Abstract
We tested the capacity of biochar (made at 450 °C from a common reed species) to neutralise pH and remove metals in two acid drainage waters (pH 2.6 and 4.6) using column leaching and batch mixing experiments. In the column experiments, the acid drainage water was neutralised upon passage through the biochar with substantial increases (4–5 pH units) in the leachate pH. In the batch experiments, the leachate pH remained above 6.5 when the drainage:biochar ratio was less than approximately 700:1 (L acid drainage:kg biochar) and 20:1 for the pH 4.6 and pH 2.6 drainage waters, respectively. Dissolved metal concentrations were reduced by 89–98 % (Fe ≈ Al > Ni ≈ Zn > Mn) in the leachate from the biochar. A key mechanism of pH neutralisation appears to be solid carbonate dissolution as calcite (CaCO3) was identified (via X-ray diffraction) in the biochar prior to contact with acid drainage, and dissolved alkalinity and Ca was observed in the leachate. Proton and metal removal by cation exchange, direct binding to oxygen-containing functional groups, and metal oxide precipitation also appears important. Further evaluation of the treatment capacity of other biochars and field trials are warranted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drainage from mine sites and acid sulphate soils creates ecological and other risks due to its low pH and high dissolved metal content. The main acid-producing process is the exposure of pyrite (FeS2) to air and water, which promotes oxidative dissolution, a reaction catalysed by microbes (Nordstrom 2011). Due to the low pH, secondary acid-dissolution of minerals and metal oxide surfaces occurs releasing potentially toxic metals such as Al, As, Mn, Ni and Zn. Typically, treatment of acid drainage is based on use of limestone (CaCO3) or hydroxide-based (eg. Ca(OH)2) products to neutralise pH and precipitate dissolved metals as solid phases. However, these chemical agents are costly, may not be able to be sourced locally, and there are significant issues with armouring (coating of the surface) of limestone by gypsum and metal oxides that greatly reduces treatment efficiency (Hammarstrom et al. 2003; Mosley et al. 2014a).
Biochar, also known as black carbon, is the carbon-rich solid product resulting from the heating of biomass in an oxygen-limited environment. Biochar is chemically and biologically more stable than the organic matter from which it is made. However, its properties may vary widely as a result of different raw biomaterials and conditions for its production (Amonett and Joseph 2009). Biochar has some characteristics that make it potentially attractive for treating (neutralising pH and removing dissolved metals) acid drainage. Kim et al. (2014) found spent coffee ground biochar raised the pH of acid mine drainage solutions from approximately 4 to 7 in 10:1 (drainage:biochar) ratios over a 2-h period. It would be useful to determine if this treatment capacity would extend to higher ratios and longer time periods. Lu et al. (2012) found sorption of Pb to a sludge-derived biochar increased as pH was raised from pH 2 to 5. Khare et al. (2013) also showed pH increased and metals were removed in synthetic multi-metal solutions and simulated acid mine drainage. It is difficult to apply these results directly to acid drainage, however, where multiple cations and anions are present and may interact. Biochar also offers some potential practical advantages compared to conventional acid drainage treatments such as limestone, as it can be made on site by pyrolysis of locally-sourced biomaterials. Hence, biochar could provide a source of acid neutralising materials where other sources are either not available or too costly.
Increases in soil pH and reductions in soil acidity have been linked to alkalinity provided by biochar (Materechera and Mkhabela 2002; Yuan and Xu 2011). This appears to be due to the presence of solid Ca and Mg carbonates formed during pyrolysis (Yuan et al. 2011a). Also, biochar has a high surface area and exchange capacity which provides an ability to remove cations (Cao et al. 2009; Uchimiya et al. 2011; Lu et al. 2012; Xu et al. 2013) and anions (Fang et al. 2014) from solution. Metal removal by biochar has been found to occur via binding to oxygen-containing (carboxylic -COO− and phenolic R-O−) functional groups (Cao et al. 2009; Uchimiya et al. 2011) and surface adsorption and coprecipitation of Al with silicate particles (Qian et al. 2013; Qian and Chen 2013).
