Abstract
A combined chemical and biological analysis of samples from a major obsolete pesticide and persistent organic pollutant (POP) dumpsite in Northern Tajikistan was carried out. The chemical analytical screening focused on a range of prioritized compounds and compounds known to be present locally. Since chemical analytics does not allow measurements of hazards in complex mixtures, we tested the use of a novel effect-based approach using a panel of quantitative high-throughput CALUX reporter assays measuring distinct biological effects relevant in hazard assessment. Assays were included for assessing effects related to estrogen, androgen, and progestin signaling, aryl hydrocarbon receptor-mediated signaling, AP1 signaling, genotoxicity, oxidative stress, chemical hypoxia, and ER stress. With this panel of assays, we first quantified the biological activities of the individual chemicals measured in chemical analytics. Next, we calculated the expected sum activity by these chemicals in the samples of the pesticide dump site and compared the results with the measured CALUX bioactivity of the total extracts of these samples. The results showed that particularly endocrine disruption-related effects were common among the samples. This was consistent with the toxicological profiles of the individual chemicals that dominated these samples. However, large discrepancies between chemical and biological analysis were found in a sample from a burn place present in this site, with biological activities that could not be explained by chemical analysis. This is likely to be caused by toxic combustion products or by spills of compounds that were not targeted in the chemical analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent organic pollutants (POPs) form a group of organic compounds, mostly from anthropogenic origin, that are persistent to biological, photolytic, and chemical degradation in the environment (Ritter et al. 1995). Due to their remarkably long half-lives (estimated from months to centuries or even longer (Weber et al. 2011)), POPs are omnipresent in environmental compartments and in addition are commonly detected in components of the everyday human diet such as, fish, meat, butter, and cheese. As a result, all humans, fish, birds, and mammals carry a burden of POPs, mainly in their fat tissue.
Exposure to and accumulation of POPs in the body have been linked to a wide range of chronic diseases and disorders, among which cancer, Alzheimer’s disease, diabetes, and reduced fertility (Mostafalou and Abdollahi 2013; De Coster and van Larebeke 2012; Gregoraszczuk and Ptak 2013; WHO 2013). Mechanistic insight in the adverse health effects of POPs and their relation to observations from epidemiological studies is often difficult to establish. This relates to issues such as confounding factors, limited insight in their molecular modes of action, and the long time frames between first exposure and manifestation of adverse effects (Gibson and Saunders 2013, Mrema et al. 2013). Moreover, and possibly most important, exposure to environmental pollutants is almost exclusively to mixtures of compounds with identical, overlapping, and sometimes counteracting modes of action, while most studies address the effects of only one or a few compounds (Kortenkamp 2007).
The production of legacy POPs is either halted or declined in most countries, but many POPs and POPs like chemicals are in current use (Scheringer et al. 2012). Due to their persistence, the current presence of POPs in the environment already forms a legacy for current and future generations. Moreover, POPs may be released into the environment via old stocks and uncontrolled storages and dumpsites of obsolete chemical and hazardous waste and poor management of obsolete and banned chemicals (FAO 2001; Weber et al. 2008). Estimates on the number of obsolete stocks around the world range from 300,000 to 500,000 tons (FAO 2001, FAO 2014). Many, if not most, of these sites are poorly controlled and lack securing measures to prevent direct contact on-site and halt migration of chemicals off-site in particular if considering long-term perspectives (Weber et al. 2008). Responsible management of these storages and dumpsites and their associated hazard forms a massive challenge for authorities and risk assessors that aim to protect environmental and public health.
Hazard assessment of POPs in pesticide storages/dumps and environmental matrices is often complicated by the complexity of the mixtures in which they may be present. In practice, most assessments focus on chemical analytical screening of a limited set of compounds that have been prioritized for their toxic potential and/or their frequent occurrence. In most cases, this approach is fast, quantitative, and relatively cost-effective but may be very non-informative as for the toxicological hazard: in addition to the uncertainty on the presence of other, non-targeted and thus non-identified, xenobiotics, limited information is usually available on the toxic potential of most compounds and their breakdown products, especially in mixtures. Therefore, identification and quantification of chemical presence by chemical analyses may give limited insight into the actual hazard.
A more complete and therefore more reliable view on the actual human health hazard of complex mixtures could be achieved by animal testing using vertebrate species. This approach has the advantage that it focuses on biological effects while comprising toxicokinetic effects, but it generally is not suitable for, e.g., assessment of contaminated sites due to the large amounts of sample needed, the length of the experiments, the low throughput, high costs, and ethical controversy. It is therefore not surprising that environmental hazard assessment by vertebrate animal testing is highly uncommon. An alternative strategy that combines important benefits from analytical chemistry and animal testing is the use of cell-based in vitro assays. In vitro assays allow for higher throughput and are more cost-effective. In vitro assays are available for the screening of POP-related toxicities such as genotoxicity, interference with hormone signaling, xenobiotic metabolism, and induction of stress signaling related to acutely toxic effects. Despite the large potential of effect-based in vitro approaches for environmental hazard and impact assessments, they are not yet commonly used for these types of application.
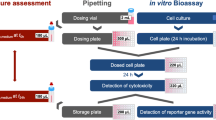
In the current study, we demonstrate the use of a set of mammalian cell-based in vitro assays for effect-based assessment of a set of well-known POPs (such as dichlorodiphenyltrichloroethanes (DDTs), polychlorinated biphenyls (PCBs), hexachlorohexanes, and drins) as well as samples from a pesticide dumpsite in the Republic of Tajikistan. The effect-based data from the dumpsite samples were subsequently compared with chemical analytical data on the same samples. While pure compounds could be directly applied to the in vitro tests, extracts were made from dumpsite samples prior to the exposure of the cells in the in vitro assays. The in vitro assays that were used are CALUX assays which are mammalian cell lines that were genetically modified to produce luciferase upon exposure to samples that evoke a biological effect mediated by a specific regulator protein. The luciferase production can be quantified by measurement of the amount of light it produces by a reaction it catalyses and is proportional to the evoked effect. Effect quantification is performed by comparison of the light signal evoked by a test sample to that of a reference compound for the respective assay. The specificity of the CALUX assays is promoted by the use of minimal promoters in front of the luciferase gene, which reduces the risk of transcriptional interference by other regulators. Moreover, the U2OS cell line on which most of the CALUX assays are based has minimal to no expression of important nuclear receptors which reduces loss of specificity by crosstalk between nuclear receptor-mediated activities (Sonneveld et al. 2005; Quaedackers et al. 2001). These features are particularly relevant in the screening of complex mixtures which may induce a wide range of transcriptional responses in the cells.
