Abstract
Temporal and spatial variations in the phytoplankton community and environmental variables were investigated from February to July 2014, in the upper lake of Shengjin Lake, China. We identified 192 species of phytoplankton belonging to 8 phyla and 84 genera, of which 46.4 % of Chlorophyta, 29.2 % of Bacillariophyta, and 12.5 % of Cyanophyta. There were 14 predominant species. Marked temporal and spatial variations were observed in the phytoplankton community. The total abundance of phytoplankton ranged from 3.66 × 105 to 867.93 × 105 cells/L and total biomass ranging from 0.40 to 20.89 mg/L. The Shannon-Wiener diversity index varied from 3.50 to 8.35 with an average of 5.58, revealing high biodiversity in the phytoplankton community. There were substantial temporal changes in the dominant species, from Bacillariophyta and Cryptophyta to Cyanophyta and Chlorophyta. Phytoplankton biomass and abundance showed a similar increasing trend from February to July. Pearson correlations and Redundancy analysis revealed that the most significant environmental factors influencing phytoplankton community were water temperature (T), transparency (SD), and nutrient concentration. The positive correlation between the key water bird areas and phytoplankton biomass indicated that the droppings of wintering water birds had an important influence on the phytoplankton community in the upper lake of Shengjin Lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoplankton are the primary producers of lake ecosystems and thus not only play a very important role in the food chain but also affect the water quality. Phytoplankton community structure is affected by multiple environmental variables and varies widely with geographical location, functional structure, and nutritional status (Yin et al. 2011; Li et al. 2009). The structure and distribution of phytoplankton populations depend upon nutrient concentrations and other ecological factors, including temperature, light, composition and quantity of organic matter, conductivity, and grazing (Chang et al. 2013). The dominant species, and the abundance and biomass of phytoplankton change considerably with increases in water temperature (Nalewajko and Murphy 2001; Heisler et al. 2008; Lu et al. 2010). Therefore, water temperature is thought to be an important environmental factor influencing phytoplankton community structure (Bierman and Dolan 1981; Masaki and Seki 1984; Tian et al. 2013a). Moreover, nutrient concentration was often regarded as the principal limiting factor for aquatic phytoplankton production (Sakamoto 1966; Smith 1982), with high nutrient concentrations resulting in an increase in phytoplankton biomass, and even the formation of phytoplankton blooms in lakes and reservoirs (Watson et al. 1997).
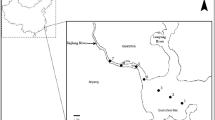
Shengjin Lake (116° 55′–117° 15′ E and 30° 15–30° 30′ N) is located on the south bank of the Yangtze River in the Anhui Province, China. The lake has a surface area of 13,300 hm2, and consists of three parts, upper, middle, and lower, covering areas of 5800, 5200, and 2300 hm2, respectively. The lake climate is subtropical monsoonal, with annual average precipitation of about 1600 mm, and most rain falling from May to August (Hu et al. 2004). This region is subject to monsoonal flooding in the summer followed by low water levels during the autumn and winter. The variable hydrological cycle creates an expansive area of multiple shallow ephemeral wetlands, with their ecosystems significantly affected by the temporally alternating flooding and drying (Cheng et al. 2009). Furthermore, the periodic hydrological changes could significantly affect phytoplankton community structure by influencing water transparency, nutrient concentrations, the water thermocline, and aquatic plant communities. Usually, there is a gradual increase in the water temperature of Shengjin Lake from February to July, reaching a peak in July. The water level rises from May onwards, reaching a peak at the end of July and into August (Zhu and Zhou 2010); thus, these shallow wetlands are usually inundated in June.
Because of the unique ecological environment of Shengjin Lake, there is widespread concern for its biological diversity. Scientists have undertaken a series of studies examining the community diversity and seasonal dynamics of wintering water birds (Zhou et al. 2009; Chen et al. 2011), their foraging behavior (Chen and Zhou 2011) and adaptation to habitat evolution (Jiang et al. 2007), as well as the plankton diversity (Shen 2008; Shen et al. 2009a, b; Zong et al. 2008, etc).
It is reported that seabirds can transport large quantities of nutrients in Arctic ponds, and the seabird-derived nutrients played a dominant role in driving aquatic primary production (Michelutti et al 2010). Ptacnik et al. (2008) also indicated that the large amount of droppings from penguins in Antarctic provided abundant nutrients for the lakes, and influenced the lake trophic levels and the phytoplankton community structure. During the low-water level season each year (from December to March), the upper part of Shengjin Lake has shallow muddy or grassy zones (Fig. 1). More than 11 km2 of shallow zones provide a habitat for wintering water birds (Yang 2011). More than 70,000 water birds dwell on these zones in the winter, among which 86 % are Anatidae. The central and southern areas of the upper lake are the most important regions for water birds, hosting 70–90 % of the birds inhabiting Shengjin Lake (Cheng et al. 2009). On average, there are about 873.6 t of droppings during the whole wintering period (from November to March) from those water birds in the central and southern areas of the upper lake, according to the correlation coefficient of ducks provided by the State Environmental Protection Administration of China No. 43 (Liu et al. 2011), Therefore, the emissions of TP, NH4 +-N, COD, and BOD from their droppings are nearly 5.4, 0.7, 40.4, and 26.2 t, respectively. It is therefore clear that water bird droppings could influence phytoplankton growth and community structure.
