Abstract
Rotenone, a natural compound derived from plants of the genera Derris and Lonchocarpus, is used worldwide as a pesticide and piscicide. This study aims to assess short-term toxicity of rotenone to early-life stages of the fish Danio rerio and Poecilia reticulata using a wide and integrative range of biomarkers (developmental, biochemical, behavioral, and histopathological). Moreover, the species sensitivity distribution (SSD) approach was used to compare rotenone acute toxicity to fish species. Toxicity tests were based on the OECD protocols, fish embryo toxicity test (for D. rerio embryos), and fish acute toxicity test (for P. reticulata juveniles). D. rerio embryos were used to estimate lethal concentrations and analyze embryonic and enzymatic alterations (activity of catalase, glutathione-S-transferase, and cholinesterase), while P. reticulata juveniles were used for the assessment of histological damage in the gills and liver. Rotenone induced significant mortality in zebrafish embryos with a 96-h lethal concentration 50 % (LC50) = 12.2 μg/L. Rotenone was embryotoxic, affecting the development of D. rerio embryos, which showed cardiac edema; tail deformities; loss of equilibrium; and a general delay characterized by lack of tail detachment, delayed somite formation, yolk sac absorption, and lack of pigmentation. Biochemical biomarker inhibition was observed for concentrations ≥1 μg/L for CAT and glutathione-S-transferase (GST) and for cholinesterase (ChE) in concentration from 10 μg/L. Behavioral changes were observed for P. reticulata juveniles exposed to concentrations equal to or above 25 μg/L of rotenone; moreover, histological damage in the liver and gills of fish exposed to concentrations equal to or above 2.5 μg/L could be observed. A hazard concentration 5 % (HC5) of 3.2 μg/L was estimated considering the acute toxicity data for different fish species (n = 49). Lethal and sublethal effects of rotenone raise a concern about its effects on nontarget fish species, especially because rotenone and its metabolite rotenolone are frequently reported in the microgram range in natural environments for several days after field applications. Rotenone should be used with caution. Given the high toxicity and wide range of sublethal effects here reported, further studies in a chronic exposure scenario are recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rotenone is a natural toxin present in plants from the genera Derris and Lonchocarpus. For centuries, natives of the Amazon basin have used this compound (locally known as timbó) to induce narcosis in fish and facilitate fishing for human consumption; this practice is still used by many Amazon riverine groups. Rotenone is also commercialized worldwide as a pesticide. In aquaculture, rotenone is used to eliminate fish and other unwanted organisms from production systems (Nanda et al. 2009). In natural aquatic ecosystems, rotenone has been employed to control invasive species of fish (Chadderton et al. 2001).
Rotenone toxicity to aquatic biota has not been well studied, and possible adverse effects on nontarget organisms, mainly at sublethal level, are still unclear. In mammals, rotenone toxicity may be related to the inhibition of mitochondrial respiration and impairment of the formation of the achromatic spindle during mitosis (Guadaño et al. 1998; Radad et al. 2006). In the fish Oncorhynchus mykiss, Cheng and Farrell (2007) showed rotenone effects on metabolic rate, oxygen consumption, and transport and decrease in swimming performance. Betarbet et al. (2000) demonstrated that rotenone may be related to the formation of free radicals and oxidative damage which, in turn, can be related to the respiratory chain dysfunction caused after exposure to rotenone. Understanding the toxicity of rotenone to nontarget species is an important step to establish safer procedures for direct applications or to determine safe discharge concentrations in effluents. Additional studies are necessary to support regulators’ decisions on the uses of rotenone and the maximum environmental concentration that would minimize harm to aquatic biota. This study aims to assess the short-term effects of rotenone on fish embryos and juveniles. Intending to present an integrative analysis of the effects, toxicity was assessed at acute, developmental, biochemical, behavioral, and histological levels.
Results were used to estimate effective concentrations of rotenone that, whilst not necessarily resulting in death, might affect health and, consequently, affect the functioning of aquatic ecosystems.
Poecilia reticulata and Danio rerio were selected as test organisms because these fish species are recommended as model organisms for toxicity studies (OECD 1992, 2013) and also because they are easily maintained in laboratory. The P. reticulata model was used to evaluate behavioral and histological alterations in the liver and gills, whereas D. rerio were used to assess the lethal, biochemical, and developmental effects of rotenone. Effective concentration values obtained for D. rerio were compared with several others from different species (Table 1) by means of a species sensitivity distribution (SSD) analysis (Posthuma et al. 2001; Pereira et al. 2014). Using the SSD approach, the responses of different fish species will be displayed as cumulative distribution functions allowing the estimation of hazardous concentrations (hazard concentration 5 % (HC5) and hazard concentration 50 % (HC50)) of rotenone that might affect the survival of organisms in the aquatic environment.
Material and methods
Chemicals
Rotenone was purchased from Sigma-Aldrich (CAS Number: 83-79-4, empirical formula: C23H22O6, ≥95 % purity).