The aim of this study was to conduct a laboratory-based assessment of the potential for biochar made from a common reed species to treat acid drainage. We hypothesised that the low pH of acid drainage water would be neutralised following passage through biochar and that dissolved metal concentrations would be substantially reduced. Column leaching and batch experiments with different drainage:biochar ratios were used to test this and gain knowledge on the potential mechanisms involved.
Materials and methods
Biochar and acid drainage description
Biochar made at 450 °C from a common reed species (Phragmites australis) growing naturally at Mypolonga (35° 02′ 36″ S 139° 19′ 34° E) in the lower River Murray (South Australia) floodplains was obtained from a commercial supplier (Clean Carbon Pty Ltd). The region where the reeds were collected has been severely affected by acid drainage as a consequence of acid sulphate soil exposure during drought (Mosley et al. 2014b). A bulk sample of acidic, oxygenated, surface drainage water were collected from the Jervois (35° 17′ 53″ S 139° 24′ 06″ °E) and Mobilong (35° 06′ 18″ S 139° 16′ 29″ °E) areas in this region in February 2014. The water was collected from the main drainage channel via grab sampling using a clean polyethylene bucket and placed in several 20-L carboys. The Jervois and Mobilong drainage waters collected for the experiments had different characteristics, in particular pH, acidity, major ion and metal concentrations (Table 1).
Experimental setup
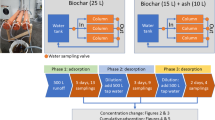
Two methods were used to assess the capacity and mechanisms of biochar to neutralise pH and remove metals in the acid drainage, (1) column leaching and (2) biochar:drainage ratio batch tests.
The column leaching method was adapted from a method for the prediction of coal mine drainage quality (USEPA 2011). We did not undertake the CO2 gas enhancement in this method as this was only relevant to sub-surface systems. Replicate 6 L columns (polyethylene, 4:1 length:diameter ratio) containing biochar were constructed. A layer of glass marbles covered by geotextile fabric was placed at the bottom of the column. The column was uniformly filled with a measured amount of biochar (approximately 1.25 kg), filled with unfiltered acid drainage (approx. 3 L) from the Jervois site and allowed to equilibrate for a period of approximately 24 h. This time period was considered sufficient for equilibration of the biochar with the proton and dissolved metal concentrations (Lu et al. 2012; Khare et al. 2013), and approximated how the biochar might be used in drainage channels with intermittent flow. Air was continually bubbled up through the columns using an aquarium bubbler, in order to maintain oxygenated (typically 4–5 mg/L dissolved oxygen, see supplementary material S1) conditions. This was designed to be similar to the well-oxygenated conditions in the surface drainage channels (Mosley et al. 2014b). Following each 24-h saturation cycle, the leachate was drained from the column and collected for analysis, and the column immediately refilled. The filling–leaching cycle was repeated 35 times using the same/bulk drainage solution.
For the drainage:biochar ratio batch tests, various amounts of biochar (0.01–100 g) were accurately weighed into a series of 1.25 L polyethylene bottles. To these bottles, a measured quantity (approximately 1 L) of drainage water from Jervois (pH 4.6) and Mobilong (pH 2.6) acid drainage was added to give drainage:biochar water ratios ranging from 10:1 to 10,000:1. In contrast at the end of the column leaching experiment, there was an approximate ratio of 80:1 (corresponding to ≈80 L acid drainage passed through ≈1 kg biochar in column). These solutions were shaken and then left to equilibrate for approximately 24 h and the leachate drained and collected for analysis. An air gap was left at the top of the bottle, and well-oxygenated conditions were maintained in all but the lowest dilution (10:1 ratio) with Mobilong water (1 mg/L dissolved oxygen, see Supplementary Material S1). Hence, redox-sensitive metals such as Mn and Fe would be present mostly in oxidised forms.