In the present study, we investigated phytoplankton species composition, dominant species, phytoplankton abundance and biomass, species diversity, and environment parameters from February to July 2014 in the upper region of Shengjin Lake. We then analyzed the main environmental factors affecting phytoplankton community structure and discuss the influence of the water environment on the phytoplankton community and biomass in key water bird areas. This study should provide baseline data for research on plankton ecology and the scientific basis for the ecological protection of Shengjin Lake.
Materials and methods
Sampling methods and water analysis
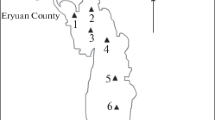
Samples were taken monthly at 13 sites in the upper region of Shengjin Lake, from February to July 2014 (Fig. 2). Shengjin Lake is a natural fishery, with more than a dozen fishing households between sites 3 and 6 all year round, who also raise poultry nearby. There is a fishery company located near site 1, which is in the main channel. Following examination of the shape of the upper lake and the distribution characteristics of the water birds, sampling points 1–3, 4–6, 7–9, 10–11, and 12–13 were selected and classed as sections A, B, C, D, and E, respectively. Water birds were concentrated in section E, and artificial disturbance was strongest in sections A and B.
Water samples were brought into the laboratory for analysis within 24 h. Water temperature ((T), HQ30d, HACH, Loveland), water depth ((WD), DY, Dayushuiwen, Weifang), pH (EL2, Mettler-Toledo, Shanghai), dissolved oxygen ((DO), HQ30d, HACH, Loveland), conductivity ((Cond), EL2, Mettler-Toledo, Shanghai), and turbidity ((Turb), 2100Q, HACH, Loveland) were measured with professional portable instruments. Transparency was measured with a Secchi disk (SD) in situ. Other parameters, namely total phosphorus (TP), NH4 +-N, NO3 −-N, NO2 −-N, and BOD5, chemical oxygen demand (COD), and suspended solids (SS) were analyzed according to standard methods (SEPB, 2002). Water samples for phytoplankton quantification and identification were stored in 1-L plastic bottles and then fixed with 1 % Lugol iodine solution. Crustacean (Crus) zooplankton biomass data were provided by Chao Wang.
Phytoplankton quantification and identification
Water samples were fixed by neutral Lugol solution and allowed to settle for 48 h. The supernatant was then siphoned off with a small-diameter silicone tube to a final volume of 30 mL, which was used for phytoplankton species identification and quantification (Eker et al. 1999). After mixing, 0.1 mL of sample was counted in a counting chamber under a light microscope (CX21, Olympus, Tokyo, Japan) at ×400 magnification. Phytoplankton species were identified based on morphology (Hu and Wei 2006). The phytoplankton biomass was calculated according to the phytoplankton cell volume, which was determined by average cell dimensions for each species (Sun and Liu 1999).
Data analysis
The dominant species of phytoplankton were determined based on the dominance value of each species, as shown in Eq. (1), and the species diversity was investigated according to the Shannon-Wiener index (Shannon and Wiener 1949), as shown in Eq. (2);
where n i is the number of individuals of species i, N is the total number of individuals of all species, n i /N represents the relative proportion of species i, f i is the occurrence frequency of species I, and s is total species. If the dominance value Y of a species was greater than 0.02, this species is considered dominant throughout the sampling periods (Xu et al. 1995; Sun et al. 2006).
Redundancy analysis (RDA) was used to evaluate the effects of environmental variables on the phytoplankton community. The measured environmental factors, namely T, pH, SD, DO, SS, BOD5, COD, TP, NH4 +-N, NO3 −-N, NO2 −-N, Cond, and Crus were used as explanatory variables. Before analysis, all parameters, except pH, were log10(x + 1)-transformed, to obtain comparable distributions. In the data matrix of species biomass, only the 23 dominant species or genera were incorporated into the analysis. Log10(x + 1) transformation was carried out on the biomass data of each dominant specie or genus before analysis. Thus, two series of matrices were produced from the measured results, one for dominant species or genera biomass and the other for environmental factors. Detrended correspondence analysis (DCA) was performed to assess the length of the dominant gradient. Redundancy analysis was appropriate for the analysis if the length of ordination axes in DCA was relatively low (< 3); otherwise, canonical correspondence analysis would be a more suitable method (Lepš and Petrš 2003). The RDA results were visualized in the ordination plots using the software Canoco for Windows 4.5.
Pearson correlation analysis was performed by the SPSS package (Version 19.0, IBM http://www.ibm.com). The interpolation map was constructed by ArcGIS software (Version 9.3, ESRI http://www.esri.com) using the inverse distance weighting method (Jiang et al. 2014).