Test organisms
D. rerio (zebrafish) and P. reticulata were maintained in aquariums with reverse osmosis and activated carbon filtered water. The temperature was maintained at 26.0 ± 1 °C, conductivity at 650 ± 100 μS/cm, pH at 7.0 ± 0.5, and dissolved oxygen equal or above 95 % saturation, and the fish were raised in a 14:10-h (light/dark) photoperiod cycle. These conditions were maintained in all the performed tests (except when other conditions are indicated). Zebrafish eggs were collected immediately after natural mating, rinsed in water, and checked under a stereomicroscope (Stereoscopic Zoom Microscope SMZ 1500, Nikon Corporation). The unfertilized eggs and those with cleavage irregularities or injuries were discarded.
D. rerio assays
FET test
The assay was based on the fish embryo toxicity (FET) test (OECD 2013) and on the embryo test described by Jesus et al. (2013). Zebrafish embryos were exposed to six treatments of rotenone 0, 5, 10, 20, 40, and 80 μg/L in 24-well microplates. Thirty eggs were used per treatment. One egg was placed per microplate well filled-up with 2 mL of the test solution. The test was performed in triplicate. Test solutions were prepared by successive dilution of the stock solution in water. Embryos and larvae were observed daily under a stereomicroscope. The test was initiated immediately after fertilization and was continued for 4 days. Developmental parameters were evaluated in embryos over the test period using a magnification of ×70 for eggs and ×40 for hatched embryos. In the embryo phase, the following parameters were evaluated: egg coagulation, otolith formation, general delay in development, eye and body pigmentation, somite formation, heartbeat, edemas, detachment of the tail bud from the yolk sac, yolk sac absorption, and hatching. After hatching, spine malformation and posture were also evaluated. All parameters were assessed in a qualitative way (observed or not observed).
Biomarker analysis
A similar toxicity test with D. rerio embryos in Petri dishes was performed using sublethal concentrations of rotenone (0, 1, 5, 10, and 20 μg/L) in order to collect samples to analyze the enzymatic activity of glutathione-S-transferase (GST), catalase (CAT), and cholinesterase (ChE). After 96 h of rotenone exposure, pools of 15 hatched embryos were collected in microtubes with 2 mL of K-phosphate buffer (0.1 M, pH 7.4), frozen in liquid nitrogen, and immediately stored at −80 °C until the day of analysis. On the day of enzymatic analysis, samples were defrosted on ice, homogenized using a homogenizer (Ystral X10/20), and centrifuged for 20 min at 10,000g in order to isolate the post-mitochondrial supernatant (PMS) (Jesus et al. 2013). Enzymatic determinations were made spectrophotometrically (Labsystem Multiskan EX microplate reader) using 96-well microplates.
GST activity was determined at 340 nm by monitoring the increase in absorbance every 20 s, during 5 min, following the general protocol described by Habig and Jakoby (1981) with modifications introduced by Frasco and Guilhermino (2002). Activity determinations were done using 100 μL of PMS from the sample and 200 μL of reaction mixture (10 mM reduced glutathione (GSH) and 60 mM 1-chloro-2.4-dinitrobenzene in K-phosphate buffer (0.05 M, pH 6.5).
CAT activity was measured at 240 nm by monitoring (every 10 s, for 2 min) the decrease of absorbance due to degradation of H2O2, as described by Clairborne (1985). Fifteen microliters of PMS was mixed with 135 μL of reaction solution (H2O2, 30 mM) and 150 μL of K-phosphate buffer (0.05 M, pH 7.0).
ChE activity was determined using acetylthiocholine as substrate and measuring at 414 nm, every 20 s, for 5 min, the conjugation product between thiocoline (a product of the degradation of acetylthiocholine) and 5,5-dithiobis-2-nitrobenzoic acid (DTNB) (absorbance increase), according to the method of Ellman et al. (1961). Activity determinations were made using 40 μL of PMS, 250 μL of reaction mixture (acetylthiocholine (75 mM), and DTNB (10 mM)) in K-phosphate buffer (0.1 M, pH 7.2).
Enzymatic activities were determined in quadruplicate and expressed as micromoles of substrate hydrolyzed per minute per milligram of protein. Protein concentration in samples was determined in quadruplicate by Bradford’s method (Bradford 1976) at 595 nm, using γ-globulin as a standard.
P. reticulata assays
Toxicity test and behavioral analysis
The toxicity test followed the OECD Guideline for Testing of Chemicals fish acute toxicity test (OECD 1992). Juveniles of P. reticulata were exposed in glass aquariums to three nominal concentrations of rotenone 0, 2.5, 25, and 250 μg/L. Tested fish had an average weight of 92 ± 28 mg and an average length of 1.51 ± 0.22 cm. Ten juvenile fish per group were placed in each aquarium containing rotenone diluted in 1 L of water and were exposed for 72 h. The experiment was conducted in duplicate. Mortality was observed after 12, 24, 48, and 72 h. In addition, the presence or absence of behavioral changes (qualitative analysis) was evaluated, such as response to mechanical stimulus, mydriasis, tremors, paralysis, loss of equilibrium, and erratic swimming.