Materials and methods
Sample site description
The Kanibadam dumpsite is a POP and obsolete pesticide dumpsite, located close to the densely populated and fertile Ferghana Valley in northern Tajikistan. The site is located just 3 km upstream of the city of Kanibadam and was at time of research (2012) not fenced, neither guarded, nor contained. It is estimated that about 4000 tons of phased out and banned solid POP pesticides were buried and liquids were burnt at Kanibadam between 1973 and 1990 (Italian Ministry for the Environment and Sea 2006). Although records were available on pesticides that were deposited to the site in the past, there were uncertainties on the current status of the site: additional deposits may have occurred, as well as illegal removals. Moreover for part of the compounds that have been dumped, the chemical identity was not known. Due to possible uncontrolled access, the vulnerability to natural hazards, and its position along water courses and vicinity of towns and cities, the Kanibadam site has been regarded as a major threat to human and environmental safety in the region.
In October 2012, a topsoil sampling campaign was carried out at the Kanibadam dumpsite to provide a view of the current contamination level of the dumpsite and its direct surroundings. Samples were taken from dust depositions, runoff in drains, and “baseline” locations (upwind locations). In total, 21 samples were chemically analyzed (a graphical representation of the site and the locations from the samples included in this particular study is presented in Fig. 1). This chemical analysis for available POPs was focused on hexachlorohexanes (HCHs), drins (dieldrin, aldrin, endrin, isodrin, telodrin), heptachlor, heptachlor epoxide, hexachlorobutadiene, DDTs and their breakdown products, endosulfans, chlordanes, and a set of PCBs. Substantial amounts of HCHs were only observed in the samples from the dumpsite itself with sum amounts ranging up to almost 1 g/kg dry weight. The drin levels were relatively low: for the samples from the surrounding area, the sum of drins was always below the detection level of 0.003 mg/kg dry weight. Inside the pesticide dump area, the highest sum amount of drins detected was 1.4 mg/kg dry weight. DDT and its degradation products DDD and DDE were detected in 19 out of 21 samples. The highest reported sum-DDT value for the dumpsite itself was 360 mg/kg dry weight. Outside the dumpsite, elevated levels of DDT were also observed. The highest sum-DDT level outside the dumpsite itself was reported for a drain sample (3.7 mg/kg dry weight). This exceeds Dutch Intervention Values for (sum) DDT which is set at 0.2 mg/kg dry weight (Dutch 2014).
Although the chemical analysis covered the major POP pesticides, it was uncertain if additional compounds may have been present that could form a substantial additional health hazard.
In order to complement the chemical profile with biological effect profiles, five soil samples that were chemically analyzed were selected for CALUX analysis (locations indicated in Fig. 1). Two of these samples originated from inside the burial site: “Source” (from the center of the site next to a waste heap) and Gully (from the top of an erosion gully at the border of the dumpsite). One sample (Burn Place) was taken from a location at the entrance of the dumpsite where between 1973 and 1990 liquid pesticides were burnt to reduce volume. Two additional samples were taken from the surrounding area (“Baseline Wind” approximately 200 m upwind from the site and “Drain” approximately 200 m down from the dumpsite). The results from the chemical analysis of these five samples are indicated in Table 1.
Assay selection
The bioassay selection for the effect-based analysis was based on previous screenings of larger sets of xenobiotic compounds on an extended CALUX reporter cell line panel comprising assays for over 25 distinct endpoints (results not shown). With an exception for the DR CALUX and the PAH CALUX (both based on the rat hepatoma cell line H4IIE; Garrison et al. 1996; Pieterse et al. 2013), all CALUX reporter assays are stable cell lines based on the human osteosarcoma cell line U2OS to which a construct was introduced in which a luciferase gene is preceded by a multimerized minimal promoter element. For the reporter cell lines for the quantification of (anti-)endocrine activities mediated by the estrogen receptor alpha (ERα CALUX), the progestin receptor (PR CALUX), and the androgen receptor (AR CALUX), the respective receptor, under control of a constitutive promoter, was also introduced into the cell line (Sonneveld et al. 2005).
The assay selection comprises three assays that relate to endocrine disruption: the ERα, the AR-anti, and the PR-anti CALUX’. These assays quantify activities mediated by the nuclear hormone receptors that play a central role of signaling by estrogens (ERα), androgens (AR), and progestins (PR), respectively (Sonneveld et al. 2005; Sonneveld et al. 2006). In the CALUX assays in which antagonistic action is addressed (indicated with “-anti”), competitive binding with a model agonist compound is assessed. The ERα CALUX was performed in the agonistic modus because of prior observations that the vast majority of compounds that could affect ERα-mediated activity did so in an agonist way. In contrast, most effects observed for the androgen and progestin receptors were antagonistic.
The DR and PAH CALUX’ (Garrison et al. 1996; Pieterse et al. 2013) both quantify aryl hydrocarbon receptor (AhR)-mediated activity. The DR CALUX was developed for the detection of stable dioxins and dioxin-like compounds. To this end, sample extracts are acid treated to remove acid-labile compounds and incubations are performed for 24 h to allow for additional removal of compounds that are less metabolically stable (Besselink et al. 2004). The PAH CALUX was specifically developed for the detection of less metabolically stable AhR ligands, in particular carcinogenic PAHs (Pieterse et al. 2013). This was achieved by selection of a cell line with reduced metabolic activity and reduction of the incubation time to 4 h (Pieterse et al. 2013).
The p53 CALUX is a reporter assay that quantifies activity mediated by the p53 protein which is the major regulator for cellular responses towards genotoxicity such as cell cycle arrest, activation of DNA repair mechanisms, and initiation of apoptosis (van der Linden et al. 2014; Jiang et al. 2010). Since various compounds may only elicit genotoxic effects after metabolic activation (for which the U2OS cell line has very limited capability; Geiger et al. 2012; Beck et al. 2013), the p53 CALUX may be complemented with an in vitro metabolizing system such as microsomal S9 fractions.
