Mar Piccolo of Taranto (Mediterranean Sea), a productive semi-enclosed coastal marine ecosystem devoted to shellfish farming. Phytoplankton dynamics were investigated in relation to environmental variables, with a particular emphasis on harmful algal blooms (HABs). Recent analyses evidenced a general reduction of the inorganic nutrient loads, except for nitrates and silicates. Also phytoplankton biomass (chlorophyll a) and abundances were characterized by a decrease of the values, except for the inner area of the basin (second inlet). The phytoplankton composition changed, with nano-sized species, indicators of oligotrophic conditions, becoming dominant over micro-sized species. If the closure of the USOs affected phytoplankton dynamics, however, it did not preserve the Mar Piccolo from HABs and anoxia crises. About 25 harmful species have been detected throughout the years, such as the potentially domoic acid producers Pseudo-nitzschia cf. galaxiae and P seudo-nitzschia cf. multistriata, identified for the first time in these waters. The presence of HABs represents a threat for human health and aquaculture. Urgent initiatives are needed to improve the communication with authorities responsible for environmental protection, economic development, and public health for a sustainable mussel culture in the Mar Piccolo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal lagoons and semi-enclosed seas possess functional and structural properties associated with their localization between land and sea. They generally show large temporal and spatial variations in hydrochemical characteristics and considerable primary productivity and fishery production (Paerl et al. 2009). The shallowness of these systems promotes a short nutrient turnover, and the effects of anthropogenic inputs are more evident. Nutrient supply to coastal waters occurs naturally as a result of geological weathering; however, in recent years, population growth and related human activities (agriculture, urban sewage runoff) have increased nutrient inputs. This kind of pollution is now considered as one of the greatest threats to coastal environmental quality (Glibert et al. 2010). Also, mussel culture, usually acting as a natural eutrophication control because of the natural capacity of molluscs to clean water (Shumway et al. 2003), in conditions of intensive production could become the source of dissolved and particulate organic matter (Bouwman et al. 2013) and consequently of regenerated nutrients. The negative effects of nutrient pollution can be further exacerbated by climate changes such as warming, enhanced vertical stratification, and increasing raining fall (Hallegraeff 2010).
Nutrient enrichment can cause major changes in nutrient ratio and has significant effects on phytoplankton community by altering its composition and dynamics and may lead to the development of blooms dominated by a single species or species group. When these blooms lead to undesirable disturbance to humans and to the environment, they are defined “harmful algal blooms” (HABs) (Glibert et al. 2010). Harmful species are those that are potential producers of toxins, which may affect negatively human health, and others are producers of allopathic substances that inhibit the growth of co-occurring species and act as a chemical defense against predatory animals (Ianora et al. 2010). However, harmful effects are also caused by the high biomass producers that cause loss of environmental quality and benthic habitat, development of hypoxia and anoxia, alteration in food web, and death of wild and farmed species (Cloern 2001). When high biomass blooms of any species cause discoloration of the sea (any color), the term “red tides” is used (Davidson et al. 2012).
The link between anthropogenic nutrient supply and the appearance of HABs has been largely discussed (Hallegraeff 1993; Anderson et al. 2002, 2008). Field examples have demonstrated evident linkages between anthropogenic nutrient supply and the marine/coastal HABs (Hallegraeff 1993; Anderson et al. 2002; Davidson et al. 2012), evidences supported also by experimental and physiological data (Glibert et al. 2006). However, other chemical–physical and biological factors may modulate the harmful species responses to nutrient loadings. In a recent review, Berdalet et al. (2014) summarized hydrobiological factors affecting HAB dynamics in stratified systems, such as the coastal and brackish environments. These factors comprise the nutritional pathways and the food web interactions, including grazing, allelopathy, and other interactions of HABs with other organisms.
The impact of HABs on public health and economy has been increasing in the last decades (Hallegraeff 2010), and deeper knowledge on processes underlying HAB events appears of critical importance to provide to the environmental and public health managers information to improve management and forecasting of these events. It is particularly important to evaluate the principles and processes responsible for phytoplankton dynamics and to discriminate natural occurring blooms from those derived from anthropogenic impact (Spatharis et al. 2007).
The Mar Piccolo (Ionian Sea, Mediterranean) is an enclosed ecosystem strongly exploited for intensive mussel commercial fishery and affected by industrial, agricultural, and sewage inputs (Caroppo et al. 2012a). Since 1938, in the Mar Piccolo, HABs occurred mainly in summer (Cerruti 1938a; Parenzan 1984). Throughout the years, urban expansion and intensive agriculture caused an increase in nutrients and organic matter levels, which exceeded the self-depurating capacities of the basin, and HABs have become increasingly more frequent than in the past (Caroppo and Cardellicchio 1995). In more recent years (between 2000 and 2005), the political authorities approved the relocation of one quarter of the urban sewage outfalls (USOs) from the Mar Piccolo to the Mar Grande with the objective of improving water quality and defending the mussel consumers’ health. The present work is aimed to study phytoplankton dynamics and its relationship with the environmental factors during three periods: before (1991–1994) and after (2007–2008) the closure of the USOs and during the sampling cruises carried out in the frame of the Italian Flagship RITMARE project (2013–2014). Special attention is given to the blooming of potentially toxic microalgae in relation to the trophic features of the Mar Piccolo. Furthermore, the implications of the HABs on the environmental quality and management of mussel farming will be discussed.
Material and methods
Study area
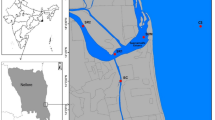
The Mar Piccolo (Ionian Sea, Mediterranean) is a shallow, nearly enclosed estuary of 21 km2 consisting of two basins separated by an intruding promontory (Fig. 1). The basins are referred to as first inlet and second inlet and have maximum depths of 13 and 10 m, respectively. The exchange with the larger semi-enclosed bay of the Mar Grande occurs through a primary artificial navigation channel (12 m) and a small natural inlet. The Mar Grande opens into the Gulf of Taranto and the northern Ionian Sea. The surrounding watershed of 555 km2 is used for horticulture (6 %) and agriculture, mainly cereals (24.1 %), olive trees (24.2 %), and vineyards (25.4 %). These cultivations involve the application of fertilizers (116,800 t year−1) and xenobiotics (1,800 t year−1) (Apulian Environmental Protection Agency 2009). The watershed is drained through a number of small tributary rivers, runoff from the surrounding agricultural soils and from freshwater springs (locally called “Citri”). The total number of the Citri is 34, comprising 20 in the first inlet and 14 in the second inlet (Cerruti 1938b).