Histopathological analysis
Among the fish that survived acute rotenone exposure for 72 h, histopathological analyses were performed on five randomly selected individuals from each treatment. Whole fish were fixed with freshly prepared Davidson fixative solution (mixed at a ratio of 9:1 fixative/acetic acid) for 24 h. The fish were then embedded in paraffin, sectioned longitudinally in 7-μm sections, stained with hematoxylin and eosin, and observed by optical microscopy (×40 Zeiss Axioskop 2).
The histopathological changes were evaluated in gills and liver. The changes observed were classified into four different groups (CS1, CS2, CS3, CS4) as proposed by Bernet et al. (1999) (see Table 2), which takes into account the importance of the lesion (0–3, see below) and the degree and extent of histological changes (0–6, see below). Ultimately, this model allows the measurement of a numeric value to indicate the overall rate of histopathological changes to the gill (ICg) and liver (ICl).
The importance factor (w) is related to the reversibility and survivability of the effect and is classified as follows: (1) lesions that could be easily reversed (minimal damage), (2) lesions that can be reversed in most cases upon neutralization of the toxic agent (moderate damage), and (3) damage that is usually irreversible, leading to partial or complete loss of organ function (severe damage).
The score value measures the degree and extent of histological changes as no change (0–1), minimal abnormalities (2–3), moderate alterations (4–5), and (6) severe alterations (diffuse injury).
SSD analysis
The SSD was performed with LC50 values from the experimental data of the present study and values from the current literature (see Table 1). All concentration values refer to the active ingredient concentration of rotenone. Only acute toxicity data from tests of 24- to 240-h exposure duration were used. When more than one value was found for the same species, the LC50 from the longest study was chosen; if the studies had the same duration, the toxicity data were summarized as geometric means. A logistic curve (log) was fitted to the data using nonlinear regression. The predicted toxicities for the 5 and 50 % most sensitive organisms were estimated (HC5 and HC50, in other words, hazardous concentration for 5 % or 50 % of the population, respectively). The SSD plot was generated using the US Environmental Protection Agency spreadsheet built over Excel (USEPA 2014).
Statistical analysis
Sigma Stat 3.5 statistical package was used for statistical analyses (SPSS 2004). The effective concentrations (50 % effective concentration (EC50)) were calculated using a nonlinear allosteric decay function in a spreadsheet built over Microsoft Excel. A one-way ANOVA was used to detect the differences between the groups for normally distributed data sets. When data did not pass the Kolmogorov–Smirnov normality test and Levene’s homogeneity of variance test, a Kruskal–Wallis test was used. However, if significant results were found, Dunnett’s or Dunn’s test (for parametric or nonparametric tests, respectively) was used to detect significant differences between the tested concentrations and the control (p < 0.05).
Results
D. rerio embryos
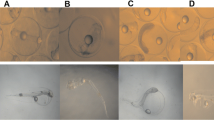
In the D. rerio assay, no significant mortality or developmental abnormalities (less than 10 %) were observed in the embryos of the control group (Figs. 1 and 2a). All the organisms exposed to concentrations higher than 40 μg/L were dead after 72 h of exposure. A 96-h LC50 of 12.2 μg/L was obtained for D. rerio embryos (Table 3).
Embryos started to hatch at 48 h (7.1 % of hatching in the control and 6.7 % in the concentration of 5 μg/L). At 96 h, 100 % of embryos had hatched in the control and in the 5 μg/L treatments, whereas a significant delay was observed in fish exposed to 20 μg/L with 13.3 % of hatching (Table 4).
Rotenone affected embryos’ behavior. After 4 days, the hatched embryos from the control group were swimming at the water surface in the microplate well and reacting to the light and mechanical stimulus. However, exposed embryos did not respond to mechanical stimulus; they showed abnormal posture, losing equilibrium and lying on the bottom of the microplate well, 96-h EC50 = 12.2 μg/L (see Table 3).
Even at very low doses, there were changes in development parameters and only otolith formation and heartbeat were not affected by rotenone exposure. Table 3 summarizes the EC50 of rotenone for each parameter analyzed during development. At 24 h, embryo development in the control and 5 μg/L treatments was normal: embryos presented a well-developed head, body, and tail (Fig. 2a). In the remaining concentrations, toxic effects were noticed, including a considerable delay in the detachment of the tail bud from the yolk sac, lack of pigmentation in the eye and body, general delay, and somite formation (Table 3). From 72 h, a significant occurrence of edemas was observed with EC50 = 19.9 μg/L. The effects on pigmentation persisted after 72 and 96 h, EC50 = 9.1 and 13.8 μg/L, respectively. Moreover, at 96 h, the exposed embryos also showed tail deformities and delayed yolk sac absorption (Fig. 2b, c). Due to these effects, exposed embryos were not able to be vertical in the water column and had trouble swimming and keeping their equilibrium.
The biomarker assays showed an inhibition of CAT for all tested concentrations (1 to 20 μg/L) (Fig. 3a). The GST was inhibited at 1, 5, and 20 μg/L (Fig. 3b). ChE was inhibited at the highest concentrations (10 and 20 μg/L, Fig. 3c).