Analyses
The raw biochar material was analysed for pH and conductivity in water (1:5 biochar:water ratio) using calibrated electrodes (methods 4A1 and 4B1, Rayment and Higginson 1992). A sub-sample of biochar was dried (at 60 °C for 48 h) and analysed for exchangeable cations (Ca, K, Mg, Na, H, Al) using a standard ammonium acetate extract (Method 15D3, Rayment and Higginson 1992) with no pretreatment for soluble salts. Acid neutralising capacity (ANC) of the biochar was assessed by addition of standardised HCl followed by back titration with standardised NaOH to pH 7 (Ahern et al. 2004). Metals were analysed in a 1:3 nitric/HCl digest by ICP-MS (method 3125, APHA 2005). Total carbon and nitrogen were analysed by dry combustion and infrared detection on a Leco CNS2000 instrument
Biochar samples (before and after acid drainage contact) were dried at room temperature for X-ray diffraction (XRD) analysis. The dried samples were ground in an agate mortar and pestle. The resulting fine powders were either gently back pressed into stainless steel sample holders prior to XRD analysis. XRD patterns of samples were collected with a PANalytical X’Pert Pro Multi-purpose diffractometer in “standard” configuration mode using iron filtered Co Kα radiation, automatic divergence slit and X’Celerator Si strip detector. The diffraction patterns were recorded in steps of 0.017° 2 theta with a 0.5-s counting time per step.
pH, temperature, specific electrical conductivity (at 25 °C), and dissolved oxygen were measured daily in the raw acid drainage and column leachate using a calibrated instrument (YSI Pro Plus). New polyethylene bottles, washed and rinsed with deionised water, were used to collect samples for laboratory analysis (every 3–4 days for the column input and leachate, all solutions in the batch experiment) of acidity, alkalinity, major ions (Ca, Mg, Na, K, Cl), and dissolved organic carbon (DOC). Acid-cleaned bottles were used to collect samples for dissolved (0.4 μm filtered) metal (Al, Mn, Fe, Ni, Zn) analysis. Laboratory analyses were undertaken within 1 week of sampling by the Australian Water Quality Centre’s National Association of Testing Authorities (NATA) accredited laboratory using Standard Methods (APHA 2005). Total alkalinity was measured by titration to a pH 4.5 end-point. Acidity was measured by titration to pH 8.3 end-point at 25 °C following hot peroxide digestion. In accordance with these operational procedures, we define acidity and alkalinity based on the proton condition, with acidity representing the equivalent sum of acids (in this context H+, metal and sulphate species that participate in protonation reactions) that are titratable with strong base to a pH endpoint of 8.3, and alkalinity the equivalent sum of bases (typically carbonates) that are titratable with strong acid to a pH of 4.5.
Ca, Mg, Na and K were measured by ICP-OES with a ferricyanide method used for Cl (method 4500-Cl- E, APHA 2005). Dissolved (<0.4 μm) metals were measured by ICP-MS (Agilent 7500 series). Dissolved organic carbon was measured by high temperature combustion and infrared detection of evolved CO2 (method 5310B, APHA 2005). Quality assurance and control checks were regularly carried out and replicates in the column and batch experiments were in close agreement (within approximately 5 %, see results below and in Supplementary Material).
Results and discussion
Acid neutralisation capacity
The results confirmed our hypothesis, namely biochar made from common reeds demonstrated a capacity to treat acid drainage water under controlled laboratory conditions. The acidic pH ≈ 4.5 of the column input water was consistently neutralised to pH 7–8 upon passage through the biochar material (Fig. 1). Slight declines in pH were observed over the sampling period. A substantial amount (1.5–3.5 meq/L) of dissolved alkalinity was created during passage of the acid drainage through the biochar material (Fig. 1). Coupled with a decrease in the amount of dissolved acidity in the column leachate, this resulted in a net alkalinity (alkalinity > acidity) being present. Our results are generally consistent with previous research on other biochar materials in contact with acidic solutions at lower drainage:biochar ratios (Khare et al. 2013; Kim et al. 2014).