Results
Environmental factors
Table 1 shows the means and standard deviations of environmental factors at the investigated sites in 2014. The maximum water temperature (approximately 34.0 °C) was recorded in July, with the minimum (10.6 °C) recorded in February. The mean fluctuation in water depth was low before June, but increased to a maximum of 5.96 m in July. Conductivity was relatively stable, with the highest mean value of 138.05 μs/cm in June. Average suspended solid concentration varied from 2.81 mg/L in July to 40.81 mg/L in February. Mean turbidity ranged from 4.90 NTU to 39.70 NTU. The pH fluctuated between 7.84 and 9.41. The mean DO concentration for the 6 months was 10.71 mg/L, with the highest average value (12.49 mg/L) recorded in February and the lowest (8.58 mg/L) in June. The mean transparency ranged from 0.33 m in February to 1.72 m in July. The average BOD5 content fluctuated from 1.508 to 3.545 mg/L. The mean TP, NH4 +-N, NO3 −-N, and NO2 −-N concentrations were 0.036, 0.470, 0.808, and 0.011 mg/L, respectively. The highest mean NH4 +-N concentrations (0.952 mg/L) were recorded in March and June (0.659 mg/L). The average concentrations of TP and NH4 +-N clearly decreased between June and July. The average COD content reached a maximum in March (18.231 mg/L), with a minimum value (13.590 mg/L) recorded in April. The total biomass of crustacean zooplankton reached its highest mean value in June (3.396 mg/L) and its lowest (0.112 mg/L) in March.
Temporal and spatial variations in the main water quality indicators of the five sections of the upper lake are displayed in Fig. 3. During the study period, nutrient concentrations in sections A, B, and E were higher than in other sections, perhaps because of inputs of human sewage discharge and droppings from poultry and wintering water birds.
Phytoplankton community composition
We identified 192 phytoplankton species from 8 phyla and 84 genera, of which 46.4 % were Chlorophyta, 29.2 % Bacillariophyta, and 12.5 % Cyanophyta. Phytoplankton species composition and the 14 predominant species or genera in the upper region of Shengjin Lake are shown in Table 2. In terms of abundance and biomass, Bacillariophyta, Chlorophyta, and Cyanophyta were more important than any other taxonomic groups. In the spring, the dominant species were mostly of the phylum Bacillariophyta and Cryptophyta, while in the summer, they were from Cyanophyta and Chlorophyta.
Phytoplankton variations in abundance, biomass, and diversity index
There were distinct temporal changes in phytoplankton abundance in the upper lake. The average abundance was 278.84 × 105cells/L, ranging from 3.66 × 105 to 867.93 × 105cells /L (Fig. 4). The minimum abundance was recorded in February and was made up of 43.98 % Bacillariophyta species and 31.24 % Chlorophyta species. The maximum abundance was observed in June, with 82.50 % Cyanophyta species and 9.58 % Chlorophyta species. From February to July, the average contributions of Cyanophyta, Bacillariophyta, Chlorophyta, and Cryptophyta species to phytoplankton abundance were 39.40, 27.68, 17.66, and 11.72 %, respectively. The relative proportions of Bacillariophyta species within the total phytoplankton abundance decreased during the sampling period, whereas Cyanophyta proportions increased rapidly, taking over from Bacillariophyta as the dominant phylum. The gradient in abundance increases rose from February to June and fell from June to July.
There were marked temporal and spatial variations in total phytoplankton biomass (Figs. 5 and 6). Highest average biomass (up to 20.89 mg/L) was observed in July, with the lowest (only 0.40 mg/L) in February. The average biomass for the upper lake was 10.66 mg/L. Biomasses of the predominant species/genera are shown in Table 3. From February to July, the average contributions of Bacillariophyta, Chlorophyta, Cryptophyta, and Pyrrophyta to phytoplankton biomass were 40.94, 23.42, 15.86, and 11.25 %, respectively. The relative proportions of Bacillariophyta to total biomass decreased during the sampling period, while Chlorophyta proportions increased. The dominant species gradually changed from Bacillariophyta to Chlorophyta during the study period. Phytoplankton biomass showed an increasing trend from February to July, with its spatial distribution varying substantially during the sampling period (Fig. 7). In February, the highest value areas of phytoplankton biomass were sections A and B, changing to section E in March. In April, the distribution of phytoplankton biomass over different sites was relatively homogeneous. From May to June, the highest value areas were mainly in section E, moving to section A in July.
The diversity index of phytoplankton in the upper lake varied from 3.50 to 8.35, with an average of 5.58 (Fig. 7). The highest diversity index was recorded at sampling site 13 in May, and the lowest at site 3 in February. The lowest mean diversity indices mainly appeared in February, and the highest in May. The average diversity indices for sections A, B, C, D, and E were 5.52, 5.53, 5.57, 5.67, and 5.68, respectively.
Correlations between phytoplankton community and environmental factors
Pearson correlation analysis was applied to evaluate interactions between phytoplankton abundance and biomass variations and environmental variables. In all periods investigated, total phytoplankton abundance showed a significant positive correlation with T, pH, Crus, SD, and COD (P < 0.01), but a negative relationship with DO, Turb, SS, and NO2 −-N (P < 0.01). Total phytoplankton biomass had a positive relationship with T, pH, SD, and Crus (P < 0.01), but a negative relationship with SS, DO, Cond, and COD (P < 0.05). Pearson correlations showed that T, pH, SD, COD, DO, SS, NO2 −-N, Turb, Cond, and Crus were the environmental factors having the greatest influence on phytoplankton in the upper lake.