Biomarker activities in D. rerio embryos (mean values ± standard error) after 96 h of exposure to rotenone: a catalase activity, b glutathione S-transferase activity, and c cholinesterase activity. Asterisk means significantly different from the respective control treatment (Dunnett’s or Dunn’s test, p < 0.05)
P. reticulata juveniles
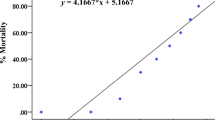
In the fish acute test with juveniles, no mortality was observed in the control group. However, a mortality of 16.7, 23.36, and 20.03 % was observed in the treatments 2.5, 25, and 250 μg/L of rotenone, respectively (Table 5). Table 5 shows that behavioral changes became more pronounced with increasing concentration of rotenone. Fish exposed to 2.5 μg/L of rotenone did not show any behavioral changes. However, fish exposed to 25 μg/L showed mydriasis. Furthermore, all the fish treated with 250 μg/L of rotenone showed no response to mechanical stimulus, mydriasis, tremors, paralysis, loss of equilibrium, and erratic swimming.
Histological damage due to rotenone was observed for all the test concentrations. Figure 4 depicts gill and liver samples from control fish and the structural changes observed in fish exposed to 250 μg/L of rotenone. The occurrence of gill and liver lesions was classified according to the type of change (Fig. 5). The ICg was significantly higher at all exposure concentrations compared to the control. Fewer liver lesions were observed, but a significant increase in ICl was noted in fish exposed to 250 μg/L of rotenone. Both organs showed more changes in the category CS2 (regressive changes in the epithelium and supporting tissues).
Gill and liver samples of P. reticulata. a Gill from control animal. Primary lamellar epithelium (square), secondary lamellar epithelium (circle), and pillar cell (arrowhead sign). b Gill exposed at 250 μg/L of rotenone. Gill hyperemia of the lamellar epithelium (arrowhead sign); aneurysm (circle); pillar cell disruption, necrosis, and sloughing of the epithelium (white arrows), change throughout architecture of lamellar epithelium; fusion of lamellar epithelium (square). c Liver from control animal. Liver tissue with normal architecture and evident sinusoids (asterisk). d Liver exposed at 250 μg/L of rotenone. Liver hepatocyte with hypertrophy/vacuolation (circle), foci of necrosis (white arrows), and vacuolar degeneration (asterisk). H & E, ×40 of magnification
SSD analysis
The SSD plot is shown in Fig. 6 with lethal values from different species. The maximum LC50 value found among all the studies with fish species was 710 μg/L (Plecostomus sp.), whereas the minimum value was 0.2 μg/L (Micropterus salmoides) (Table 1). The predicted toxicities for the 5 % and 50 % most sensitive species are, respectively, 3.2 μg/L (upper limit = 6.6 and lower limit = 1.6 μg/L) and 42.2 μg/L (upper limit = 85 and lower limit = 21 μg/L) of rotenone.
Discussion
The data from this study show that rotenone displays very steep dose-response curves for both lethal and sublethal adverse effects. The threshold between the concentrations that induce effects and the ineffective concentrations is very narrow. Rotenone caused significant mortality and developmental and behavioral alterations in zebrafish embryos for concentrations as low as 20 μg/L. Effects on the biochemical biomarkers were observed for concentrations above 1 μg/L. In addition, behavioral changes were observed for P. reticulata juveniles exposed to concentrations equal to or above 25 μg/L of rotenone; moreover, histological damage in the liver was observed in fish exposed to concentrations equal to or above 2.5 μg/L.
Analyses of developmental parameters in fish have been gaining space in the assessment of the effects of synthetic and natural products (Crawford et al. 2011; Hung et al. 2012). The 48-h LC50 value obtained for D. rerio embryos is in line with the results of a previous study reporting a 48-h LC50 = 27 μg/L (Hanisch et al. 2010). No data on developmental alterations caused by rotenone on fish were found in the literature. Reproductive and developmental studies of rotenone are available only for mammals and birds (Haag 1931; Rao and Chauhan 1971). Short-term exposure to rotenone can provoke abnormal body pigmentation, abnormal posture, spine deformities, and cardiac edema (Table 3). Those morphological changes might be related to the mutagenic and cytotoxic action of rotenone. Melo et al. (2014) reported induction of micronuclei (MN) frequency in Oreochromis niloticus exposed to rotenone. Additionally, by means of the fluorescence in situ hybridization technique, it was possible to understand that MN formation occurred due to an aneugenic effect of rotenone probably caused by mitotic spindle disturbances. The cytotoxic activity of rotenone is attributed to binding directly to tubulin and, thus, the inhibition of microtubule assembly arresting cells in mitosis (Srivastava and Panda 2007).