Dilutions of different amounts of biochar with acid drainage were used to extend the range of dilutions past that achieved in the column experiment (≈80:1 L/kg) up to 100,000:1 and 1000:1 (L/kg) for the Jervois and Mobilong drainage waters, respectively. Mixing of acidic drainage water with the biochar raised the solution pH above 6.5 (guideline value to protect aquatic ecosystems, ANZECC 2000) when the drainage:biochar ratio was less than approximately 700:1 (Jervois water, initial pH 4.6) and 20:1 (Mobilong water, initial pH 2.6) (Fig. 2). Hence, the apparent treatment capacity was substantially reduced for the lower pH, higher dissolved metal and Mobilong acid drainage water. The biochar still showed some ability to raise the pH of the acid drainage water up to ratios of approximately 10:000:1 and 1000:1 for the Jervois and Mobilong water, respectively. Similar to the column experiments, appreciable alkalinity generation occurred in the batch experiments that decreased as the drainage water to biochar ratio increased (Fig. 2).
pH, acidity, alkalinity and dissolved metals (Al, Fe, Mn, Ni, Zn) in the water from the biochar batch experiments with Jervois and Mobilong acid drainage water. The concentration in the raw acid drainage water (infinite dilution, α) and ANZECC (2000) water quality guideline (dashed line) to protect aquatic ecosystems is also shown. The WQG is for 95 % species protection applicable to freshwaters. For Al, WQGs is for waters with pH > 6.5, no WQG exists for Fe, and WQGs for Ni and Zn are hardness adjusted (90 mg CaCO3/L)
The measured ANC of the biochar (1.45 mol H+/kg) corresponded to only about 7 % of the theoretical ANC of pure limestone (20 mol H+/kg). Using the dilution factors above (to achieve pH > 6.5 leachate) and an average acidity of these drainage waters (1 and 6 meq/L, respectively) gives an effective ANC for the biochar of 1–1.3 mol H+/kg. This is quite close to the theoretical ANC. The effective ANC of limestone is often much lower than the theoretical ANC due to armouring (e.g. Mosley et al. 2014a, ≈25 % effective ANC). Based on this preliminary assessment, biochar treatment is likely to be most useful when limestone is unavailable or expensive to source. However, direct comparisons of biochar versus limestone treatment capacity for the same acid drainage waters would be useful.
Metal removal
Passage through the biochar column reduced all the dissolved metal concentrations in the acid drainage on average by 89–98 % (Fe, 98 % ≈ Al 97 % > Ni 94 % ≈ Zn 93 % > Mn 89 %; see Supplementary Material Fig. S2 and note the effectiveness of Mn and Ni removal decreased slightly over time). Substantial metal removal also occurred in the batch experiments that decreased as the drainage water to biochar ratio increased (Fig. 2). One to two orders of magnitude (compared to raw water) reductions in dissolved metal concentrations occurred up to an approximately 1000:1 (L/kg) dilution for the biochar mixed with Jervois acid drainage but only to about 50:1 for the more acidic Mobilong drainage. This was the range where pH was maintained >6.5 and alkalinity was present (Fig. 2). In this dilution range, the char-associated decline in dissolved metals produced leachate that met respective water quality guidelines for protection of aquatic ecosystems (Fig. 2, dashed line represents guideline values).
Potential treatment mechanisms
The chemical and physical properties of the common reed (Phragmites sp.) biochar that are relevant to its treatment capacity are shown in Table 2. A 1:5 biochar:water solution had an approximately neutral pH and moderate conductivity of 4680 μS/cm. The total effective cation exchange capacity (CEC) in the biochar was high (relative to typical global surface soil values, 76–501 mmol/kg, Essington 2004) and mostly comprised of Ca, Mg, K, and Na, with very little exchangeable H and Al. The biochar had a high (32 %) total carbon content but a low (1 %) nitrogen content. The bulk density of the material was very low, on the order of 0.27 kg/L. The metal content of the biochar is in the order Fe > Al > Mn > Zn > Ni (Table 1).
Without detailed mechanistic research at the molecular level, it is difficult to precisely define the treatment mechanism(s). Nevertheless, based on our results, and findings from previous research, there are some strong indications of potential mechanisms. A conceptual diagram with four potential mechanisms (carbonate dissolution, cation exchange, complexation on oxygen-containing functional groups, metal oxide formation) is shown in Fig. 4 and discussed below in reference to our results.