The nrf2 CALUX (van der Linden et al. 2014) measures activity of the nrf2 transcription factor that initiates transcription of genes involved in detoxification and the anti-oxidant stress response (Ma 2013). In combination with the p53-CALUX, the nrf2-CALUX enables identification of compounds that elicit part of their genotoxic effect by oxidative stress (van der Linden et al. 2014).
The AP-1 CALUX indicates transcription activation by activator protein 1 mediated by binding to the TPA (12-O-tetradecanoylphorbol-13-acetate) DNA response element. AP-1 is involved in differentiation, proliferation, and promotion (Ameyar et al. 2003). This transcription factor has been implicated in the onset of various forms of cancer (Ashida et al. 2005). AP-1-mediated activity may be directly related to apoptotic effects (Shaulian and Karin 2002; Pyrzynska et al. 2000) and may therefore be used in combination with the cytotoxicity CALUX for the distinction between cytotoxic effects by apoptosis versus necrosis.
The Hif-1 CALUX detects activity mediated by transcription factor Hif-1 which is involved in the expression of genes that play a role in angiogenesis, embryogenesis, growth, and survival (Semenza et al. 2000; Xia et al. 2009). Chemical hypoxia, in which chemicals directly mimic the effect of hypoxia mediated by the Hif-1 transcription factor, may play a role in their carcinogenic potential (Salnikow et al. 2003).
The ESRE CALUX indicates endoplasmic reticulum stress by quantification of expression mediated by the regulatory proteins ATF6 and XBP1.
Test chemicals
CAS registry numbers of the test chemicals are depicted in Table 2. Polychlorinated biphenyls (PCBs) were obtained from LGC standards (Almere, The Netherlands). Polycyclic aromatic hydrocarbons (PAHs) were obtained from Ultra Scientific (Wesel, Germany). All other compounds were obtained from Sigma-Aldrich (Zwijndrecht, The Netherlands).
Sampling
Sandy soil samples from the obsolete pesticide dumpsite and surroundings were obtained from spots without vegetation. Samples were taken from the upper 20 cm of the soil and were sieved (0.2 × 0.2 cm) and homogenized in the field.
Chemicals analysis
Chemical analysis for quantification of targeted POPs (presented in Table 1) was performed by Eurofins Analytico B.V. (Barneveld, The Netherlands), according to methods compliant with NEN-6980 of the Netherlands Standardization Institute (Netherlands Standardization Institute NEN 2010). In short, an acetone/hexane-based extract was made, after which cleanup was performed using Florisil (U.S. Silica Co.). The purified extracts were analyzed by capillary gas chromatography (GC) coupled to a mass spectrometer (MS; either single-quad MS or triple-quad MS-MS). Compound identifications were based on retention times and their qualifier and target ions. Quantifications were based on peak size.
Sample preparation for CALUX analysis
For the testing of pure compounds dilution series of the compound in dimethylsulfoxide (DMSO) were directly used for exposure of the CALUX cells.
The first step of the soil sample preparation for CALUX analyses was a dichloromethane-based pressurized liquid extraction (PLE) which is suitable for the extraction of water-insoluble and slightly water-soluble compounds (Dionex application note 318/USEPA Method 3545). A second step was included to remove the co-extracted sample matrix based on size exclusion. To this end, gel permeation chromatography (GPC) was performed with dichloromethane on two Styragel HR 0.5 columns (Waters, Etten-Leur, The Netherlands). The collection window was determined by the elution profile of a broad range of test compounds (e.g., reference compounds of the CALUX assays and a set of POPs; data not shown). Subsequently, the GPC extracts were divided into two portions. The larger portion was directly dissolved in DMSO for exposure of all but the DR CALUX cell line. The smaller portion of each extract (∼10 %) was used for the preparation of chemically stable extracts containing mainly non-polar, acid-stable, dioxin and dioxin-like compounds using a multilayer sulfuric acid silica gel column (Besselink et al. 2004). These samples were also dissolved in DMSO. As procedure blank clean sea sand (J.T. Baker, Deventer, The Netherlands) went through the same extraction and cleanup as described above.
CALUX analyses
CALUX assays were performed in triplicate as previously described (Piersma et al. 2013). In short, cells were seeded in 384-well plates and cultured for 24 h after which they were exposed to a dilution series of 13 dilutions with 0.5 log unit increments of the compound or extract in DMSO (final concentration in the well was 1 %). The highest concentrations tested were 0.1 or 10 mM of the compound in the well (with an exception for the following: 2,3,7,8-TCDD, highest concentration 10 nM; benzo[a]pyrene, highest concentration tested 10 μM; estradiol, highest concentration 3 nM) or the equivalence of 1 g dry weight soil per ml culture medium. Along with the test samples, a concentration series of a reference compound (indicated in Table 2) was included on the same well plate. After 24 h of exposure (PAH CALUX 4 h.) cells were lysed, and luciferase activity was quantified using a luminometer (Berthold Technologies, Bad Wildbad, Germany) that adds substrate to each well and subsequently measures luminescence for 1 s per well.
Data handling
Agonistic effects on the CALUX assays were scored as PC10 values. This is the concentration at which a compound or sample evokes 10 % of the maximum signal of the reference compound (PC: positive control) minus the background signal. Response curves were fit using a log agonist versus response algorithm in GraphPad Prism (version 5.03, GraphPad Software, San Diego, USA). For antagonistic effects, PC20 values were established, being the concentration of the test compound or sample at which a 20 % reduction of a baseline signal evoked by an EC50 concentration of the reference agonist could be observed. The baseline signals were established using a log antagonist versus response algorithm in GraphPad Prism. In order to avoid allocation of differential light signals to non-specific (cytotoxic) events, effects were not scored at concentrations higher than the concentration that evoked a 10 % reduction of the signal in the cytotoxicity CALUX for the assays on antagonist action or a 50 % reduction of the signal on the cytotoxicity CALUX in case of the other assays. The Z scores of all assays are between 0.5 and 1.0. The inter-day coefficient of variation for the curve fits, characterized by the log(EC50) values, is below 5 % (Van der Burg et al. 2014). The relative standard deviation of the log(PC10) and log(PC20) values of the test compounds was always below 5 %.