Hatching delay was observed in all concentrations from ≥20 μg/L. Despite the lack of information on the effects of rotenone in fish embryos, development studies with other vertebrate models have highlighted the embryotoxic effects of rotenone. For instance, Haag (1931) conducted a single-generation reproduction study in guinea pigs. At a dietary concentration of 150 mg/kg, all young were either born dead or died within 5 days of birth. In a chick embryo-screening assay, Rao and Chauhan (1971) noted a complete arrest of embryo development at 1 μg/L but no effect at 0.1 μg/L.
Rotenone exposure caused a general inhibition of biochemical markers of enzymatic activity. The oxidative stress enzymes CAT and GST were inhibited at concentrations ≥1 μg/L (Fig. 3a, b). In spite of the lack of studies on rotenone effects on oxidative stress parameters of fish, the inhibition of CAT and GST in zebrafish embryos is consistent with rotenone effects on in vitro models. In a study by Siddiqui et al. (2013), rotenone depicted a dose-dependent cytotoxic response in HepG2 cells and simultaneously caused a decrease in the activity of oxidative stress biomarkers, such as glutathione, CAT, and superoxide dismutase. The alterations in normal oxidative status in zebrafish embryos induce cellular damage and might lead to the death or morphological aberrations. The activity of ChE, a neurological biomarker, was inhibited in zebrafish embryos exposed to concentrations ≥10 μg/L of rotenone. ChE activity inhibition is consistent with previous studies, suggesting clinical signs of neurotoxicity, including tremors, prostration, and breathing difficulties, observed following acute oral exposure to rotenone (Turner et al. 2007). Rotenone crosses the blood-brain barrier, causing neurotoxic effects by uncoupling the mitochondrial electron transport chain, releasing reactive oxygen species, which contributes to apoptosis (Swarnkar et al. 2013). The neurotoxic action of rotenone was also demonstrated by several studies relating rotenone exposure to the pathology of Parkinson’s disease, a chronic degenerative disease that affects the central nervous system (Betarbet et al. 2000; Giasson and Lee 2000).
Behavioral changes were observed in both P. reticulata juveniles and zebrafish embryos exposed to rotenone. Loss of equilibrium was observed in P. reticulata juveniles exposed to rotenone concentrations ≥250 μg/L, whereas for the same effect, an EC50 = 12.2 μg/L was observed for zebrafish embryos. This suggests a higher sensitivity of embryos to rotenone and/or an interspecies variability in the sensitivity to rotenone. Our results are in agreement with previous results by Chadderton et al. (2001) who reported behavioral alterations, including loss of equilibrium, in six different species of fish exposed to 200 μg/L of rotenone (nominal concentration). Changes in the behavioral patterns of fish might be related to the neurotoxic action of rotenone, which interferes in the normal status of the central nervous system including ChE activity, as supported by our results.
Histological alterations are commonly determined in two key organs: the gills, which interact directly with the aquatic environment and are therefore considered primary exposure sites (Cengiz 2006), and the liver that has the function of metabolizing and excreting xenobiotics. In the present study, the histopathological parameters highlight the effect of rotenone on the fish target tissues. Depending on the distribution and severity of the injury, exposure to toxic substances can cause pathological changes and affect organ function. Here, we determined rotenone-induced changes in the gills and liver of rotenone-exposed fish. The gills are known to perform a variety of functions, including gas exchange, osmoregulation, excretion, and hormone metabolism (Da Cuna et al. 2011). On the other hand, the liver is involved in metabolism, excretion of xenobiotics, and protein synthesis (Ferguson 2006). We observed rotenone-induced changes in both organs, but the damage was more pronounced in the gills. As gills represent the uptake site for rotenone, they are typically sensitive to toxicity and are often the first organ to show structural and functional responses to an agent (Ba-Omar et al. 2011). In contrast, the liver usually shows toxic effects after chronic (prolonged) exposure. Since the present study examined only the short-term toxicity of rotenone, it remains possible that more pronounced liver damage could be seen following a longer exposure to this chemical.
Acute toxicity tests assess the rapid and severe effects of a given chemical on a given organism over a short period of time. The most common result is LC50—the concentration value lethal to 50 % of the organisms, which is the basis of risk assessment procedures to evaluate the relative sensitivities of aquatic organisms to chemicals. In general, zebrafish ranks among the most rotenone-sensitive fish species, with a 48-h LC50 = 22.6 μg/L and a 96-h LC50 value of 12.2 μg/L (Table 3). Such high sensitivity is also highlighted in the SSD analysis, where a HC5 value of 3.2 μg/L indicates rotenone as extremely toxic to fish species. This may reflect the route of contamination, as the breathing mechanism of a fish (the gills) comes into direct contact with the contaminated water, allowing rotenone to pass directly into the bloodstream. Even so, tolerance to rotenone is highly variable among fish species, and this difference in sensitivity can be due to the variation in the levels of liver enzymes responsible for the decomposition of rotenone. The liver is the main organ responsible for the detoxification of xenobiotics, and the formation of bile is a major detoxification pathway and one of the most likely routes for excretion. Another reason for differential rotenone tolerance is the production of alternative substrates in ATP synthesis; these substrates bypass complex I of the mitochondrial respiratory chain affected by rotenone (Ling 2003). Plhalová et al. (2010) showed that P. reticulata is more sensitive to terbutryn than D. rerio. Their results confirmed that the acute toxicity of a toxicant depends not only on age but also on the species studied. Depending on the tested substance, larvae or adults can be more sensitive, because after hatching, the embryo loses the protection of the chorion membrane, becoming fully exposed. Even not tested, juvenile stages of D. rerio could still be more sensitive than embryos.