Consistent with findings of the alkalinity in biochar ameliorating acidic soils (Yuan and Xu 2011), carbonate dissolution appears to be a key mechanism for acid neutralisation in our experiments. We observed that when acid drainage water contacted the biochar, a significant amount of dissolved alkalinity was generated (Figs. 1 and 2). We also identified calcite by XRD in the biochar (Fig. 5, pre-acid treatment only) which is consistent with previous research (Yuan et al. 2011b; Bruun et al. 2014). It is likely protons (H+) in the acid drainage dissolve solid phase carbonate/calcite in the biochar to form dissolved alkalinity/HCO3 − and Ca2+ in the leachate water (Fig. 3) via the reaction (Mosley et al. 2014a):
Change in concentration ([output]−[input]) of major ions (Na, K, Cl, Ca, Mg), HCO3 −(from measured alkalinity and presuming this is dominant species at pH 7–8), dissolved metals (Al, Fe, Mn) and protons (H+ ≈ 10-pH), and dissolved organic carbon (DOC) after passage through the columns containing biochar. A positive value indicates flushing, desorption or cation exchange off the biochar into the leachate, whilst a negative value indicates retention of a solution component by the biochar in the column
Formation of carbonate minerals in biochar has been found to depend on pyrolysis temperature (Yuan et al. 2011b), so optimizing this process would likely be beneficial for potential future applications of biochar for treating acid drainage.
Cation exchange is another mechanism that is potentially important for acid neutralisation and metal removal (Lu et al. 2012). Passage of the drainage water through the biochar resulted in the initial binding of Ca but when the drainage water/biochar ratio exceeded approximately 30:1 (L/kg), it was released from the biochar (Fig. 3). In contrast, Mg was consistently released off the biochar material throughout the experiment. The release of dissolved Ca and Mg in our column experiment was much greater than the retention of protons and dissolved metals and more similar to alkalinity/HCO3- (Fig. 3). Hence, a major source of these ions, at least for Ca, may be from carbonate mineral dissolution (as described above) rather than cation exchange.
Based on the known redox and acid–base chemistry of acid drainage waters (including the water used in these experiments; Simpson et al. 2013; Mosley et al. 2014), if oxygenated conditions are maintained and pH is neutralised, solid metal oxide phases will rapidly form. Once formed, these oxides may adsorb to negatively-charged functional groups on the biochar (Lu et al. 2012) as shown conceptually in Fig. 4. Our XRD results (post-acid treatment, Fig. 5) provide some support for this as they showed that Fe oxides (hematite) formed on the biochar during the column experiment.
Conceptual diagram of four processes proposed to be important for acid neutralisation and metal removal by biochar. The main mechanism of pH increase appears to be solid carbonate dissolution, whilst cation exchange, metal oxide formation and binding, and direct binding to oxygen-containing functional groups and silicates are also likely important for dissolved metal removal
Direct binding of protons and dissolved metals to oxygen containing functional groups (Cao et al. 2009; Uchimiya et al. 2011) and silicates for Al (Qian and Chen 2013) has been reported. It is likely that, based on this previous research, direct binding to the biochar functional groups was at least a partial reason for observations of metal removal in our experiments (Figs. 2 and 3). Metal binding was also pH dependent (Fig. 2), which is consistent with involvement of carboxylic acid and hydroxyl groups (Lu et al. 2012). Development of geochemical models to predict proton and metal binding to biochar, and carbonate dissolution, would be useful. This requires further specific research on proton/metal binding affinities and competitive effects.
Observations of flushing of salt and DOC from biochar
Large amounts of soluble salt (Na, K, Cl) were flushed off the biochar early in the column experiment (Fig. 2), and this approximately tripled the conductivity of the leachate water (Fig. 1). The Na and Cl likely came from dissolution of halite (NaCl), which we identified by XRD (Fig. 5). The P. australis reeds used for our experimental biochar were grown in a very saline soil so these salt flushing effects may not be representative of other biochars. Further investigation of salt concentrations in leachate from different biochars from less saline areas is warranted.