Relative potencies of test compounds were calculated by dividing the PC10 (/PC 20 for antagonistic assays) value of the reference compound by the PC10 (/PC20) value of the test compound. All relative potencies that were used for calculation of the expected activities in the extracts (based on the chemical analytical data) could be established from effect concentrations at which no cytotoxic effects were observed. Expected activities on the CALUX assays expressed in equivalences of the reference compounds were calculated by adding up the molar concentration of the individual compounds in a sample (as determined by chemical analysis) multiplied by their relative potency.
Activities in the soil samples expressed in equivalences of the reference compound/kg dry weight were established by multiplication of the dilution factor at which a PC10 signal was observed (obtained by interpolation of the response curve) with the PC10 (or PC20 in the case of antagonist assays) of the reference compound and subsequent multiplication with the concentration factor of the sample in the solvent (DMSO).
Expected and measured activities for the extracts were presented in weight units of the reference compound per kg dry weight of soil by multiplication of the molar concentration by the molecular weight of the respective compound.
Results and discussion
Effect profiling of pure compounds
In this study, a set of pure chemical compounds were tested for their potency to evoke an effect on different CALUX assays. The compounds that were included in this screening were selected either for their presence in the samples from the pesticide dumpsite and its surroundings or for the fact that they are well-known persistent organic pollutants. Many of these compounds are also among the first POPs list from which the use and production were to be reduced or eliminated according to the Stockholm Convention from the United Nations Environment Programme (Stockholm Convention 2001). An overview of the observed effect concentrations (PC10/PC20) is depicted in Table 2. Lower effect concentrations (more negative) indicate a higher biological potency of the compound with respect to the effect that is addressed.
The screening of pure compounds on the selected assays frequently indicated interference with the ERα, AR, and PR hormone receptors. Endocrine disruptive mechanisms have an implicated role in various adverse outcomes among which carcinogenicity, genital malformations, endometriosis, reduced semen quality, and changes in pubertal onset (Mostafalou and Abdollahi 2013). Direct effects on hormone receptors have been reported for many chemicals that are abundantly present in the environment (De Coster and Van Larebeke 2012). Xenobiotic compounds may have either agonistic or antagonistic effects on the various hormone receptors. Because of observations from previous screenings with these assays (results not shown), we focused on agonistic interaction on the ERα receptor, and antagonistic interaction on the AR, and PR hormone receptors. Agonistic effects on the ERα receptor were observed for the vast majority of test compounds. PC10 concentrations were in the range from 0.1 to 10 μM. The PCBs that were tested did not evoke a response in the ERα CALUX. It has been suggested that PCBs may only become estrogenic after hydroxylation by cytochrome P450 mono-oxygenases (Matsusue et al. 1996; Vakharia and Gierthy 2000). By itself, the U2OS cell line has limited metabolic capacities (Beck et al. 2013; Geiger et al. 2012.), due to which no detectable levels of hydroxylated PCB-metabolites will be formed. Complementation with an in vitro metabolizing system may be used for the detection of effects by metabolization products. In the CALUX assays for anti-androgenic and anti-progestagenic activities, a 20 % reduction of the signal evoked by an EC50 concentration of the model agonist compound was observed by concentrations of test compounds in the range from 0.06 to 10 μM. Notably for all compounds that were tested, antagonistic effects on the androgen receptor co-occurred with antagonistic effects on the progestin receptor. This observation can be explained by the high amino acid sequence homology between the ligand binding domains of both receptors that has previously been described. The related conformational homology of both ligand binding domains is also reflected by the natural ligands of AR and PR, testosterone and progesterone, which only differ in a 17β-substitution (Marhefka et al. 2001; Vivat et al. 1997).
The DR and PAH CALUX’ both indicate ligand binding to the Ah receptor (AhR). While the DR CALUX was specifically developed for the detection of the highly stable dioxins and dioxin-like PCBs, the PAH CALUX is more dedicated to the detection of PAHs and PAH-like compounds (Pieterse et al. 2013). AhR is involved in transcription initiation of genes encoding various phase I and phase II metabolizing enzymes upon activation by ligands such as dioxins, PAHs, PCBs, polybrominated diphenylethers (PBDEs), and benzofurans. Moreover, AhR has been implicated as a central mediator of toxic effects associated with these compounds among which developmental disorders and cancer (Feng et al. 2013; Marlowe and Puga 2005; White and Birnbaum 2009; Nakatsuru et al. 2004). Binding to the Ah receptor as measured with the DR CALUX and the PAH CALUX was observed for the PCB-118 and PCB-126 and the high-MW PAHs benzo[a]pyrene and, dibenzo[a,h]pyrene) which are well-known agonists for this receptor. The potency of PCBs and PAHs for agonist binding to AhR have implications for their toxicological profile: PCBs that act as agonist on the Ah-receptor are typically referred to as dioxin-like PCBs and are associated with dioxin-like health effects such as immunosuppression and reproductive and developmental toxicity (Giesy and Kannan 1998). Screenings of larger sets of PAHs on the PAH CALUX showed that the assay particularly detects higher molecular weight PAHs, most of which are classified as (possible/probable) carcinogenic to humans (Pieterse et al. 2013). In general, lower PC10 concentrations are observed for the PAHs on the DR CALUX as compared to the PAH CALUX which is to be attributed to higher metabolic activity in the DR CALUX cell line. The small difference in PC10 value for dibenzo[a,h]pyrene on the DR CALUX compared to the PAH CALUX indicates a relatively high metabolic stability for this PAH.
The p53 CALUX reports activity mediated by the p53 protein, which is the major regulator of cellular responses towards genotoxicity (van der Linden et al. 2014). Mutations in p53 are commonly observed in various types of cancer and are associated with loss of repair and growth-control functions and may lead to the acquirement of oncogenic functions (Muller and Vousden 2013). The genotoxicity screening using the p53 CALUX indicated no effects for any of the tested compounds. It should be noted that p53 activity was measured in absence of components for metabolic activation. Except for β-HCH, δ-HCH, 4,4-DDT, dibenzo[a,h]pyrene, and the PCBs, genotoxicity data were available for comparison in the in vitro mutagenesis in Salmonella typhimurium database (ISSTY; Benigni et al., 2013). The only classifications in the ISSTY database that contradicted with the CALUX results are those for chlordane and toxaphene which were both indicated as direct mutagens in S. typhimurium. Nevertheless, although chlordane produces liver tumors in mice, weight of evidence indicates that it is not genotoxic (WHO 2011). Toxaphene as well, although indicated as a weak mutagen in the Ames Salmonella test, was not classified as such when tested in a mammalian system based on Chinese hamster V79 lung fibroblasts with metabolic activation provided by human HepG2 hepatoma cells (Schrader et al. 1998).