RDA was also used to assess relationships between phytoplankton and environmental factors. The RDA ordination triplot of the upper lake, which includes 23 dominant species and 13 environmental variables, is shown in Fig. 8. The eigenvalues of SPE AX1 and SPE AX2 were 0.524 and 0.186, respectively. Together, these axes explained 71.0 % of the total variance in species data (Table 4). The correlation between dominant species and environment was strong, 0.912 and 0.875 for the first and second axes, respectively. The positions of each species with respect to the first two environmental axes are shown in Fig. 8. Variables such as SD (r = +0.8723), T (r = 0.5791), NO2 −-N (r = 0.5604), Cond (r = −0.5583), BOD5 (r = 0.5160), and SS (r = −0.5004) were significantly related to axis 1, whereas NO3 −-N (r = 0.4840), and conductivity (r = −0.3224) were strongly associated with axis 2 (Fig. 8). The RDA ordination clearly showed that the dominant phytoplankton species changed significantly over time, and these changes were statistically related to many environmental variables, including SD, T, NO2 −-N, Cond, BOD5, SS, and NO3 −-N.
Phytoplankton species and environmental factor biplot based on RDA. 1. Navicula bacillum, 2. Synedra acus, 3. Melosira granulata var. angustissima, 4. Melosira granulata var. angustissima f. spiralis, 5. Cyclotella sp., 6. Melosira italica, 7. Dinobryon sp., 8. Anabaena azotica, 9. Raphidiopsis sp., 10. Merismopedia marssonii, 11. Anabaena inaequalis, 12. Planktolyngbya subtilis, 13. Merismopedia minima, 14. Phormidium angustissimum, 15. Scenedesmus platydiscus, 16. Chlorella sp., 17. Chlamydomonas sp., 18. Scenedesmus quadricauda, 19. Ankistrodesmus angustus, 20. Mougeotia sp., 21. Chroomonas acuta, 22. Cryptomonas ovata, and 23. Cryptomonas erosa. Species data are represented by the triangle symbol. Environmental variables are represented by hidden lines with arrows. Variables with lines close to each other and headed in the same direction are positively correlated, while those headed in opposite directions are negatively correlated. Two lines at a 90° angle indicate that the corresponding variables are uncorrelated
During the study, the predominant species clustered with the variables SD, NO3 −-N, and TP. Predominant species, including Anabaena azotica, Raphidiopsis sp., Planktolyngbya subtilis, Mougeotia sp., and Phormidium angustissimum, showed a significantly positive correlation with SD (Fig. 8). Other dominant species, Navicula bacillum, Melosira granulata var. angustissima, and Melosira italica presented a notably positive relationship with TP. Prepotent species such as M. granulata var. angustissima f. Spiralis, Chroomonas acuta, and Chlorella sp. were positively correlated with NO3 −-N. Synedra acus showed a positive relationship with T. Merismopedia minima correlated with Cond. Finally, Scenedesmus platydiscus was significantly positively correlated with Crus. In conclusion, phytoplankton community structure was closely linked to changes in SD, T, Cond, NO3 −-N, and TP throughout the sampling period in the upper lake, with SD and T identified as the most important environmental factors.
Discussion
Temporal and spatial variations in phytoplankton community
Phytoplankton community structure and composition in the upper lake changed temporally over the course of the study. The dominant phytoplankton species changed from Bacillariophyta and Cryptophyta to Cyanophyta and Chlorophyta in this research. The dominant species and its temporal variations agree with earlier research by Shen (2008). They reported that the Bacillariophyta community developed in the winter and spring, and made a significant contribution to phytoplankton community biomass (Rolland et al. 2009; Xiao et al. 2013). Sommer (1985) indicated that the Cryptophyta were a fast-growing and opportunistic species. Cyanophyta and Chlorophyta were found to be dominant in the summer (Zhang et al. 2010; Xiao et al. 2013), which we also observed in our study (Fig. 4).
A high diversity index (H') indicates a healthy ecosystem, while a low value denotes a less healthy or degraded ecosystem (Wilhm 1970). In this study, the average phytoplankton diversity indices for all five sections were high, implying that the upper lake had a relatively healthy ecosystem. Karydis and Tsirtsis (1996) indicated that diversity indices of phytoplankton could be used as indicators of water quality. The mean diversity index was lowest in February, whereas water quality was not obviously worse during this month (Table 1). Meanwhile, section E in March had the highest concentration of TP, BOD5, and COD (Fig. 3) and showed the highest mean diversity index. Thus, it can be concluded that the high diversity in the upper region of Shengjin Lake did not necessarily indicate good water quality for this lake.