Elevated DOC levels were also observed early in the column experiments (Fig. 3). This is likely due to the release of some water-soluble organic carbon from the biochar when it is first wetted. Kim et al. (2014) also found that a large amount of DOC was mobilized from spent coffee ground biochar following contact with acidic solutions. Despite leaching some DOC, the biochar does not appear to have a particularly high oxygen demand as we only observed low dissolved oxygen in the lowest dilution of biochar (10:1) with the Mobilong acid drainage water (see supplementary material, Fig. S1). This is likely due to the material being quite resistant to microbial breakdown. Nevertheless, the potential for elevated conductivity and DOC in leachate warrants further consideration in any field application.
Environmental management implications
We believe our results are encouraging enough to support further laboratory-based and pilot-scale field testing of the use of biochar for treating acid drainage. It is recommended further tests are conducted with various other types of biochars (e.g. different plant materials, pyrolysis temperatures) and acid drainage waters. The results indicate that biochar is a potential alternative for acid drainage treatment, particularly where more conventional acid treatment products such as limestone are unavailable or too costly. The technique may be most suitable to smaller-scale or diffuse acid sulphate soil or mine drainage problems, also in developing countries, where the biomaterials can be locally grown and processed into biochar.
In our local context, we envision that the biochar could be placed in adequately scaled porous containers/bags along sections of the acid drainage channels. In the Lower Murray river floodplains, it is potentially feasible to grow large areas of Phragmites species. There is a large area (approximately 2000 ha) of salt-affected land that is retired from farming, and this species is naturally occurring over this landscape. The biomass production of Phragmites at the trial site that supplied the biochar for these experiments was measured at at 9 tonnes/ha/year (2 harvests per year), resulting in a potential of 18,000 tonnes of biomass per year over the 2000 ha area. With a biomass to biochar conversion of 34 %, that equates to 6,120 tonnes of biochar per year. Based on the Jervois acid drainage (most representative of average pH and acidity in the region), and a treatment ratio of 700:1 L/kg (this project), approximately 4300 ML of water could be treated using biomass produced from the region. Given an average acid drainage volume of approximately 2.5 ML/ha/year (Mosley et al. 2014c), biochar made from locally-sourced reeds has potential to treat much of the acid draining from the 2000 ha area. There may be additional benefits to growing reeds for biochar production such as for carbon sequestration and habitat enhancement. The cost effectiveness of a potential biochar treatment approach requires further assessment however. An additional important consideration for any field application will be appropriate disposal or reuse (e.g. as an alkaline soil amendment) of the biochar when its treatment capacity is exhausted. The potential leaching of protons and metals from spent biochar (e.g. following rainfall) requires further research.
Conclusions
This study has demonstrated the capacity of biochar made from common reeds to neutralise pH and remove metals from acid drainage. Contact of acid drainage with a sufficient amount of biochar neutralised the pH and produced alkalinity in the leachate. Metals were also reduced by >97 %, whilst the pH of the biochar leachate was maintained greater than 6.5. A major mechanism responsible for the pH neutralisation effects appears to be carbonate dissolution. Metal removal may also occur via cation exchange, direct binding to oxygen containing functional groups and silicates, and metal oxide formation and adsorption. Biochar is an alternative to conventional limestone and lime treatment. The practicality and costs of biochar compared to conventional treatment requires further assessment however.