Positive results were observed on the AP1-CALUX for benzo[a]pyrene, PCB-156, 2,4′-DDT, and 4,4′-DDT. While, with an exception for benzo[a]pyrene, these effects were observed at concentrations between the 10 and 50 % cytotoxicity concentrations, several other compounds also appeared to evoke small dose responsive effects but only at concentrations above the 50 % cytotoxicity concentration.
For the PAHs, benzo[a]pyrene and dibenzo[a,h]pyrene induction was observed on the ESRE CALUX, indicative for endoplasmic reticulum stress. Endoplasmic reticulum stress in human lungs has previously been related to cigarette smoke (Wei et al. 2013), in which PAHs form an important component. Disturbance of the endoplasmic reticulum can lead to accumulation of misfolded and unfolded proteins. Prolonged ER stress may be involved in the development of multiple, particularly chronic, diseases among which Parkinson, atherosclerosis, and renal failure. Various pesticides have been reported to induce endoplasmic reticulum stress-related responses (Mostafalou and Abdollahi 2013). For the pesticides involved in this study, no indication for ER stress were observed.
None of the compounds evoked an effect on the Hif-1 CALUX (chemical hypoxia). Also on the nrf2 CALUX (oxidative stress), no effects were observed by any of the test compounds on the nrf2 CALUX, which may be explained by the stable, non-reactive nature of the compounds.
Effect profiling of pesticide dumpsite samples
Effect profiles of the samples from the dumpsite and its surrounding were generated by two approaches: the first is an indirect, theoretical approach for which expected sum-activities were calculated from the concentrations of identified compounds and their individual relative potencies on the different CALUX assays. The second is a direct approach for which sum-activities were measured by exposure of the CALUX cells to the extracts of the samples. Expectedly, comparison of the values that were calculated from the chemical analytical data and the relative potencies of the individual compounds with the actual bioassay measurements of the extracts from the dumpsite provides insight in whether or not the chemical analysis gives a representative view of the sum of bioactive compounds in these samples. Activities were expressed in equivalents of the reference compound (reference compounds are indicated in Table 2) on the respective assays per gram of dry weight soil. For the direct approach, cells were effectively exposed to dilution series of the extract. The highest concentrations tested contained the extracted material of approximately 1 kg of dry weight from the soil samples per liter exposure medium. While no cytotoxic effects were observed up to the highest concentrations tested for the Baseline Wind and the Drain sample, the cytotoxicity threshold for the other samples was passed at concentrations with the equivalence of 31 (Burn Place), 11 (Gully), and 1 (Source) grams dry weight of the soils per liter exposure medium.
Measured values on the endocrine CALUX assays ERα-, AR-anti-, and PR-anti CALUX were consistently low for the Baseline Wind and the Drain sample as compared to the other samples (Fig. 2). While the observed activities for the Source and Gully samples on these assays are in line with what was expected from the chemical analytical data, remarkably higher values than expected were observed for the Burn Place sample.
A distinction was made for AhR-mediated activities by measurement of PAH-like compounds on the PAH CALUX and measurement of the highly stable dioxin-like compounds, after an additional clean-up procedure, on the DR CALUX. The activities that were observed on the PAH CALUX were relatively low for all samples: the highest observed activity on the PAH CALUX corresponded with 6 mg benzo[a]pyrene equivalences/kg dry weight which, to put it in perspective, is below the threshold values for PAHs in soils in the Netherlands (Dutch 2014). In contrast, substantial dioxin-like activities were observed in the Drain, Source, and Gully samples. The observed values exceed 40–580 times the toxicity equivalence 55 ng TCDD/kg dry weight as indicated as acceptable background level in the Netherlands (Dutch 2014). The only dioxin-like PCB that was addressed in the chemical analysis was PCB 118. Dioxins, other dioxin-like compounds, and PAHs were not included in the chemical analytical screening.
None of the extracts evoked activities during the measurements on the p53, ESRE-, and hif1 CALUX’ which corresponds with expectations based on the effect profiles of individual compounds that were identified in these samples by chemical analysis.
Nrf2-mediated activity, which is a measure for oxidative stress, was shown for the Source, Gully, and Burn Place samples. This activity could not be related to the presence of the organic compounds that were identified by the chemical analysis. An important class of environmental toxicants from which various representatives could generate oxidative stress are the heavy metals (Simmons et al. 2011; unpublished results). In a separate chemical analysis of the Gully sample, substantial amounts of arsenic (800 mg/kg dry weight) were observed. Although the dichloromethane-based extraction protocol that was used for the bioassay analyses is not particularly suitable for the extraction of polar compounds, traces of arsenic may have been present in the extracts.
For the Source, Gully, and Burn Place samples, activities were measured in the AP-1 CALUX. For the Source and Gully samples, these activities were already expected, based on the available 2,4′-DDT and 4,4′-DDT. For the Burn Place sample on the other hand, the measured values were much higher than expected. The AP-1-mediated activities were observed at concentrations above the concentrations at which 10 % reduction in signal was observed in the cytotoxicity CALUX. Possibly AP-1 served a mediating role in chemically induced apoptosis.
From the samples that were analyzed with the CALUX bioassays, the biggest difference between the measured versus the expected activities (based on the biological potencies of the compounds that were identified by chemical analysis) was observed for the Burn Place sample. While the chemical analytical data suggested substantially lower pollution levels at the Burn Place as compared to the Source and Gully locations, the activities in various CALUX assays were mostly in the same range for these three samples. Apparently, a substantial amount of bioactive compounds from this location were not identified in the chemical analysis. This could either indicate that the chemicals that were dealt with at the Burn Place are not among the standard set that were targeted in the chemical analysis or that new products were formed during the burning process that harbor these bioactive potential. Notably, no substantial amounts of dioxin- or PAH-like activities were detected in the Burn Place sample. Although, arguably, substantial amounts of PAHs may have been formed during combustions in the past, their levels may have decreased during the last two decades (Shuttleworth and Cerniglia 1995). Whereas, with an exception for PCB-118, no dioxin-like compounds were addressed in the chemical analysis, substantial dioxin-like activities were observed in the Source, Gully, and Drain samples. These observations may be explained by polychlorinated dibenzo-p-dioxins and dibenzofurans impurities in pesticides (Holt et al. 2010, Huang et al. 2014, UNEP 2013) or formed from degradation of pesticides (Holt et al. 2012) or other dioxin-like compounds present in pesticides (Huang et al. 2014, Zahov et al. 2014).