Correlations between phytoplankton community and environmental factors
Water temperature is thought to be a vitally important environmental factor affecting phytoplankton community structure (Bierman and Dolan 1981; Masaki and Seki 1984). In Tsuchiura Harbor Lake, temperature was the limiting factor controlling multiplication rate and standing stock of phytoplankton during the spring bloom of 1980 (Tsuchida et al. 1984). Tian et al. (2013a) found that temperature was the key factor driving changes in phytoplankton community composition in Nansi Lake. Similarly, Lv et al. (2011) reported that water temperature and TP were the principal limiting factors for phytoplankton growth on an annual basis. In our research, phytoplankton abundance and biomass were significantly positively correlated with water temperature. Rising water temperature favored the growth of phytoplankton, which led to increases in phytoplankton abundance and biomass. Increased abundance was due mainly to the increase in Cyanophyta and Chlorophyta species, which preferred high temperatures. And the rise in phytoplankton biomass was the result of increased abundance (Li et al. 2007). In our study, the changing trend of phytoplankton abundance differed from biomass variations from June to July (Figs. 4 and 5). Phytoplankton abundance declined, but its biomass continued to rise. The decrease in phytoplankton abundance might be due to the following: (1) a drastic change in water depth and (2) an increase in food consumption by phytophagous fish (Fang et al. 2014; Li et al. 2014). Shengjin Lake is an important natural fishery, yielding approximately 2000 t per year, with Hypophthalmichthys molitrix and Aristichthys nobilis identified as the dominant species in recent years. The increase in phytoplankton biomass may also be the result of variations in size among different varieties of phytoplankton (Hu et al. 2008). The water dilution and the increase of food consumption led to a substantial reduction in total phytoplankton abundance in July, but the emergence of many Pyrrophyta, with their larger size causing an increase in the total biomass.
Transparency (SD) is also an important environmental factor affecting phytoplankton communities (Peng et al. 2013; Tian et al. 2013b). It was reported that SD reduced exponentially in accordance with phytoplankton biomass (Tu et al. 1990). From our RDA and Pearson correlation analysis, total abundance and biomass were found to be strongly influenced by SD. The SD was mostly driven by SS in the water. Phytoplankton blooms and their decomposed particles could induce increases in the concentration of suspended solids, and then reduce the transparency of freshwater (Cai and Gao 1997). Thus, our study also explained why phytoplankton abundance and biomass had a significant negative correlation with SS.
Generally, physical-chemical parameters, including WD, Cond, pH, DO, COD, TP, NO3 −-N, and NO2-N, are important environmental factors influencing the growth of phytoplankton. Li et al. (2012) revealed that WD, T, TP, SD, oxidation reduction potential, and Cond were the environmental factors affecting phytoplankton community structure in Datong Lake. Abuzer et al. (2014) indicated that pH, T, DO, and redox potential played a significant role in phytoplankton species distribution. In Lake Baiyangdian, positive correlations were reported between total phytoplankton abundance and COD but negative relationships between phytoplankton abundance and DO/SD (Liu et al. 2010). In contrast, phosphorus was the limiting nutrient for phytoplankton growth in Lake Washington (Arhonditsis et al. 2003). Finally, Zina et al. (2010) found that NO3 −-N and NO2 −-N had positive relationships with phytoplankton concentration. Similarly, in our study, the phytoplankton community structure was significantly correlated to pH, SD, DO, NO2 −-N, Cond, COD, and Turb in the upper lake.
Crustacean zooplankton community structure and abundance are also closely linked with phytoplankton community structure and quantity. They can influence phytoplankton populations and quantity, changing the phytoplankton community structure through grazing (Brett et al. 1994; Kagami et al. 2002; Lau and Lane 2006). Deng et al. (2007) suggested that the presence of small crustacean zooplankton favored the development of inedible filamentous or colony-forming cyanobacteria. In our investigation, crustacean zooplankton biomass showed a notably positive relationship with total phytoplankton abundance and biomass. From the RDA results, a significant positive correlation between crustacean zooplankton biomass and S. platydiscus biomass was observed in the upper lake.
Impacts of wintering water birds on phytoplankton community
It was reported that high nutrient concentrations result in increases in phytoplankton biomass and influence the distribution of the phytoplankton community (Sakamoto 1966; Watson et al., 1997; Li et al. 2013). Animal waste contains multifarious nutrient components, including phosphorus and ammonia nitrogen. Phytoplankton species and other aquatic organisms can easily use these nutrients, and thus, their concentrations significantly influence the density and biomass of phytoplankton. Nearly 56,000 migratory water birds winter at the central and southern areas of the upper lake every year, congregating primarily in sections D and E, and producing a huge number of droppings. In March, the water level rose and flooded some muddy places, and thus, the nutrients from water bird droppings were mixed with water under hydrodynamic forcing, making phytoplankton biomass higher in section E than in other sections. From May onwards, water began to slowly cover the grassy areas, which were completely covered by June. As in March, water bird droppings produced the higher phytoplankton biomass in section E in June. The area with the highest biomass changed to section A in July, for two potential reasons. Nutrients from droppings in section E were completely consumed, and the mean WD rose quickly from June to July to around 4 m, which influenced the vertical profile of water temperature, and the stratification and availability of nutrients (Feng et al. 2014). There are a dozen fishing households near sections A and B, with waste and poultry discharging from these households causing the highest phytoplankton biomass in other months. Consequently, the water bird droppings favored phytoplankton growth, and led to an area of high phytoplankton biomass. However, the high value area shifted away, following the consumption of nutrients from the droppings and significant changes in other environmental factors.