Map of the study area with the location of sampling stations and urban sewage outfalls (USOs). In the period 2000–2006, 6 out of 13 USOs were closed (blue circles), while today seven are still active (yellow-red circles). Note that in the first inlet, the two urban USOs “via delle Fornaci” and “Quartiere Paolo VI” with the highest depuration capacity (bigger circles) have been closed
The exchange with the Mar Grande has been modified in 1985 by the installation of a water-scooping machine (0.15 M m3 day−1) to provide cooling water for the iron and steel industry. A more important disturbance factor has been represented by the presence of USOs collecting wastewaters from the northern area of Taranto and eight nearby towns.
Sample collection and abiotic factors
Surface seawater samples were collected monthly with an acid-rinsed 5-L Niskin bottle, equipped with silicon elastic and red silicon O-ring. Samplings were carried out from March 1991 to February 1994 (period I) and from January 2007 to December 2008 (period II) at two stations located in the first inlet (1E) and in the second one (2C), respectively (Fig. 1). In 2013–2014 (period III), seasonal samples were collected at the surface and bottom at four stations in the first inlet (1B, 1E, 1G, and 1I) and at two stations in the second one (2B and 2C) (Fig. 1 and Table 1). These samplings were carried out in June and October 2013 and in February and April 2014 in the framework of the Italian project RITM-ARE. A total of 168 water samples has been observed.
CTD profiles were obtained by different multiparametric probes according to the sampling period: Idronaut Ocean Seven 501 (March 1991–February 1992), Idromar IM5260 (January 2007–December 2008), Seabird 19 PlusSeacat (June 2013 and April 2014), and Idromar IPO5OD (October 2013 and February 2014).
Nutrient (N-NH4, N-NO2, N-NO3, P-PO4, and Si-Si(OH)4) concentrations were measured by the spectrophotometric method according to Strickland and Parsons (1972). Chlorophyll a was determined by spectrophotometric method (Parsons et al. 1984).
As concerning the other abiotic parameters acquired during the RITMARE cruises, dissolved inorganic carbon (DIC) and dissolved organic carbon (DOC) were measured using the Shimadzu TOC-V CSH analyzer following the method of Sugimura and Suzuki (1988). Particulate organic carbon (POC) and particulate nitrogen (PN) were determined using an elemental analyzer CHNO-S Costech mod. ECS 4010 applying the method of Pella and Colombo (1973) and Sharp (1974). All these parameters are described in detail by Kralj et al. (2015; this issue).
Phytoplankton counts and identification
Samples, freshly collected during all three periods, were fixed with Lugol’s iodine solution and examined by an inverted microscope (Labovert FS Leitz) equipped with phase contrast at a magnification of ×400 and ×630. Depending on phytoplankton densities, subsamples varying from 50 to 100 ml were allowed to settle for 24–48 h and examined following the Utermöhl method (Utermöhl 1958). Counting was performed along transects (1–4) or in random fields (30–60); in addition, half of the Utermöhl chamber was also examined at a magnification of ×200, to obtain a more correct evaluation of less abundant microphytoplankton taxa. Nanophytoplankton (2–20 μm) was counted in 15 randomly selected fields with a magnifications of ×630.
For the identification at species level of diatoms belonging to the potentially toxic genus Pseudo-nitzschia, ultrastructural observations of the frustules were carried out using a transmission electron microscope (TEM). Fixed samples were treated with nitric and sulfuric acids (1:1:4, sample/HNO3/H2SO4), shortly boiled to remove the organic matter, and washed with distilled water until all the acid was removed (von Stosch 1974). A drop of the cleaned material was placed on a Formvar-coated grid and observed with a JEOL JEM-100S and a Philips EM208 electron microscope.
Statistical analyses
Differences in the distribution of environmental variables and in the abundances of the main phytoplankton groups (diatoms, dinoflagellates, coccolithophores, and other flagellates) among different sampling time and stations (two inlets) were assessed through analysis of variance (Kruskal-Wallis ANOVA) using Statistica (Statsoft) software. When significant differences for the main effects were observed (p < 0.05), post hoc comparisons of mean ranks of all pairs of groups (Siegel and Castellan 1988) were also performed to further assess these statistically significant differences.
Square root-transformed abundances of the main phytoplankton groups were used to estimate Bray-Curtis similarity and perform a cluster (complete linkage method) analysis and a non-metric multidimensional scaling ordination (nMDS) (Kruskal and Wish 1978) to unveil temporal patterns in the community structure. In the latter case, the environmental variables were fitted as supplementary variables (vectors) onto ordination spaces to investigate their effects on the community structure. When the nMDS analysis was carried out for environmental data, the ordination was based on a Euclidean distance matrix derived from normalized (z-standardization) physical–chemical data. Analysis of similarity (one-way ANOSIM) was also carried out (10,000 permutations) to test for the significance of similarities in biological and physical–chemical features among the three periods. These analyses were performed using PRIMER software package (v. 7).
Results
Environmental data
Average values of the main abiotic and biotic parameters monitored in the three periods and at the two stations 1E and 2C of the Mar Piccolo are listed in Table 2. Temperature showed comparable values at the two stations, and its seasonal cycle was characterized by minima in December–January (up to 8.39 °C, December 1991) and maxima in summer (up to 29.39 °C, June 2007) (Fig. 2a, e). Salinity usually showed the lowest values in winter and spring (up to 34.32) and the highest in late summer–fall (up to 37.59) (Fig. 2b, f). Percentages of oxygen saturation ranged from 62.2 to 153.50 %. The water masses of the Mar Piccolo were generally oversaturated in oxygen (usually over 110 %) throughout the year, except for the late summer–fall period when values were strongly reduced, especially in the second inlet.
Nutrient concentrations were usually higher in period I than in period II, except for phosphates and silicates (Table 2). Statistical analyses revealed significant differences between the two periods for all nutrients (p < 0.001), with the exception of nitrates. In period I, ammonium reached high concentrations in summer and late fall period (December), particularly in the second inlet, where the maximum value of 19.95 μM was detected. In period II, the highest concentrations were recorded in September in both inlets (up to 10.48 μM), about half of the value registered in period I. In general, higher ammonium values were recorded in the second inlet than in the first one (H = 6.57, p < 0.05). Nitrite and nitrate seasonal patterns were similar at the two stations with maxima observed in autumn and winter. Mean concentrations of phosphates and silicates increased after the closure of the USOs. As concerning phosphates, their seasonal pattern changed, too. Particularly, in period I, phosphates showed very low and comparable concentrations throughout the year. In contrast, in period II, an increase of phosphates was observed in late fall and winter periods (up to 1.29 μM). In both inlets, the maxima of silicates were usually detected in summer (up to 25.97 μM) and the minima in spring (around 2 μM). The nutrient pattern resulted in higher N to P (H = 28.49, p < 0.001) (Fig. 2c, g) and N to Si (H = 28.75, p < 0.001) (Fig. 2d, h) ratios in period I than in period II.