Two samples from outside the dumpsite were included in the CALUX-based analysis: the Baseline Wind sample and the Drain sample. The bioassay data confirm that the Baseline Wind location is beyond suspicion. The observed activities for the Drain sample are generally lower than those for the samples from the dumpsite. Nevertheless, elevated levels of dioxin-like activities were observed for this sample, which may indicate runoff to some extent.
The data from the nrf2 CALUX and the cytotoxicity CALUX indicate that the dumpsite could form an acute health risk in case of direct exposure. At least for the Gully and the Source locations, this is hardly surprising, considering the toxicant concentrations that were already measured by chemical analysis. The endocrine disruptive activities that were also indicated are arguably more relevant for the longer-term health risks. Endocrine disruption is considered an important health risk by organizations such as the World Health Organisation (WHO and UNEP 2013). The actual health impact of endocrine disruptive pollutants is a matter of discussion, complicated by a lack of mechanistic insight, confounding factors in epidemiological studies, and the long time window that could occur between first exposure and the manifestation of adverse health outcomes. Nevertheless, the combined facts that these compounds have been frequently observed to accumulate in the body and their bioactive traits in vitro alone may already be considered an important reason to protect humans and the environment.
Bioassay data are typically expressed relative to the activity evoked by a reference compound. Whereas some activities, such as, e.g., AhR-mediated activities, may be directly related to the presence of compounds for which threshold values are available in terms of (sum) concentrations, threshold values based on activities of other important cellular pathways are usually not available. Recently, effect-based trigger values were proposed for estrogenic, androgenic, progestagenic, and glucocorticoid activities in drinking water and its sources in a study by a Dutch water research institute (Brand et al. 2013). Development of likewise trigger values for other matrices will further promote effect-based assessment approaches.
Conclusions
We here demonstrated endocrine and other activities for some key POPs, such as DDTs, PCBs, hexachlorohexanes, and drins, listed in the Stockholm Convention. Notably, the selection of pure compounds that were screened in the current study represents a fraction of the wide range of compounds present in the environment and harboring these activities. While the concentration of many compounds in the environment may be too low to trigger a measurable effect, the combined exposure to a wide range of compounds with overlapping effect profiles is more likely to exert an effect. This may explain epidemiological observations that relate endocrine and other effects to exposures to environmental toxicants. This is why hazard assessors should ideally aim for approaches in which exposure to mixtures, overlapping modes of action, and persistence (as well in the environment as in the body) are considered and addressed. Effect-based screening methods could facilitate such an approach.
The presented results indicate that the POP pesticide dumpsite and its surrounding have areas of high-risk exposure. For one specific location (Burn Place), this was only apparent from the bioassay data indicating the presence of other pollutants than those that were included in the chemical analysis. Clearly, protective measures such as fencing are necessary as an immediate countermeasure with adequate planning of long-term measures for this area with further securing and remediation.
This study demonstrates the strong potential of effect-based screening as complementary or, for some applications, even alternative method to chemical analytical assessment of POP pesticide-polluted sites. Obvious advantages of effect-based screening for adverse activities in complex samples are
-
1.
The strong relation to key endocrine receptors and therefore with human health
-
2.
The (semi-)quantitative nature of the data
-
3.
A reduced risk of underestimation of the actual hazard by directly measuring sum activities instead of focusing the presence of individual compounds that are among the “usual suspects”
A similar approach as the one presented here for soil quality assessment may also be used for other environmental compartments, and other types of samples such as sediments, food and feed, and animal and human tissue (e.g., Suzuki et al. 2011; Papadopoulou et al. 2013; Porpora et al. 2009).
References
Ameyar M, Wisniewska M, Weitzman JB (2003) A role for AP-1 in apoptosis: the case for and against. Biochimie 85:747–752
Ashida R, Tominaga K, Sasaki E, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K, Mitsuyama S, Iwao H, Arakawa T (2005) AP-1 and colorectal cancer. Inflammopharmacology 13:113–125
Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Benigni R, Battistelli CL, Bossa C, Tcheremenskaia O, Crettaz P (2013) New perspectives in toxicological information management, and the role of ISSTOX databases in assessing chemical mutagenicity and carcinogenicity. Mutagenesis 28:401–409. doi:10.1093/mutage/get016
Benigni R, Battistelli CL, Bossa C, Tcheremenskaia O, Crettaz P (2013) New perspectives in toxicological information management, and the role of ISSTOX databases in assessing chemical mutagenicity and carcinogenicity. Mutagenesis 28(4):401–9. doi:10.1093/mutage/get016
Besselink HT, Schipper C, Klamer H, Leonards P, Verhaar H, Felzel E, Murk AJ, Thain J, Hosoe K, Schoeters G, Legler J, Brouwer B (2004) Intra- and interlaboratory calibration of the DR CALUX bioassay for the analysis of dioxins and dioxin-like chemicals in sediments. Environ Toxicol Chem 23(12):2781–2789