Conclusions
In this study, the upper lake of Shengjin Lake was found to have a high diversity of phytoplankton species, with 192 species from 8 phyla and 84 genera identified during the study period. There were 14 predominant species, with substantial temporal changes in the dominant species from Bacillariophyta and Cryptophyta to Cyanophyta and Chlorophyta. The average abundance and biomass of phytoplankton in the upper lake were 278.84 × 105 cells/L and 10.66 mg/L. Phytoplankton biomass and abundance showed a similar increasing trend from February to July, but changes in phytoplankton abundance differed from biomass variations from June to July. The Shannon Wiener diversity index varied from 3.50 to 8.35, with an average of 5.58, revealing a highly diverse phytoplankton community. The high diversity showed in the upper lake does not necessary indicate good water quality. Statistical analysis revealed that water temperature (T), transparency (SD), and nutrient concentration were the most significant environmental factors influencing phytoplankton community, with other environmental factors such as pH, DO, SS, NO2 −-N, COD, turbidity, conductivity, and crustacean zooplankton, which were also important. The correlation between the key water bird areas and phytoplankton biomass indicated that the droppings from the wintering water birds had an important influence on the phytoplankton community in the upper lake. The water bird droppings favored phytoplankton growth, forming an area of high phytoplankton biomass. However, the high value area shifted away following the consumption of nutrients from the droppings and significant changes in other environmental factors.
References
Abuzer C, Baki Ö, Mehme K (2014) Relationship between phytoplankton composition and environmental variables in artificial pond. Algal Res 5:37–41
Arhonditsis G, Brett MT, Frodge J (2003) Environmental control and limnological impacts of a large recurrent spring bloom in Lake Washington, USA. Environ Manag 31:603–618
Bierman V Jr, Dolan DM (1981) Modeling of phytoplankton-nutrient dynamics in Saginaw Bay, Lake Huron. J Great Lakes Res 7:409–439
Brett MT, Wiackowski K, Lubnow FS, Mueller-Solger A, Eller JJ, Goldman CR (1994) Species-dependent effects of zooplankton on planktonic ecosystem processes in castle lake, California. Ecology 75(8):2243–2254
Cai Q-M, Gao X-Y (1997) Dynamic variations of water quality in Taihu Lake and multivariate analysis of its influential factors. Chin Geogr Sci 7:72–82
Chang T, Xue T-L, Hai Y-P, Wen R-H, Jun X (2013) Seasonal dynamics of phytoplankton and its relationship with the environmental factors in Dongping Lake, China. Environ Monit Assess 185:2627–2645
Chen J-Y, Zhou L-Z (2011) Guild structure of wintering waterbird assemblages in shallow lakes along Yangtze River in Anhui Province, China. Acta Ecol Sin 31(18):5323–5331 (in Chinese)
Chen J-Y, Zhou L-Z, Zhou B, Xu R-X, Zhu W-Z, Xu W-B (2011) Seasonal dynamics of wintering waterbirds in two shallow lakes along Yangtze River in Anhui Province. Zool Res 32(5):540–548 (in Chinese)
Cheng Y-Q, Cao L, Mark B, Xu W-B et al (2009) Wintering waterbirds survey at the Anhui Shengjin lake national nature reserve, China 2008/2009. University of Science and Technology of China, Hefei
Deng D-G, Xie P, Zhou Q, Yang H, Guo L-G (2007) Studies on temporal and spatial variations of phytoplankton in Lake Chaohu. J Integr Plant Biol 49(4):409–418
Eker E, Georgieva L, Senichkina L, Kideys AE (1999) Phytoplankton distribution in the western and eastern Black Sea in spring and autumn 1995. Mar Sci (Suppl) 56:15–22
Fang L-J, Liu D-F, Zhang J-L, Yang Z-J, Tian Z-B (2014) Phytoplankton community structure of Xiangxi Bay before and after the 175m impoundment of three gorges reservoir. J Hydroecol 35(3):1–9 (in Chinese)
Feng Y, Xiao L-J, Wei G-F, Han B-P (2014) Responses of phytoplankton community to increasing water level in the lacustrine zone near the dam of a large tropical reservoir: Gaozhou Reservoir, South China. Chin J Appl Environ Biol 20(3):529–536 (in Chinese)
Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennisonet WC et al (2008) Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8:3–13
Hu H-J, Wei Y-X (2006) The freshwater algae of China: systematic, taxonomy and ecology. Science Press, Beijing, in Chinese
Hu W-H, Yu G-Q, Liu Z-Y (2004) The ecology environment and its protection of Shengjin Lake wetland. Territor Nat Resour Stud 4:70–71 (in Chinese)