In period II, the chlorophyll a was characterized by a slight decrease (not statistically significant) in the first inlet and an increase in the second one (H = 4.70, p < 0.05), compared to period I (Table 2). Moreover, in period I, the chlorophyll a seasonal dynamics showed peaks in spring and late summer in both inlets and also in fall in the second one. In period II, the highest concentrations were reached in late fall (December), up to 3.99 and 7.26 μg L−1 in the first and second inlets, respectively.
Data collected within the project RITMARE, even if sampled seasonally, confirmed the general reduction of the inorganic nutrient load already detected in period II, except for nitrates and silicates in the second inlet (Table 2). The higher concentrations of the monitored nutrients were observed in February in the second inlet. Ammonium concentration varied from undetectable values to 3.14 μM, nitrite from 0.03 to 0.54 μM and nitrates from 0.06 to 11.21 μM. Phosphates decreased compared to period II and were comprised between 0.03 and 0.64 μM. Silicates ranged between of 6.44 and 19.61 μM, with high concentrations in February and October. DOC concentrations (range 1.03–1.96 μM) were higher in June while DIC (range 31.78–40.36 μM) in October. POC and PN ranged from 114.57 to 644.58 μM and from 22.93 to 110.52 μM, respectively, with maxima in October and minima in February.
Chlorophyll a data were not comparable with those of the previous period, and concentrations varied between 0.92 (February, first inlet) and 2.55 μg L−1 (October, second inlet). In the first inlet, concentrations were lower, while in the second, they were higher than those observed in 1991–1992.
A complete description of the physical–chemical parameters registered during the RITMARE cruises is reported in Kralj et al. (2015; this issue).
Phytoplankton communities
Average abundance values of the total phytoplankton and the main groups detected at the two stations of the Mar Piccolo are listed in Table 2.
Non-metric multidimensional scaling based on transformed biological and normalized physical–chemical data distinguished the period I (1991–1992) from the other two (2007–2008 and 2013–2014) (Fig. 3). This separation was confirmed by cluster analyses, too (Fig. 3). Although the ANOSIM result gave a global R value of 0.41 indicating an overlapping among the three periods (p < 0.001), a certain degree of differences was nevertheless revealed by this analysis. The pairwise comparison indeed showed slight differences between periods I and III and more pronounced ones between periods I and II (p < 0.001), while no differences were observed between periods II and III.
Period I: 1991–1994
Phytoplankton abundance displayed high variability in the first inlet, ranging from 45.6 to 2,775.7 × 103 cells L−1 (Fig. 4a). In this period, the highest densities were observed in spring (April) and in winter (up to 1.99 × 106 cells L−1, January 1993). Blooms were mainly due to diatoms. Particularly, Pseudo-nitzschia delicatissima group accounted for 1.6 × 106 cells L−1 (69.3 % of the total phytoplankton abundance) and 1.4 × 106 cells L−1 (51.9 % of the total) in April 1991 and 1992, respectively. Hemiaulus hauckii alone reached the concentration of 1.7 × 106 cells L−1 (85 % of the total) in January 1993.
By considering the entire period, diatoms always dominated the phytoplankton (Fig. 4a). The species detected more frequently were, besides the P. delicatissima group, Chaetoceros spp., Ceratoneis closterium, Guinardia striata, Proboscia alata, Skeletonema marinoi, and Leptocylindrus danicus. Dinoflagellates were responsible for red tides in August–September 1991, due to the bloom the of the species Scrippsiella trochoidea (up to 0.4 × 106 cells L−1), Prorocentrum micans, P rorocentrum triestinum, Heterocapsa niei, H eterocapsa triquetra, Alexandrium spp., and, at the end of the phenomenon, Lingulodinium polyedrum (up to 0.12 × 106 cells L−1), potentially producer of the yessotoxin, which seems to have any effect on humans. Finally, coccolitophores were scarcely represented with the species Emiliania huxleyi, Syracosphaera sp., and Calciosolenia brasiliensis.
In the second inlet, phytoplankton abundances showed marked temporal variations with values comprised between 30.6 and 2,184.3 × 103 cells L−1 (Fig. 4b). The seasonal trend was characterized by an increase of the concentrations in spring (up to 2.2 × 106 cells L−1, May 1991) and summer (up 1.21 × 106 cells L−1, August 1991). In spring, diatoms were the most abundant component of the community. Particularly, in May 1991, the P. delicatissima group reached the concentration of 1.71 × 106 cells L−1 (78.2 % of the total phytoplankton), while in April 1991, the same species, together with the other diatom Skeletonema marinoi, represented the 68.2 % of the total cell counts. In summer 1991, undetermined phytoflagellates and the diatoms Thalassionema nitzschioides, L. danicus, L eptocylindrus minimus, and the P. delicatissima group dominated the community. In September 1992, dinoflagellates were the most important components of the assemblages (52.7 % of the total) and were represented by H. niei, Gyrodinium glaucum, G yrodinium lachryma, Heterodinium sp., Gymnodinium spp. Phytoflagellates were the second group, in terms of abundance (41.5 % of the total). In September 1993, a bloom of T. nitzschioides occurred and reached the concentration of 1.11 × 106 cells L−1 (93 % of the total).
Throughout the years, also in the second inlet, diatoms were the most abundant components of the assemblages (Fig. 4b).
Period II: 2007–2008
After the USOs closure, in the first inlet, phytoplankton abundances displayed decreasing values ranging between 57.3 and 923.3 × 103 cells L−1 (Fig. 4a). Higher values were observed in April 2007 and 2008 (up to 774.1 × 103 cells L−1) as well in June 2008 (923.3 × 103 cells L−1) and in December 2007 (719.1 × 103 cells L−1). In April, diatoms, represented mainly by the P. delicatissima group, were the most abundant component of the community (up to 60.5 % of the total abundances). Phytoflagellates, and particularly undetermined cryptophytes, became dominant during the late fall (December 2007) and summer (June 2008) blooms. In the second inlet, phytoplankton abundances increased compared to the period I, with values ranging between 67.5 and 5,850.8 × 103 cells L−1 (Fig. 4b). The seasonal trend was characterized by marked temporal variations, and peaks were detected throughout the year, except in winter. Particularly in fall, phytoflagellates bloomed reaching the highest concentrations (up to 5.9 × 106 cells L−1) and accounting up to the 98.0 % of the total counts. In spring (April 2008, 1.47 × 106 cells L−1) and in summer (July 2008, 3.24 × 106 cells L−1), diatoms were dominant and represented 81.5 and 92.1 % of the total abundances, respectively. In April 2008, P. delicatissima group was responsible for the bloom (77.3 % of the total), while in July 2008, C. closterium represented the 90.1 % of the community. From a qualitative point of view, phytoflagellates were the most abundant component of the population both in the first and second inlet (Fig. 4a and b).