Rotenone is widely used in the management of fisheries, to eliminate undesirable fish, but after application, rotenone and its main metabolite rotenolone can persist in the water, sediments, and biota of the aquatic environment (Finlayson 2001; Vasquez et al. 2012; Finlayson et al. 2014). Regardless of the high sensitivity of rotenone to photolysis on the top layer of surface water (half-life of 21 h in the top 1 cm of water), at 2 m deep, the toxin can persist for 191 days (USEPA 2006). It is essential to have detailed knowledge of the thresholds of rotenone toxicity against nontarget species (such as fish, zooplankton, amphibians, and reptiles). Thus, only a careful use of rotenone for selective elimination, associated with comprehensive knowledge of thresholds of toxicity for both selected and nontarget species, will permit the safe use of this pesticide without compromising the whole ecosystem.
Conclusion
Rotenone is an extremely toxic pesticide, and its use should be carefully regulated. Exposure to low concentrations of rotenone induced biochemical alterations inhibiting the activities of the oxidative stress markers CAT and GST (lowest-observed-effect concentration (LOEC) = 1 μg/L) and the neurologic biomarker ChE (LOEC = 10 μg/L). A link between ChE inhibition and behavioral alterations is suggested. Histological changes could be related to oxidative damage trigged by rotenone exposure. Rotenone is embryotoxic, provoking developmental changes in D. rerio embryos, namely lack of tail detachment, delayed somite formation, lack of pigmentation, cardiac edema, tail deformities, and delay in yolk sac absorption. Moreover, the SSD analysis showed that rotenone is extremely toxic to several fish species (HC5 = 3.2 μg/L), suggesting that after an application, effects on nontarget organisms might occur even in concentrations below micrograms per liter.
References
Ba-Omar TA, Al-Jardani S, Victor R (2011) Effects of pesticide temephos on the gills of Aphanius dispar (Pisces: Cyprinodontidae). Tissue Cell 43:29–38
Bernet D, Schmidt H, Meier W et al (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34
Betarbet R, Sherer TB, MacKenzie G et al (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Bills TD, Marking LL, Mauck WL (1981) Polychlorinated biphenyl (Aroclor 1254) residues in rainbow trout: effects on sensitivity to nine fishery chemicals. N Am J Fish Manag 1:200–203
Boogaard MA, Bills TD, Selgeby JH, Johnson DA (1996) Evaluation of piscicides for control of ruffe. N Am J Fish Manag 16:600–607
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bridges WR, Cope OB (1965) The relative toxicities of similar formulations of pyrethrum and rotenone to fish and immature stoneflies.
Broderius SJ, Kahl MD, Hoglund MD (1995) Use of joint toxic response to define the primary mode of toxic action for diverse industrial organic chemicals. Environ Toxicol Chem 14:1591–1605
Cengiz EI (2006) Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ Toxicol Pharmacol 22:200–204
Chadderton L, Kelleher S, Brow A et al (2001) Testing the efficacy of rotenone as a piscicide for New Zealand pest fish species. Manag. invasive Freshw. fish New Zealand. Proc. a Work. hosted by Dep. Conserv 10–12
Cheng WW, Farrell AP (2007) Acute and sublethal toxicities of rotenone in juvenile rainbow trout (Oncorhynchus mykiss): swimming performance and oxygen consumption. Arch Environ Contam Toxicol 52:388–396
Clairborne A (1985) Catalase activity. In: Greenwald RA (Ed.) CRC handbook of methods for oxygen radical research. Boca Raton, FL, USA. CRC Press, p 283–284
Clemens HP, Sneed KE (1959) Lethal doses of several commercial chemicals for fingerling channel catfish. US Department of the Interior, Fish and Wildlife Service
Cohen JM, Kamphake LJ, Lemke AE et al (1960) Effect of fish poisons on water supplies, part 1—removal of toxic materials. J Am Water Work Assoc 52:1551–1566
Crawford AD, Liekens S, Kamuhabwa AR et al (2011) Zebrafish bioassay-guided natural product discovery: isolation of angiogenesis inhibitors from East African medicinal plants. PLoS One 6:e14694
Cruz-Lacierda ER (1992) Toxicity of rotenone to milkfish, Chanos chanos, and tilapia, Oreochromis mossambicus. In: Shariff M, Subasinghe RP, Arthur JR (Eds). Diseases in Asian Aquaculture I. Proc First Symp Dis Asian Aquac. Manilla, Philippines. Asian Fisheries Society, pp 419–423
Da Cuna RH, Rey Vázquez G, Piol MN et al (2011) Assessment of the acute toxicity of the organochlorine pesticide endosulfan in Cichlasoma dimerus (Teleostei, Perciformes). Ecotoxicol Environ Saf 74:1065–1073