Hu R, Lei L-M, Han B-P (2008) Phytoplankton assemblage and seasonal dynamics in the large oligotrophic Xinfengjiang reservoir in southern China. Acta Ecol Sin 28(10):4652–4664 (in Chinese)
Jiang H-X, Xu W-B, Qian F-W, Chu G-Z (2007) Impact of habitat evolvement and human disturbance on wintering water birds in Shengjin lake. Chin J Appl Ecol 18(8):1832–1836 (in Chinese)
Jiang Y-J, He W, Liu W-X, Qin N, Ouyang H-L, Wang Q-M, Kong X-Z, He Q-S, Yang C, Yang B, Xu F-L (2014) The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecol Indic 40:58–67
Kagami M, Yoshida T, Gurung TB, Urabe J (2002) Direct and indirect effects of zooplankton on algal composition in situ grazing experiments. Oecologia 133:356–363
Karydis M, Tsirtsis G (1996) Ecological indices: a biometric approach for assessing eutrophication levels in the marine environment. Sci Total Environ 186:209–219
Lau SSS, Lane SN (2006) Nutrient and grazing factors in relation to phytoplankton level in a eutrophic shallow lake: the effect of low macrophyte. Water Res 36:3593–3601
Lepš J, Petrš M (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Li Q-H, Hu R, Han B-P (2007) Spring dynamics of the phytoplankton community of oligotrophic reservoir in the southern subtropics of China. J Plant Ecol 31(2):313–319 (in Chinese)
Li Q-H, He W-T, Chen H (2009) Characteristics of the phytoplankton community in wetlands of Macao. J Plant Ecol 33(4):689–697 (in Chinese)
Li D-L, Zhang T, Xiao T-Y, Yu J-B, Wang H-Q, Chen K-J, Liu A-M, Z-J LI (2012) Phytoplankton community structure and its relationships with environmental factors in an aquaculture lake, Datong Lake of China. Chin J Appl Ecol 23(8):2107–2113 (in Chinese)
Li S-Y, Han Z-Y, Xu Z-C, Zhao X-M, Yao L-A, Wei D-Y, Zhang J, Hu F (2013) Study on the structure of phytoplankton community and its relationships with environmental factors in Gaozhou Reservoir. J Hydroecol 34(5):16–24 (in Chinese)
Li R-R, Zhang G-X, Zhang L (2014) Multivariate analysis of the relations between phytoplankton assemblages and environmental factors in Chagan Lake Wetland. Acta Ecol Sin 34(10):2663–2673 (in Chinese)
Liu C-Q, Liu L-S, Shen H-T (2010) Seasonal variations of phytoplankton community structure in relation to physico-chemical factors in Lake Baiyangdian, China. Procedia Environ Sci 2:1622–1631
Liu J-L, Hu X-B, Tian Z-F, Ning G-H, Xie J-Z (2011) Effect of sodium butyrate on growth performance of Broiler Duck and reduction of emission of pollutants with its faeces. J Ecol Rural Environ 27(1):39–43 (in Chinese)
Lu N, Yin H-B, Deng J-C, Gao F, Hu W-P, Gao J-F (2010) Spring community structure of phytoplankton from Lake Chaohu and its relationship to environmental factors. J Lake Sci 22(6):950–956, in Chinese
Lv J, Wu H-J, Chen M-Q (2011) Effects of nitrogen and phosphorus on phytoplankton composition and biomass in 15 subtropical, urban shallow lakes in Wuhan, China. Limnologica 41:48–56
Masaki A, Seki H (1984) Spring bloom in a hypereutrophic lake, Lake Kasumigaura, Japan—IV: inductive factors for phytoplankton bloom. Water Res 18:869–876
Michelutti N, Blais JM, Cumming BF, Paterson AM, Riihland K, Wolfe AP, Smol JP (2010) Do spectrally inferred determinations of chlorophyll a reflect trends in lake trophic status? J Paleolimnol 43:205–217
Nalewajko C, Murphy TP (2001) Effects of temperature and availability of nitrogen and phosphorus on the abundance of Anabaena and Microcystis in Lake Biwa, Japan: an experimental approach. Limnology 2(1):45–48
Peng C-G, Zhang L, Zheng Y-Z, Li D-H (2013) Seasonal succession of phytoplankton in response to the variation of environmental factors in the Gaolan River, Three Gorges Reservoir, China. Chin J Oceanol Limnol 31(4):737–749
Ptacnik R, Solimini AG et al (2008) Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc Natl Acad Sci 105:5134–5138
Rolland A, Bertrand F, Maumy M, Jacquet S (2009) Assessing phytoplankton structure and spatio-temporal dynamics in a freshwater ecosystem using a powerful multiway statistical analysis. Water Res 43:3155–3168
Sakamoto M (1966) Primary production by phytoplankton community in some Japanese lakes and its dependence on lake depth. Arch Hydrobiol 62:1–28
Shannon CE, Wiener W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Shen J (2008) Anhui Shengjin Lake phytoplankton diversity and ecological factors. Dissertation, Anhui university (in Chinese)
Shen J, Xu R-X, Zhou Z-Z, Chen Y-Q, Sun Q-Y, Xu L-L, Zhao X-X, Zong M (2009a) Study on winter phytoplankton diversity of Shengjin lake, Anhui. J Biol 26(3):16–18 (in Chinese)