Period III: 2013–2014
Phytoplankton abundances detected at the six stations of the Mar Piccolo were comprised between 66.9 and 4,362.8 × 103 cells L−1 (Fig. 5). In the second inlet, higher cell concentrations (855.5 ± 1,231.8 × 103 cells L−1) were detected than in the first one (467.0 ± 621.4 × 103 cells L−1), although this difference was not statistically significant.
Furthermore, at the surface, slightly higher abundances (631.7 ± 810.4 × 103 cells L−1) were observed than at the bottom (561.2 ± 959.5 × 103 cells L−1), but also in this case, differences were not statistically significant. Considerably higher abundances (H = 39.90, p < 0.001) were recorded during the cruise carried out in April 2014 (1.78 ± 1.11 × 106 cells L−1) compared to the other surveys (mean value 202.3 ± 98.6 × 103 cells L−1), especially by approaching the second inlet (st. 2B and st. 2C) (Fig. 5d). In this sampling, phytoflagellates and cryptophytes reached the 60.8 % and diatoms the 33.6 % of the entire community. The most abundant species were Pseudo-nitzschia cf. galaxiae and nano-sized Chaetoceros spp.
By considering the four sampling cruises, the phytoplankton community consisted mainly of phytoflagellates (Fig. 6), which represented on average about the 53.1 ± 21.7 % of the total abundances. Diatoms and dinoflagellates accounted for 24.1 ± 18.0 and 16.8 ± 13.3 % of total, respectively. The most common diatoms were Chaetoceros spp., Pseudo-nitzschia spp., Dactyliosolen blavyanus, D actyliosolen fragilissimus, L. danicus, L. minimus, and Lioloma pacificum. Among dinoflagellates, besides typically autotrophic dinoflagellates, mixotrophic species were also detected: Akashiwo sanguinea (max concentration 102.1 × 103 cells L−1), Alexandrium minutum, P. micans, P. triestinum, Ceratium furca, and L. polyedrum. Coccolithophores (e.g., E. huxleyi, Syracosphaera pulchra, Calciosolenia murrayi) were present in very low percentages by representing the 6.0 ± 12.4 % of total counts.
Non-metric multidimensional scaling based on abundance data also clearly distinguished assemblages of April from the other months (Fig. 7a), characterized by a lower diatom abundance (Fig. 7b). June and October assemblages were more similar but still distinct, and clearly separated from the February one, when a well-diversified community developed with a high number of dinoflagellates (Fig. 7b).
Harmful algal species
The harmful species detected in the Mar Piccolo in Taranto from 1991 to 2014 are listed in Table 3. All the listed species are potentially producers of toxins, except for C. closterium and A. sanguinea, which are high biomass producers. The first species was responsible for anoxia crises and death of mussels in July 2007. Non-metric multidimensional scaling based on harmful species abundance data allowed to distinguish the three examined periods characterized by different assemblages of harmful species (Fig. 8). Period I is characterized by the presence of P. delicatissima group (species with a diameter <2 μm), Dinophysis, and Lingulodinium polyedra. In period II, the number of the harmful species increases with Pseudo-nitzschia spp. (>2 μm), C. closterium, Alexandrium spp., A. sanguinea, and Prorocentrum cordatum. In period III, besides the species detected in the previous period, P. cf. galaxiae and P. cf. multistriata have been identified.
Non-metric multidimensional scaling (nMDS) ordination plot of the RITMARE cruises. The length of arrows indicates the correlations between the environmental variables and the ordination axes (a). The abundances (percentage of range) of the main phytoplankton groups are represented as segmented bubble plot (b). Abbreviations: Temp temperature, Sal salinity, Chl a chorophyll a, Phaeo phaeopigments
The diatoms belonging to the Pseudo-nitzschia genus were abundant in the Mar Piccolo since 1991, the year of the beginning of our monitoring, and were responsible for the annual spring bloom and showed a wide temporal distribution. Also during the RITMARE cruises, different Pseudo-nitzschia species were detected (Fig. 9). Among them, P. cf. galaxiae and P. cf. multistriata were identified for the first time in this basin. Due to the difficulties encountered in the identification of different species belonging to this genus, only the ultrastructural observations of the frustule morphology allowed us to confirm the presence of P. cf. galaxiae in the Mar Piccolo. This particular species is characterized by a clearly distinctive valve ultrastructure, with a high number of striae in 10 μm and the lack of poroids that are typical for other Pseudo-nitzschia species (Lundholm and Moestrup 2002) (Fig. 9c, d). This species reached high concentrations in April 2014 (up to 1.3 × 106 cells L−1, Table 3).
Harmful dinoflagellates comprised a high number of species, and except for L. polyedrum and A. sanguinea, they did not reach high concentrations.
Discussion
Phytoplankton dynamics and trophic state
In coastal and estuarine areas throughout the world, the interactions among topography, hydrodynamic regime, and highly variable continental water inputs drive a large interannual, seasonal, and short-term diversity in the distributional patterns of phytoplankton (Cloern and Jassby 2010). In this study, we analyzed the phytoplankton dynamics in a highly impacted coastal, quasi-enclosed area in different periods before and after the USOs closure. The results confirmed phytoplankton as a good indicator of the trophic status of the Mar Piccolo and a key element for assessing the environmental quality, according to the recent directives, namely the Water Framework Directive (2000/60/EC, WFD) (Ferreira et al. 2007) and the Marine Strategy Framework Directive (2008/56/EC, MSFD) (Garmendia et al. 2013).