References
Ahern CR, McElnea AE, Sullivan LA (2004) Acid sulfate soils laboratory methods guidelines. Queensland Department for Natural Resources Mines and Energy, Australia
ANZECC (2000) Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association and American Water Works Association and Water Environment Federation, Washington
Amonett J, Joseph S (2009) Characteristics of biochar, Microchemical properties. In: Lehmann J, Joseph S (eds) Biochar for environmental management. Earthscan Publications Ltd. ISBN: 9781844076581 pp 33–52
Bruun S, Clauson-Kaas S, Bobulská I, Thomsen K (2014) Carbon dioxide emissions from biochar in soil: role of clay, microorganisms and carbonates. Eur J Soil Sci 65:52–59
Cao X, Ma L, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Essington ME (2004) Soil and water chemistry: an integrative approach. CRC Press 534 pp
Fang Q, Chen B, Lin Y, Guan Y (2014) Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen containing organic groups. Environ Sci Technol 48:279–288
Hammarstrom JM, Sibrell PL, Belkin HE (2003) Characterization of limestone reacted with acid-mine drainage in a pulsed limestone bed treatment system at the Friendship Hill National Historical Site, Pennsylvania. USA Appl Geochem 18:1705–1721
Khare P, Dilshad U, Rout PK, Yadav V, Jain S (2013) Plant refuses driven biochar: Application as metal adsorbent from acidic solutions. Arabian J Chem special issue
Kim MS, Min HG, Koo N, Park J, Lee SH, Bak GI, Kim JG (2014) The effectiveness of spent coffee grounds and its biochar on the amelioration of heavy metals-contaminated water and soil using chemical and biological assessments. J Environ Manage 146:124–130
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res 46(3):854–862
Materechera SA, Mkhabela TS (2002) The effectiveness of lime, chicken manure and leaf litter ash in ameliorating acidity in a soil previously under black wattle (Acacia mearnsii) plantation. Bioresour Technol 85:9–16
Mosley LM, Shand P, Self P, Fitzpatrick R (2014a) The geochemistry during management of lake acidification caused by the rewetting of sulfuric (pH < 4) acid sulfate soils. Appl Geochem 41:49–61
Mosley LM, Palmer D, Leyden E, Fitzpatrick R, Shand P (2014b) Acidification of floodplains due to river level decline during drought. J Contam Hydrol 161:10–23
Mosley LM, Palmer D, Leyden E, Fitzpatrick R, Shand P (2014c) Changes in acidity and metal geochemistry in soils, groundwater, drain and river water in the Lower Murray River after a severe drought. Sci Tot Environ 485–486:281–291
Nordstrom DK (2011) Mine waters: acidic to circumneutral. Elements 7:393–398
Qian L, Chen B, Hu D (2013) Effective alleviation of aluminum phytotoxicity by manure-derived biochar. Environ Sci Technol 47(6):2737–2745
Qian L, Chen B (2013) The dual role of biochars as adsorbents for aluminum: The effects of oxygen-containing organic components and the scattering of silicate particles. Environ Sci Technol 47(15):8759–8768
Rayment GE, Higginson FR (1992) Australian Laboratory Handbook of soil and water chemical methods. Inkata Press
Simpson SL, Vardanega CR, Jarolimek C, Jolley DF, Angel BM, Mosley LM (2013) Metal speciation and bioavailability changes during discharge and neutralisation of acidic drainage water. Chemosphere 103:172–180
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190:432–441
USEPA (2011) Method 1627: Kinetic test procedure for the prediction of coal mine drainage quality, United States Environmental Protection Agency, http://water.epa.gov/scitech/methods/cwa/upload/Method-1627-Kinetic-Test-Method-for-the-Prediction-of-Mine-Drainage-Quality.pdf Accessed 27 Mar 2015
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20(1):358–368
Yuan JH, Xu RK (2011) The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manage 27:110–115
Yuan J, Xu RK, Wang N, Li JY (2011a) Amendment of acid soils with crop residues and biochars. Pedosphere 21(3):302–308
Yuan JH, Xu RK, Zhang H (2011b) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresource Technol 102(3):3488–3497
Acknowledgments
The part-funding of the Australian Government is gratefully acknowledged as is the support of Wayne Brown (Environments by Design). We kindly thank Mark Raven (CSIRO) for his XRD analysis. We thank Prof. Petra Marschner (University of Adelaide) for useful discussions and comments on an earlier draft of the manuscript. We also thank the anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Mosley, L.M., Willson, P., Hamilton, B. et al. The capacity of biochar made from common reeds to neutralise pH and remove dissolved metals in acid drainage. Environ Sci Pollut Res 22, 15113–15122 (2015). https://doi.org/10.1007/s11356-015-4735-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4735-9