Brand W, de Jongh CM, van der Linden SC, Mennes W, Puijker LM, van Leeuwen CJ, van Wezel AP, Schriks M, Heringa MB (2013) Trigger values for investigation of hormonal activity in drinking water and its sources using CALUX bioassays. Environ Int 55:109–118
De Coster S, van Larebeke N (2012) Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health 2012:713696
Dutch Government (2014) Annex B to the Dutch guidelines on soil qualities: background values and maximum values for soil and dredging sludge. http://wetten.overheid.nl/BWBR0023085/BijlageB/geldigheidsdatum_15-05-2014
Feng S, Cao Z, Wang X (2013) Role of aryl hydrocarbon receptor in cancer. Biochim Biophys Acta 1836:197–210. doi:10.1016/j.bbcan.2013.05.001
Food and Agriculture Organization of the United Nations (FAO) (2001) The ticking time bomb: toxic pesticide waste dumps. Newsroom 9 http://www.fao.org/english/newsroom/highlights/2001/010502-e.htm
Food and Agriculture Organization of the United Nations (FAO) (2014) Where are the stocks? http://www.fao.org/agriculture/crops/obsolete-pesticides/where-stocks/en/
Garrison PM, Tullis K, Aarts JM, Brouwer A, Giesy JP, Denison MS (1996) Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam Appl Toxicol 30(2):194–203
Geiger T, Wehner A, Schaab C, Cox J, Mann M (2012) Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol Cell Proteomics 11(3):M111.014050. doi:10.1074/mcp.M111.014050
Gibson DA, Saunders PT (2013) Endocrine disruption of estrogen action and female reproductive tract cancers. Endocr Relat Cancer. Oct 25. [Epub ahead of print]
Giesy JP, Kannan K (1998) Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Crit Rev Toxicol 28(6):511–569
Gregoraszczuk EL, Ptak A (2013) Endocrine-disrupting chemicals: some actions of POPs on female reproduction. Int J Endocrinol 2013:828532
Holt E, Weber R, Stevenson G, Gaus C (2010) Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) impurities in pesticides: a neglected source of contemporary relevance. Environ Sci Technol 44(14):5409–5415. doi:10.1021/es903915k
Holt E, Weber R, Stevenson G, Gaus C (2012) Formation of dioxins during exposure of pesticide formulations to sunlight. Chemosphere 88:364–370
Huang J, Gao J, Yu G, Yamazaki N, Deng S, Wang B, Weber R (2014) Unintentional formed PCDDs, PCDFs and DL-PCBs as impurities in Chinese pentachloronitrobenzene products. Environ Sci Pollut Res in print
Italian Ministry for the Environment, Land and Sea (2006) Transboundary risk assessment on the hazardous waste sites of Kanibadam, Khaidarken, Kadamjai in the Ferghana Valley, Regional Synthesis Report. 2006. http://enrin.grida.no/htmls/ferghana_valley/ferghana_valley_soe/pdf/rehra-eng.pdf
Jiang L, Sheikh MS, Huang Y (2010) Decision making by p53: life versus death. Mol Cell Pharmacol 2:69–77
Kortenkamp A (2007) Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect 115(Suppl 1):98–105. doi:10.1289/ehp.9357
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. doi:10.1146/annurev-pharmtox-011112-140320
Marhefka CA, Moore BM 2nd, Bishop TC, Kirkovsky L, Mukherjee A, Dalton JT, Miller DD (2001) Homology modeling using multiple molecular dynamics simulations and docking studies of the human androgen receptor ligand binding domain bound to testosterone and nonsteroidal ligands. J Med Chem 24:1729–1740
Marlowe JL, Puga A (2005) Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J Cell Biochem 96(6):1174–1184
Matsusue K, Ariyoshi N, Oguri K, Koga N, Yoshimura H (1996) Involvement of cytochrome b5 in the metabolism of tetrachlorobiphenyls catalyzed by CYP2B1 and CYP1A1. Chemosphere 32(3):517–523
Mostafalou S, Abdollahi M (2013) Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol 268:157–177. doi:10.1016/j.taap.2013.01.025
Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C (2013) Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 307:74–88. doi:10.1016/j.tox.2012.11.015
Muller PA, Vousden KH (2013) p53 mutations in cancer. Nat Cell Biol 15(1):2–8. doi:10.1038/ncb2641
Nakatsuru Y, Wakabayashi K, Fujii-Kuriyama Y, Ishikawa T, Kusama K, Ide F (2004) Dibenzo[A, L]pyrene-induced genotoxic and carcinogenic responses are dramatically suppressed in aryl hydrocarbon receptor-deficient mice. Int J Cancer 112:179–183
Netherlands Standardization Institute (NEN) (2010) NEN 6980:2008+C1:2010+C2:2011 nl: Soil—quantitative analysis of organochlorine pesticides, pentachlorobiphenyls, and semi-volatile chlorobenzenes by gas chromatography. (in Dutch). http://www.nen.nl/NEN-Shop/Norm/NEN-69802008C12010C22011-nl-1.htm
Papadopoulou E, Vafeiadi M, Agramunt S, Mathianaki K, Karakosta P, Spanaki A, Besselink H, Kiviranta H, Rantakokko P, Sarri K, Koutis A, Chatzi L, Kogevinas M (2013) Maternal diet, prenatal exposure to dioxins and other persistent organic pollutants and anogenital distance in children. Sci Total Environ 461–462:222–229
Piersma AH, Bosgra S, van Duursen MB, Hermsen SA, Jonker LR, Kroese ED, van der Linden SC, Man H, Roelofs MJ, Schulpen SH, Schwarz M, Uibel F, van Vugt-Lussenburg BM, Westerhout J, Wolterbeek AP, van der Burg B (2013) Evaluation of an alternative in vitro test battery for detecting reproductive toxicants. Reprod Toxicol 38:53–64. doi:10.1016/j.reprotox.2013.03.002
Pieterse B, Felzel E, Winter R, van der Burg B, Brouwer A (2013) PAH-CALUX, an optimized bioassay for AhR-mediated hazard identification of polycyclic aromatic hydrocarbons (PAHs) as individual compounds and in complex mixtures. Environ Sci Technol 47:11651–11659. doi:10.1021/es403810w
Porpora MG, Medda E, Abballe A, Bolli S, De Angelis I, di Domenico A, Ferro A, Ingelido AM, Maggi A, Panici PB, De Felip E (2009) Endometriosis and organochlorinated environmental pollutants: a case–control study on Italian women of reproductive age. Environ Health Perspect 117(7):1070–1075
Pyrzynska B, Mosieniak G, Kaminska B (2000) Changes of the trans-activating potential of AP-1 transcription factor during cyclosporin A-induced apoptosis of glioma cells are mediated by phosphorylation and alterations of AP-1 composition. J Neurochem 74(1):42–51