In condition of wastewater pollution, during the first period of investigation, phytoplankton dynamics showed a typical seasonal succession of temperate coastal waters, characterized by the dominance of diatoms in the winter–spring period in both inlets. According to the classic “phytoplankton Mandala” model, proposed by Margalef (1978), a fertilization event like the seasonal mixing of the water column favored diatoms, which grow rapidly in nutrient-rich waters. Diatoms assimilate available nutrients and bloom, then sink and decompose. During warmer summer months, ammonium was released following the decomposition processes occurred in the sediments, which gave rise to blooms dominated by dinoflagellates, including potentially toxic species (L. polyedrum) and red tide dinoflagellates, responsible for benthic faunal kills and damages to the mussels’ cultivation (Parenzan 1984; Caroppo and Cardellicchio 1995). In the second inlet that is poorly flushed and characterized by limited water exchange, summer blooms were dominated by diatoms (T. nitzschioides and P. delicatissima group). In this case, factors such as high nutrient availability and favorable light conditions could have prevailed over stratification that usually favors dinoflagellates capable of reaching deeper and nutrient-enriched waters and favored species capable of rapid utilization of the available resources (r-strategy diatom species).
The closure of USOs in the 2000–2005 period and the following inorganic nutrient decrease seemed to affect phytoplankton composition since nano-sized components, indicators of oligotrophic conditions, became dominant over the micro-sized components. Data collected during the RITMARE cruises confirmed the results obtained in the 2007–2008 period. In most recent years, spring blooms were composed of diatoms such as the P. delicatissima group and the nano-sized Chaetoceros sp., together with phytoflagellates and cryptophytes. The conspicuous presence of smaller species in the study area was also confirmed by the observation of small Chaetoceros spores in sediments in April 2014 (Rubino et al. Microbenthic community structure and trophic status of sediments in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea) (under review)).
In addition, another study carried out within the RITMARE project and focussed on the planktonic trophic web revealed an increase of the picophytoplankton abundances (Karuza et al. Planktonic trophic web and its relantionships with the mussel farms in the Mar Piccolo of Taranto (Ionian Sea, Italy) (under review)), when compared with those of some years before (Caroppo et al. 2006). The increase of picocyanobacteria represents a common feature of many sites devoted to the mussel culture, such as, for example, the Thau lagoon, where the proliferation of Synechococcus was associated with increased temperatures and a reduced inorganic phosphorus load (Collos et al. 2009). This proliferation of cyanobacteria, which are not assimilated by mussel, was co-responsible, with the concomitant bloom of the potentially toxic dinoflagellate Alexandrium catenella, of severe economic loss, because cyanobacteria represent a conspicuous source of available biomass for A. catenella, whose capacity for preying on cyanobacteria has recently been demonstrated (Jeong et al. 2010).
Also in the Mar Piccolo, our data on phytoplankton species composition evidenced, in more recent years, the increasing presence of heterotrophic and mixotrophic dinoflagellates like A. sanguinea, P. micans, P. triestinum, and C. furca and the potentially toxic species A. minutum and L. polyedrum. Mixotrophy occurs in many harmful algae which respond directly to nutrient inputs and indirectly through high abundance of preys (bacteria, autotrophic and heterotrophic flagellates) and researches on the nutritional factors that control these harmful algae are necessary to predict the occurrence of their blooms in estuarine and coastal waters (Burkholder et al. 2008).
If the closure of the USOs affected phytoplankton dynamics, it did not prevent the occurrence of harmful algal blooms and anoxia crises. The apparently limited role of nutrients in HAB dynamics could be due to at least two factors. Firstly, water nutrient levels provide less information than their fluxes, which in contrast enable a dynamic interpretation of production and consumption processes. Furthermore, environmental nutrient concentrations could be underestimated if we consider only inorganic compounds and not dissolved organic matter. The sources of organic nutrient in the Mar Piccolo are presumably due to the intensive mussel culture carried out in the basin, and the nutrient inputs resulted from the watershed. The capacity of phytoplankton to assimilate dissolved organic matter has been generally accepted (Heisler et al. 2008), and the role of urea, which is increasingly used in agriculture as an N fertilizer, on HABs and non HAB species, is widely discussed (Davidson et al. 2012). Particulate organic carbon could be important for mixotrophic and heterotrophic dinoflagellates which consume predominantly particulate rather than dissolved nutrients (Jeong et al. 2010), which are becoming more abundant in the Mar Piccolo waters in more recent years. Today, our data are insufficient to demonstrate this kind of relationships, but in the future, the monitoring of these variables will be needed to understand the HAB dynamics in the Mar Piccolo.
Harmful algal dynamics
In the Mar Piccolo, high biomass blooming species and potential producers of toxins represent a threat to the environmental quality and the health of humans and animals. High biomass producers were represented by red tide dinoflagellates and diatoms. Particularly in summer and in the second inlet, HABs caused anoxia crises and mussel kills since 1938 (Cerruti 1938a; Parenzan 1984; Caroppo and Cardellicchio 1995). Also in recent years (July 2008), a bloom of the diatom identified as Cylindrotheca closterium (=Ceratoneis closterium) was responsible for a heavy reduction (~35 %) of the total commercial mussel harvest with a valued loss of ~€ 13 millions. Furthermore, the loss of seeds compromised the harvest of the following year (Caroppo et al. 2013).
C. closterium is very similar to P. cf. galaxiae, potentially capable of producing the neurotoxin domoic acid and identified for the first time in the Mar Piccolo during the RITMARE cruises when it was responsible for the spring bloom in both Inlets. This species has been detected for the first time in Mexican and Australian waters (Lundholm and Moestrup 2002), but it is widely distributed in the Mediterranean coastal waters (Quijano-Scheggia et al. 2010). P. cf. galaxiae is characterized by a considerable morphological variability with three differently sized morphotypes (Cerino et al. 2005). Misidentification of the longer morphotype, even at the genus level, could be possible if based solely on light microscopy observations, and it is very plausible that the blooming species in summer 2008 was P. cf. galaxiae. We have no information on the toxicity of this species on the mussel flesh, because, to the best of our knowledge, it has not been tested yet.
Micrographs of Pseudo-nitzschia species from the Mar Piccolo during the RITMARE cruise in April 2014 at light (a and c) and transmission electron (b and d) microscopes. Pseudo-nitzschia delicatissima group sp. (a and b) (note in a the presence of heterotrophic flagellates) and P. cf. galaxiae (c and d). Scale bar: a and c 20 μm, b 1 μm, d 2 μm
Pseudo-nitzschia species are commonly detected in the coastal phytoplankton communities throughout the world and have been frequently reported as abundant or dominating in certain seasons (Trainer et al. 2012). Sedimentological data showed an increase in Pseudo-nitzschia abundance, which reflected a documented development of the eutrophication in Louisiana coastal waters (Parsons and Dortch 2002). In the Mar Piccolo, P. cf. galaxiae bloom was associated to low levels of phosphates, and the possibility that the species could assimilate also organic forms of nutrients, extending the mesocosms’ results reported by Trainer et al. (2012) to the natural environment, should be considered.