Ellman GL, Courtney KD, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fabacher DL, Chambers H (1972) Rotenone tolerance in mosquitofish. Environ Pollut 3:139–141
Ferguson HW (2006) Systemic pathology of fish. A text and atlas of normal tissues in teleosts and their responses in disease. Scotian Press, London
Finlayson BJ (2001) Introduction: Proceedings of the Symposium Rotenone in Fisheries. In: Cailteux et al. 2001, pp. 1–3. Available at: http://www.fisheries.org/units/rotenone/rewards/00intro.pdf[Internet
Finlayson BJ, Eilers JM, Huchko HA (2014) Fate and behavior of rotenone in Diamond Lake, Oregon, USA following invasive tui chub eradication. Environ Toxicol Chem 33:1650–1655
Frasco MF, Guilhermino L (2002) Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata. Fish Physiol Biochem 26:149–156
Geiger DL, Brooke LT CD (1990) Acute toxicities of organic chemicals to fathead minnows (Pimephales promelas), Volume V. Center for Lake Superior Environment Studies, University of Wisconsin-Superior
Geiger D L, Poirier S H, Brooke L T CDJ (1986) Acute toxicities of organic chemicals to fathead minnows (Pimephales promelas), Volume III. Center for Lake Superior Environment Studies, University of Wisconsin-Superior
Giasson BI, Lee VM-Y (2000) A new link between pesticides and Parkinson’s. Nat Neurosci 3:1227
Gilderhus PA (1982) Effects of an aquatic plant and suspended clay on the activity of fish toxicants. N Am J Fish Manag 2:301–306
Gingerich WH, Rach JJ (1985) Uptake, biotransformation, and elimination of rotenone by bluegills (Lepomis macrochirus). Aquat Toxicol 6:179–196
Guadaño A, González-Coloma A, de la Peña E (1998) Genotoxicity of the insecticide rotenone in cultured human lymphocytes. Mutat Res Gen Toxicol Environ Mutagen 414(1):1–7
Haag HB (1931) Toxicological studies of Derris elliptica and its constituents I. Rotenone. J Pharmacol Exp Ther 43:193–208
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione transferases. Methods Enzymol 77:398–405
Hanisch K, Küster E, Altenburger R, Gündel U (2010) Proteomic signatures of the zebrafish (Danio rerio) embryo: sensitivity and specificity in toxicity assessment of chemicals. Int J Proteomics 2010:1–13
Hashimoto Y, Nishuichi Y (1981) Establishment of bioassay methods for evaluation of acute toxicity of pesticides to aquatic organisms. J Pestic Sci 6:257–264
Hinton MJ, Eversole AG (1978) Toxicity of ten commonly used chemicals to American eels. Proc Annu Conf Southeast Assoc Fish Wildl Agencies 32:599–604
Hinton MJ, Eversole AG (1979) Toxicity of ten chemicals commonly used in aquaculture to the black eel stage of the American eel. Proc World Maric Soc Wiley Online Library, pp 554–560
Holcombe GW, Phipps GL, Sulaiman AH, Hoffman AD (1987) Simultaneous multiple species testing: acute toxicity of 13 chemicals to 12 diverse freshwater amphibian, fish, and invertebrate families. Arch Environ Contam Toxicol 16:697–710
Howland RM (1969) Interaction of antimycin A and rotenone in fish bioassays. Prog Fish Cult 31:33–34
Hung MW, Zhang ZJ, Li S et al (2012) From omics to drug metabolism and high content screen of natural product in zebrafish: a new model for discovery of neuroactive compound. Evid Based Complement Altern Med 2012:1–20
Jesus FT, Oliveira R, Silva A et al (2013) Lethal and sub lethal effects of the biocide chlorhexidine on aquatic organisms. Ecotoxicology 22:1348–1358
Ling N (2003) Rotenone: a review of its toxicity and use for fisheries management. Sci Conserv 211:1–40
Marking LL, Bills TD (1976) Toxicity of rotenone to fish in standardized laboratory tests. US Fish. Wildlife Serv. Investigation in Fish Control 72:1–11
Marking LL, Bills TD (1981) Sensitivity of four species of carp to selected fish toxicants. N Am J Fish Manag 1:51–54
Marking LL, Bills TD, Crowther JR (1984) Effects of five diets on sensitivity of rainbow trout to eleven chemicals. Prog Fish Cult 46:1–5
Mascaro UCP, Rodrigues LA, Bastos JK, Santos E, Chaves da Costa JP (1998) Valores de DL50 em peixes e no rato tratados com pó de raízes de Derris spp e suas implicações ecotoxicológicas. Pesqui Vet Bras 18:53–56
Mayer FLJ (1974) Pesticides as pollutants. In: Liptak BG (ed) Environmental engineer’s handbook. Chilton Book Co., Radnor, PA, USA, pp 405–418
Mayer FL, Ellersieck MR (1986) Manual of acute toxicity: interpretation and data base for 410 chemicals and 66 species of freshwater animals. US Department of the Interior, Fish and Wildlife Service, Washington DC