Quaedackers ME, Van Den Brink CE, Wissink S, Schreurs RH, Gustafsson JA, Van Der Saag PT, Van Der Burg BB (2001) 4-hydroxytamoxifen trans-represses nuclear factor-kappa B activity in human osteoblastic U2-OS cells through estrogen receptor (ER)alpha, and not through ER beta. Endocrinology 142:1156–1166
Ritter L, Solomon KR, Forget J (1995) A review on selected persistent organic pollutants. Prepared for The International Programme on Chemical Safety (IPCS). http://cdrwww.who.int/ipcs/assessment/en/pcs_95_39_2004_05_13.pdf
Salnikow K, Davidson T, Zhang Q, Chen LC, Su W, Costa M (2003) The involvement of hypoxia-inducible transcription factor-1-dependent pathway in nickel carcinogenesis. Cancer Res 63(13):3524–3530
Scheringer M, Strempel S, Hukari S, Ng CA, Blepp M, Hungerbühler K (2012) How many persistent organic pollutants should we expect? Atmos Pollut Res 3:383–391
Schrader TJ, Boyes BG, Matula TI, Héroux-Metcalf C, Langlois I, Downie RH (1998) In vitro investigation of toxaphene genotoxicity in S. typhimurium and Chinese hamster V79 lung fibroblasts. Mutat Res 413:159–168
Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A (2000) Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol 475:123–130
Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4(5):E131–E136
Shuttleworth KL, Cerniglia CE (1995) Environmental aspects of PAH biodegradation. Appl Biochem Biotechnol 54(1–3):291–302
Simmons SO, Fan CY, Yeoman K, Wakefield J, Ramabhadran R (2011) Nrf2 oxidative stress induced by heavy metals is cell type dependent. Curr Chem Genom 5:1–12. doi:10.2174/1875397301105010001
Sonneveld E, Jansen HJ, Riteco JA, Brouwer A, van der Burg B (2005) Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol Sci 83(1):136–148
Sonneveld E, Riteco JA, Jansen HJ, Pieterse B, Brouwer A, Schoonen WG, van der Burg B (2006) Comparison of in vitro and in vivo screening models for androgenic and estrogenic activities. Toxicol Sci 89(1):173–187
Stockholm Convention (2001) Stockholm Convention on persistent organic pollutants. http://www.pops.int/documents/convtext/convtext_en.pdf
Suzuki G, Tue NM, van der Linden S, Brouwer A, van der Burg B, van Velzen M, Lamoree M, Someya M, Takahashi S, Isobe T, Tajima Y, Yamada TK, Takigami H, Tanabe S (2011) Identification of major dioxin-like compounds and androgen receptor antagonist in acid-treated tissue extracts of high trophic-level animals. Environ Sci Technol 45(23):10203–10211
UNEP (2013) Toolkit for identification and quantification of releases of dioxins, furans and other unintentional POPs under Article 5 of the Stockholm Convention on Persistent Organic Pollutants. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-TOOLKIT-TOOLK-PCDD-PCDF-2012.En.pdf
Vakharia DD, Gierthy JF (2000) Use of a combined human liver microsome-estrogen receptor binding assay to assess potential estrogen modulating activity of PCB metabolites. Toxicol Lett 114(1–3):55–65
Van der Burg B, Pieterse B, Buist H, Lewin G, Van der Linden SC, Man HY, Rorije E, Piersma AH, Mangelsdorf I, Wolterbeek AP, Kroese ED, Van Vugt-Lussenburg B (2014) A high throughput screening system for predicting chemically-induced reproductive organ deformities. Reprod Toxicol. doi:10.1016/j.reprotox.2014.11.011
van der Linden SC, von Bergh AR, van Vught-Lussenburg BM, Jonker LR, Teunis M, Krul CA, van der Burg B (2014) Development of a panel of high-throughput reporter-gene assays to detect genotoxicity and oxidative stress. Mutat Res 760:23–32
Vivat V, Gofflo D, Garcia T, Wurtz JM, Bourguet W, Philibert D, Gronemeyer H (1997) Sequences in the ligand-binding domains of the human androgen and progesterone receptors which determine their distinct ligand identities. J Mol Endocrinol 18(2):147–160
Weber R, Gaus C, Tysklind M, Johnston P, Forter M, Hollert H, Heinisch H, Holoubek I, Lloyd-Smith M, Masunaga S, Moccarelli P, Santillo D, Seike N, Symons R, Torres JPM, Verta M, Varbelow G, Vijgen J, Watson A, Costner P, Wölz J, Wycisk P, Zennegg M (2008) Dioxin- and POP-contaminated sites—contemporary and future relevance and challenges. Environ Sci Pollut Res 15:363–393
Weber R, Watson A, Forter M, Oliaei F (2011) Persistent organic pollutants and landfills—a review of past experiences and future challenges. Waste Manag Res 29(1):107–121
Wei J, Rahman S, Ayaub EA, Dickhout JG, Ask K (2013) Protein misfolding and endoplasmic reticulum stress in chronic lung disease. Chest 143(4):1098–1105. doi:10.1378/chest.12-2133
White SS, Birnbaum LS (2009) An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27:197–211. doi:10.1080/10590500903310047
WHO, UNEP (2013) State of the science of endocrine disrupting chemicals-2012. http://unep.org/pdf/9789241505031_eng.pdf
World Health Organization (2011) Guidelines for drinking water-quality; chemical fact sheets. http://www.who.int/water_sanitation_health/publications/2011/9789241548151_ch12.pdf?ua=1
Xia M, Huang R, Sun Y, Semenza GL, Aldred SF, Witt KL, Inglese J, Tice RR, Austin CP (2009) Identification of chemical compounds that induce HIF-1alpha activity. Toxicol Sci 112(1):153–163. doi:10.1093/toxsci/kfp123
Zahov S, Hollert H, Seiler T-B, Weber R, Holt E (2014) Distinguishing between the mutagenicity, cytotoxicity and dioxin-like activity of pesticides and their impurities. Env. Sci Pollut Res submitted
Acknowledgments
Part of this work was funded by the BE-Basic foundation, which was granted a FES subsidy from the Dutch Ministry of Economic Affairs, Agriculture and Innovation (EL&I).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Pieterse, B., Rijk, I.J.C., Simon, E. et al. Effect-based assessment of persistent organic pollutant and pesticide dumpsite using mammalian CALUX reporter cell lines. Environ Sci Pollut Res 22, 14442–14454 (2015). https://doi.org/10.1007/s11356-015-4739-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4739-5