In the Mar Piccolo, Pseudo-nitzschia is presumably represented by different species; however, the only use of light microscopy does not allow for a definitive species identification, with the exception of few species. So, it is very plausible that the number of these species could increase in future if more specific techniques will be applied (electronic microscopy and genetic probes). No episode of toxicity is known, also because in April mussels usually do not reach the market and therefore toxicity analyses on the mussels’ flesh are not mandatory.
Harmful dinoflagellates were not only represented by red tide producers that in summer affected negatively mussel culture, but also potential producers of toxins that could cause illness even at low detected cell concentrations (<103 cells L−1). A typical example is represented by Dinophysis species (D. saccula and D. caudata comprised), which produce the diarrhetic shellfish poisoning (DSP) and affect negatively the economy of many shellfish production areas of Northern Japan, Chile, and Europe, in terms of days of harvesting bans (Reguera et al. 2014).
Another species that has never attained high concentrations in water was Ostreopsis cf. ovata, which is a benthic species detected in the water column after mixing episodes. Ostreopsis species are producers of palytoxin-like toxins (putative palytoxin and ovatoxins) and can cause respiratory illness (rhinorrhea, cough, fever, and bronchoconstriction) in beachgoers, who often required hospital treatment, and irritations due to contact (skin irritations) have been also recorded. This species could have also effects at cellular level; in fact, one strain isolated in the Mar Grande of Taranto revealed cytotoxic effects, cytoskeletal rearrangement, and apoptosis on the human-derived HeLa cells (Pagliara et al. 2015).
Furthermore, HABs can have effects on the recruitment of planktonic and benthic species. As an example, in laboratory experiments, P. delicatissima has produced oxylipins that induce low hatching success and apoptosis in the offspring of the copepod of Calanus helgolandicus (Ianora et al. 2010). Furthermore, extracts of Ostreopsis cf. ovata negatively affect the development of larvae of sea urchins (Paracentrotus lividus) (Pagliara and Caroppo 2012).
Studies on the effects of toxicity of HABs on marine recruitment in the Mar Piccolo should be encouraged, taking into account that generally in Italian waters, and in recent years also in the basin, it is difficult to capture abundant wild mussel seed (ISMEA 2013).
Management of the HABs and implication on mussel culture
The relocation of the main USOs had positive effects on the environment, as it reduced sewage detritus and nutrient loadings in the Mar Piccolo by 25 % and increased the water transparency (from annual average Secchi disc readings of 3.5 to 4.5 m), leading to a recovery of shallow water phytobenthos, such as seagrasses (Caroppo et al. 2012a), which are indicators of good environmental status. The reduction of the sewage inputs has resulted also in a species shift in dominance from micro-sized diatoms to nano- and pico-sized organisms, with negative effects on the quality and productivity of mussels. These molluscs are filter feeders, and their growth is largely controlled by food availability, which in turn is affected by seston concentration, composition, and transport rate (Berg and Newel 1986).
The effects of phytoplankton community changes on mussel physiology have been recently modelled (Caroppo et al. 2012a). The model was calibrated by using data on trophic carbon budgets (phytoplankton and mussels) and available biometric data of two periods characterized by a good quality (1975, before the closure of USOs) and bad quality of mussels (2003, during the closure of USOs), respectively. The obtained model demonstrated that a diet mainly based on diatoms generate better growth compared to a diet based on detritus or on smaller phytoplankton components (nano- and pico-sized species). This preliminary model supported the recent findings on the quality of mussels, which is declining for the intensive techniques of culture.
HABs represent a significant challenge for the coastal zone management, aimed to protect fisheries, minimize economic and ecosystem losses, and protect public and environmental health. Management strategies imply mitigation, prevention, and control (Anderson 2009). Mitigation is represented by the routine monitoring programs for toxins in shellfish which avoid that contaminated shellfish reach the market.
Prevention implies research on all the aspects of HABs, including their diversity, ecology, and physiology. Monitoring will be useful to understand the physics, biology, and chemistry of the environment. All these data are necessary to develop models to advance toward prediction of HABs. Particularly, pieces of information on physiology of the species of interest, trophic interactions, and benthic–pelagic coupling are needed. Although the Mar Piccolo represents one of the most important farming sites in Italy, which together with the northern Adriatic Sea covered up to 50 % of the national mussel production in the 2000–2005 period (FAO 2010), such routine monitoring is not carried out.
A strategy to control HABs in the Mar Piccolo has been studied through laboratory experiments (Caroppo et al. 2012b), which evaluated the ability of the filter feeder polychaete Sabella spallanzanii to remove the toxic dinoflagellate Amphidinium carterae. The use of polychaete filter feeders for the purification of the water column from these toxic microalgae is a novel approach. Some previous studies (Stabili et al. 2006) allowed to evaluate the filtration effect of S. spallanzanii on the density of bacterioplankton. The preliminary results obtained in this work encouraged the use of this polychaete as potential bioremediator capable of filtering microalgae in the presence of a possible blooming.
In conclusion, the policy changes implemented in the Mar Piccolo have resulted in a significant effect on the phytoplankton communities. Nano-sized components such as Chaetoceros sp., phytoflagellates, and cryptophyceans, indicators of oligotrophic conditions, became dominant over the micro-sized species. But the closure of the USOs did not avoid the occurrence of HABs and anoxia crises, mainly in summer periods and in the inner area (second inlet) of the Mar Piccolo. These findings suggest that nutrient fluxes, more than nutrient concentrations and more data on dissolved and particulate organic matter, could provide more information on HAB dynamics. Other sources of pollution such as chemical fertilizer in agriculture and aquaculture wastes should be considered. If the role of runoff of agriculturally derived urea will be established, a debate should be generated in order to address the conflict between the need to use urea in intensive terrestrial agriculture and the possible impact of HABs. The monitoring of the HABs in the Mar Piccolo should be implemented; in fact, tests for biotoxins in flesh represent the regulatory tools for commercially harvested shellfish, but these are not supported by systematic phytoplankton and environmental data. Particularly, it will be important to establish the role of different inorganic and organic nutrient pools prior to or during HAB events. Further studies should be carried out to unravel the influence of nutrient cycling on their occurrence. The use of electron microscopy and molecular methodologies should be encouraged in order to investigate the diversity and seasonal occurrence of species belonging for example to the Pseudo-nitzschia genus (McDonald et al. 2007; Rhodes et al. 2013), which represent a significant threat for human health. Models should be built for the prediction of HABs and the development of tools useful for decision makers. Initiatives are urgently needed to improve the communication with authorities responsible for environmental protection, economic development, and public health, to continue previous experiences in the frame of the sustainable mussel culture in the Mar Piccolo initiated in 2007 (Caroppo et al. 2012a), and to protect the ecosystem and mainly human health. Citizens have already demonstrated a high level of awareness in relation to the mussels’ quality, and they could be also involved in routine monitoring, mainly in summer periods.