Shen J, Zhou Z-Z, Chen Y-Q, Xu L-L, Zhao X-X, Zong M (2009b) Autumn phytoplankton diversity and water quality evaluation in Shengjin Lake, Anhui. J Hydroecol 2(3):17–21 (in Chinese)
Smith VH (1982) The nitrogen and phosphorus dependence of algal biomass in lakes: an empirical and theoretical analysis. Limnol Oceanogr 27:1101–1112
Sommer U (1985) Differential migration of cryptophyceae in lake constance. Mar Sci 17:166–175
State Environmental Protection Bureau (SEPB) (2002) Methods of monitoring and analysis for water and wastewater (4th Edn.). China Environmental Science Press, Beijing, in Chinese
Sun J, Liu D-Y (1999) Study on phytoplankton biomass: I. Phytoplankton measurement biomass from cell volume or plasma volume. Acta Oceanol Sin 21(2):75–85 (in Chinese)
Sun C-C, Wang Y-S, Sun S, Zhang F-Q (2006) Analysis dynamics of phytoplankton community characteristics in Daya Bay. Acta Ecol Sin 26(12):3948–3958 (in Chinese)
Tian C, Pei H-Y, Hu W-R, Xie J (2013a) Phytoplankton variation and its relationship with the environmental factors in Nansi Lake, China. Environ Monit Assess 185:295–310
Tian C, Lu X-T, Pei H-Y, Hu W-R, Xie J (2013b) Seasonal dynamics of phytoplankton and its relationship with the environmental factors in Dongping Lake, China. Environ Monit Assess 185:2627–2645
Tsuchida A, Hara Y, Seki H (1984) Spring bloom in a hypereutrophic lake, Lake Kasumigaura, Japan-V: factors controlling natural population of phytoplankters. Water Res 18:877–883
Tu Q-Y, Gu D-X, Yin C-Q, Xu Z-R, Han J-Z (1990) The Chao Lake—study on eutrophication. China Science and Technology Press, Hefei (in Chinese)
Watson SB, McCauley E, Downing JA (1997) Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol Oceanogr 42(3):487–495
Wilhm JL (1970) Range of diversity index in benthic macroinvertebrate populations. J Water Pollut Control Fed 42:221–224
Xiao R, Su S-L, Anas G, Wu J-P (2013) Spatial analysis of phytoplankton patterns in relation to environmental factors across the southern Taihu basin, China. Stoch Environ Res Risk Assess 27:1347–1357
Xu Z-L, Wang Y-L, Chen Y-Q, Shen H-T (1995) An ecological study on zooplankton in maximum turbid zone of estuarine area of Changjiang (Yangtze) River. J Fish Sci China 2(1):39–48 (in Chinese)
Yang X-L (2011) Research on the numbers, distribution, feeding behavior and diet of Great White Fronted Geese at Shengjin lake, the national natural reserves in Anhui Province. Dissertation, University of Science and Technology of China (in Chinese)
Yin D-C, Zheng L-L, Song L-R (2011) Spatiotemporal distribution of phytoplankton in the Dan jiangkou Reservoir, a water source area for the South-to-North Water Diversion Project (Middle Route), China. Chin J Oceanol Limnol 29(3):531–540
Zhang Y, Feng L, Li J, Luo L, Yin Y, Liu M, Li Y (2010) Seasonal-spatial variation and remote sensing of phytoplankton absorption in Lake Taihu, a large eutrophic and shallow lake in China. J Plankton Res 32:1023–1037
Zhou B, Zhou L-Z, Chen J-Y, Xu W-B, Cheng Y-Q (2009) Assemblage dynamics and territorial behavior of hooded crane in winter on Shengjin Lake. ChinJ Wildl 30(3):133–136 (in Chinese)
Zhu W-Z, Zhou L-Z (2010) Biodiversity and conservation in AnQing floodplain wetlands. Hefei university of technology Press, Hefei (in Chinese)
Zina A, Elbahri T, Souad T, Béchir B, Naceur BM (2010) Seasonal phytoplankton responses to environmental factors in a shallow Mediterranean lagoon. J Mar Sci Technol 15:417–426
Zong M, Zhong Z-Z, Chen Y-Q, Sun Q-Y, Xu L-L, Shen J, Zhao X-X (2008) Primary studies on type combination and its ecologica1 factor of planktonic Rotifers in Shengjin lake. J Biol 25(5):17–19 (in Chinese)
Acknowledgments
We are grateful to Mr. John Olsen for his help with the English language. This research was supported by the Chinese Specialized Research Fund for Doctoral Programs in Higher Education (Grant No. 20123401110005), the National Natural Science Foundation of China (Grant Nos. 41072251, 31370470), and the National Science and Technology support project (Grant No. 2012BAC10B02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Braunbeck
Rights and permissions
About this article
Cite this article
Wang, L., Wang, C., Deng, D. et al. Temporal and spatial variations in phytoplankton: correlations with environmental factors in Shengjin Lake, China. Environ Sci Pollut Res 22, 14144–14156 (2015). https://doi.org/10.1007/s11356-015-4640-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4640-2