References
Anderson DM (2009) Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast Manag 52:342–347
Anderson DM, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication nutrient sources, composition, and consequences. Estuaries 25(4b):704–726
Anderson DM, Burkholder JM, Cochlan WP, Glibert PM, Gobler CJ, Heil CA, Kudela RM, Parsons ML, Rensel JEJ, Townsend DW, Trainer VL, Vargo GA (2008) Harmful algal blooms and eutrophication: examining linkages from selected coastal regions of the United States. Harmful Algae 8:39–53
Apulian Environmental Protection Agency (2009) Agriculture. In RSA 2009, Bari (Italy): 16–157 (in Italian)
Berdalet E, McManus MA, Ross ON, Burchard H, Chavez FP, Jaffe JS, Jenkinson IR, Kudela R, Lips I, Lips U, Lucas A, Rivas D, Ruiz-de la Torre MC, Ryan J, Sullivan JM, Yamazaki H (2014) Understanding harmful algae in stratified systems: review of progress and future directions. Deep-Sea Res II 101:4–20
Berg JA, Newell RIE (1986) Temporal and spatial variations in the composition of the seston available to the suspension feeder Crassostrea virginica. Estuar Coast Shelf Sci 23:375–386
Bouwman L, Beusen A, Glibert PM, Overbeek C, Pawlowski M, Herrera J, Mulsow SYR, Zhou M (2013) Mariculture: significant and expanding cause of coastal nutrient enrichment. Environ Res Lett. doi:10.1088/1748-9326/8/4/044026
Burkholder JM, Glibert PM, Skelton HM (2008) Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8:77–93
Caroppo C, Cardellicchio N (1995) Preliminary studies on phytoplankton communities of Mar Piccolo in Taranto (Jonian Sea). Oebalia 21(2):61–76
Caroppo C, Giordano L, Palmieri N, Bellio G, Bisci AP, Portacci G, Sclafani P, Hopkins TS (2012a) Progress toward sustainable mussel aquaculture in Mar Piccolo, Italy. Ecol Soc 1 17(3):10, http://www.ecologyandsociety.org/vol17/iss3/art10/
Caroppo C, Licciano M, Giangrande A, Stabili L (2012b) Filtrazione della microalga Amphidinium carterae (Hulburt, 1957) da parte del Sabellide Polichete Sabella spallanzanii (Gmelin, 1791). Biol Mar Medit 19(1):232–233
Caroppo C, Portacci G, Alabiso G (2013) Summer harmful algal blooms (HABs) in a coastal basin devoted to the mussel culture and implication for their management. Rapp Comm Int Mer Médit 40:377
Caroppo C, Turicchia S, Margheri MC (2006) Phytoplankton assemblages in coastal waters of the Northern Ionian Sea (eastern Mediterranean), with special reference to cyanobacteria. J Mar Biol Assoc UK 86:927--937. doi:10.1017/S0025315406013889
Cerino F, Orsini L, Sarno D, Dell’Aversano C, Tartaglione L, Zingone A (2005) The alternation of different morphotypes in the seasonal cycle of the toxic diatom Pseudo-nitzschia cf. galaxiae. Harmful Algae 4:33–48
Cerruti A (1938a) Le condizioni oceanografiche e biologiche del mar Piccolo durante l’agosto 1938. Boll Pesca Pisc Idrobiol 14:711–751 (in Italian)
Cerruti A (1938b) Le sorgenti sottomarine (Citri) del Mar Grande e Mar Piccolo di Taranto. Ann Ist Sup Nav Napoli 7:171–196 (in Italian)
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Cloern JE, Jassby AD (2010) Patterns and scales of phytoplankton variability in estuarine–coastal ecosystems. Estuar Coast. doi:10.1007/s12237-009-9195-3
Collos Y, Bec B, Jauzein C, Abadie E, Laugier T, Pastoureaud A, Souchu P, Vaquer A, Lautier J (2009) Oligotrophication and emergence of picocyanobacteria and a toxic dinoflagellate in Thau lagoon, southern France. J Sea Res 61:68–75
Davidson K, Gowen RJ, Tett P, Bresnan E, Harrison PJ, McKinney A, Milligan S, Mills DK, Silke J, Crooks AM (2012) Harmful algal blooms: how strong is the evidence that nutrient ratios and forms influence their occurrence? Estuar Coast Shelf Sci 115:399–413
FAO (2010) Fishery and aquaculture statistics. Statistics and Information Service of the Fisheries and Aquaculture Department/Service, Rome, 72 pp
Ferreira JG, Vale C, Soares CV, Salas F, Stacey PE, Bricker SB, Silva MC, Marques JC (2007) Monitoring of coastal and transitional waters under the E.U. Water Framework Directive. Environ Monit Assess 135:195–216. doi:10.1007/s10661-007-9643-0
Garmendia M, Borja Á, Franco J, Revilla M (2013) Phytoplankton composition indicators for the assessment of eutrophication in marine waters: present state and challenges within the European directives. Mar Pollut Bull 66:7–16
Glibert PM, Burkholder JM, Parrow MW, Lewitus AJ, Gustafson DE (2006) Direct uptake of nitrogen by Pfiesteria piscicida and Pfiesteria shumwayae, and nitrogen nutritional preferences. Harmful Algae 5:380–394
Glibert PM, Allen JI, Bouwman AF, Brown CW, Flynn KJ, Lewitus AJ, Madden CJ (2010) Modeling of HABs and eutrophication: status, advances, challenges. J Mar Syst 83:262–275
Hallegraeff GM (1993) A review of harmful algal blooms and their apparent global increase. Phycologia 32:79–99
Hallegraeff GM (2010) Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. J Phycol 46:220–235. doi:10.1111/j.1529-8817.2010.00815.x
Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, Dortch Q, Gobler CJ, Heil CA, Humphries E, Lewitus A, Magnien R, Marshall HG, Sellner K, Stockwell DA, Stoecker DK, Suddleson M (2008) Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8:3–13
Ianora A, Miralto A (2010) Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: a review. Ecotoxicology 19:493–511. doi:10.1007/s10646-009-0434-y
ISMEA (2013) The fishing industry in Italy—check up 2013. Institute for Studies, […] Research and Information on the Agricultural market, 129 pp. http://www.ismea.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/8845. Accessed 27 May 2015
Jeong HJ, Yoo YD, Kim JS, Seong KA, Kang NS, Kim TH (2010) Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J 45(2):65–91. doi:10.1007/s12601-010-0007-2
Kralj M, Comici C, De Vittor C, Alabiso G et al. (2015) Recent evolution of the physical-chemical characteristics of a Site of National Interest - the Mar Piccolo of Taranto (Ionian Sea) - and changes over the last 20 years (this issue)
Kruskal JB, Wish M (1978) Multidimensional scaling. Sage Publications, Beverly Hills, 96 pp
Lundholm N, Moestrup Ø (2002) The marine diatom Pseudo-nitzschia galaxiae sp. nov. (Bacillariophyceae): morphology and phylogenetic relationships. Phycologia 41(6):594–605
Margalef R (1978) Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol Acta 4:493–509
McDonald SM, Sarno D, Zingone A (2007) Identifying Pseudo-nitzschia species in natural samples using genus-specific PCR primers and clone libraries. Harmful Algae 6:849–860
Paerl HW, Rossignol KL, Hall SN, Peierls BL, Wetz MS (2009) Phytoplankton community indicators of short- and long-term ecological change in the anthropogenically and climatically impacted Neuse River Estuary, North Carolina, USA. Estuar Coast. doi:10.1007/s12237-009-9137-0
Pagliara P, Caroppo C (2012) Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon 60(6):1203–1214. doi:10.1016/j.toxicon.2012.08.005
Pagliara P, Scarano A, Barca A, Verri T, Zuppone S, Caroppo C (2015) Ostreopsis cf. ovata induces cytoskeletal disorganization, apoptosis and gene expression disregulation on HeLa cells. J Appl Phycol 27. doi:10.1007/s10811-014-0515-z
Parenzan, P (1984) Il Mar Piccolo di Taranto. Camera di Commercio Artigianato e Agricoltura di Taranto, 319 pp (in Italian)
Parsons ML, Dortch Q (2002) Sedimentological evidence of an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication. Limnol Oceanogr 47(2):551–558
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, 173 pp
Pella E, Colombo B (1973) Study of carbon, hydrogen and nitrogen determination by combustion-gas chromatography. Mikrochim Acta 5:697–719
Quijano-Scheggia S, Garcés E, Karl BA, De La Iglesia P, Diogène J, Jose-Fortuño M, Camp J (2010) Pseudo-nitzschia species on the Catalan coast: characterization and contribution to the current knowledge of the distribution of this genus in the Mediterranean Sea. Sci Mar 74(2):395–410. doi:10.3989/scimar.2010.74n2395
Reguera B, Riobó P, Rodríguez F, Díaz PA, Pizarro G, Franco JM, Blanco J (2014) Dinophysis toxins: causative organisms, distribution and fate in shellfish. Mar Drugs 12:394–461. doi:10.3390/md12010394
Rhodes L, Smith K, Moisan C (2013) Shifts and stasis in marine HAB monitoring in New Zealand. Environ Sci Pollut Res 20:6872–6877. doi:10.1007/s11356-012-0898-9
Sharp JH (1974) Improved analysis for “particulate” organic carbon and nitrogen from seawater. Limnol Oceanogr 19(6):984–989
Shumway SE, Davis C, Downey R, Karney R, Kraeuter J, Parson J, Rheault R, Wikfors G (2003) Shellfish aquaculture—in praise of sustainable economies and environments. World Aquacult 34:15–17
Siegel S, Castellan NJ (1988) Nonparametric statistics for the behavioural sciences, 2nd edn. McGraw-Hill, New York
Spatharis S, Tsirtsis G, Danielidis DB, Do Chi T, Mouillot D (2007) Effects of pulsed nutrient inputs on phytoplankton assemblage structure and blooms in an enclosed coastal area. Estuar Coast Shelf Sci 73:807–815
Stabili L, Licciano M, Giangrande A, Fanelli G, Cavallo RA (2006) Sabella spallanzanii filter-feeding on bacterial community: ecological implications and applications. Mar Environ Res 61:74–92
Strickland YDH, Parsons T.R (1972) A practical handbook of seawater analysis. Bull Fish res B Canada, 167, 311 pp
Sugimura Y, Suzuki Y (1988) A high temperature catalytic oxidation method for the determination of non-volatile dissolved organic carbon in seawater by direct injection of liquid sample. Mar Chem 24:105–131
Trainer VL, Bates SS, Lundholm N, Thessen AE, Cochlan WP, Adams NG, Trick CG (2012) Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 14:271–300
Utermöhl H (1958) Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt Int Ver Theor Angew Limnol 9:1–38
von Stosch HA (1974) Pleurax, seine synthese und seine verwendung zur einbettung und darstellung der zellwand von diatomeen, peridineen und anderen algen, sowie fur eine neue methode zur elektivffbung von dinoflagellaten-panzern. (Pleurax: its synthesis and its application to the mounting and clearing for cell walls of diatoms, dinoflagellates and other algae, as well as for a new method of electively staining dinoflagellate armours). Arch Protistenkd 116:132–141
Acknowledgments
The authors wish to thank Roberta Bortul (Dipartimento Universitario Clinico di Scienze mediche, chirurgiche e della salute, University of Trieste) and Paolo Bertoncin (Dipartimento di Scienze della Vita, University of Trieste) for technical assistance with TEM. Financial support for the activities carried out in 2007–2008 was provided by the Project VulnErability of the Italian coastal area and marine Ecosystems to Climatic changes and Their rOle in the Mediterranean caRbon cycles (V.E.C.T.O.R.) funded by Ministry of Education, University and Research; Ministry of Economy and Finance; Ministry of the Environment and Protection of Natural Resources; and Ministry of Agriculture and Forestry, with Integrated Special Fund for Research (FISR)-Call 2001. Researches carried out in 2013–2014 were funded by the Project Bandiera RITMARE-La Ricerca Italiana per il Mare coordinated by the National Research Council and funded by the Ministry for Education, University and Research within the National Research Programme 2011–2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Caroppo, C., Cerino, F., Auriemma, R. et al. Phytoplankton dynamics with a special emphasis on harmful algal blooms in the Mar Piccolo of Taranto (Ionian Sea, Italy). Environ Sci Pollut Res 23, 12691–12706 (2016). https://doi.org/10.1007/s11356-015-5000-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5000-y