Meadows BS (1973) Toxicity of rotenone to some species of coarse fish and invertebrates. J Fish Biol 5:155–163
Melo KM, Grisolia CK, Pieczarka JC et al (2014) FISH in micronucleus test demonstrates aneugenic action of rotenone in a common freshwater fish species, Nile tilapia (Oreochromis niloticus). Mutagenesis 29:215–219
Nanda NBP, Das PC, Jena J (2009) Use of rotenone as piscicide: toxicity levels in a few common freshwater predatory and weed fishes. J Appl Aquac 21:241–249
OECD (1992) Guideline for testing chemicals. Fish Acute Toxicity Test 203:1–9
OECD (2013) Test 236: Fish Embryo Acute Toxicity (FET) Test. OECD Guideline for the Testing of Chemicals,Section 2, OECD Publishing, Paris, France. doi:10.1787\9789264203709-en
Olson LE, Marking LL (1975) Toxicity of four toxicants to green eggs of salmonids. Prog Fish Cult 37:143–147
Orciari RD (1979) Rotenone resistance of golden shiners from a periodically reclaimed pond. Trans Am Fish Soc 108:641–645
Pereira SPP, Oliveira R, Coelho S et al (2014) From sub cellular to community level: toxicity of glutaraldehyde to several aquatic organisms. Sci Total Environ 470:147–158
Plhalová L, Mácová S, Dolezelová P et al (2010) Comparison of Terbutryn acute toxicity to Danio rerio and Poecilia reticulata. Acta Vet Brno 79:593–598
Posthuma L, Suter GW II, Traas TP (2001) Species sensitivity distributions in ecotoxicology. Lewis Publishers, Boca Raton, FL, USA
Radad K, Rausch WD, Gille G (2006) Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochem Int 49:379–386
Rao KV, Chauhan SPS (1971) Teratogenic effects of rotenone on the early development of chick embryos in vitro. Teratology 4:191–197
Rowe-Rowe DT (1971) Rotenone tolerances of some freshwater fishes of Natal. Prog Fish Cult 33:206–209
Siddiqui MA, Ahmad J, Farshori NN et al (2013) Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells. Mol Cell Biochem 384:59–69
Skadsen JM, Webb PM, Kostecki PT (1980) Measurement of sublethal metabolic stress in rainbow trout (Salmo gairdneri) using automated respirometry. J Environ Sci Health B 15:193–206
SPSS (2004) Sigma Stat for Windows (version 3.10).
Srivastava P, Panda D (2007) Rotenone inhibits mammalian cell proliferation by inhibiting microtubule assembly through tubulin binding. FEBS J 274:4788–4801
Swarnkar S, Goswami P, Kamat PK et al (2013) Rotenone-induced neurotoxicity in rat brain areas: a study on neuronal and neuronal supportive cells. Neuroscience 230:172–183
Turner L, Jacobson S, Shoemaker L (2007) Risk assessment for piscicidal formulations of rotenone. Compliance Services International, Lakewood
USEPA (2006) Enviromental fate and ecological risk assessment for the registration of rotenone. Prepared by Phillips T, Steeger T, Jones R D. Washington DC, 203. Environ Fate Eff Div U S EPA
USEPA (2014) Pesticide ecotoxicity database, formerly: environmental effects database (EEDB). Environ Fate Eff Div U S EPA
Vasquez ME, Rinderneck J, Newman J, McMillin S, Finlayson B, Mekebri A, Crane D, Tjeerdema S (2012) Rotenone formulation fate in Lake Davis following the 2007 treatment. Environ Toxicol Chem 31:1032–1041
Acknowledgments
The authors acknowledge the National Council of Technological and Scientific Development (CNPq), Coordination of Improvement of Higher Education Personnel (CAPES; scholarship provided to Karina Motta Melo), Ministry of Education, and Ministry of Science and Technology of Brazil through the program Science without Borders (CNPq; BJT-A scholarship provided to Rhaul Oliveira). The authors also acknowledge the Science Support Foundation of Para (FAPESPA) for financial support through the National Program of Research Excellence (PRONEX, 011/2008) and the Portuguese Fundação para Ciência e Tecnologia (FCT) for the financial support through the grants for the authors (SFRH/BPD/31752/2006, SFRH/BD/62605/2009). CYN, JCP, and CKG are grateful to CNPq for the Bolsas de Produtividade. This study is part of the Master’s Dissertation of KMM. In memoriam José de Souza Filho.
Ethical standards
The experiments are in accordance with the current laws of the country in which they were performed. The study was approved by the ethics committee, at the Federal University of Para (reference BIO036-12).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Highlights
- Rotenone is extremely toxic for fish with a HC5 = 3.2 μg/L.
- Behavioral, structural, and developmental changes occur after exposure to low concentrations of rotenone.
- Biomarkers of oxidative (CAT and GST) and neurological (ChE) stress are inhibited by rotenone.
In memoriam of José de Souza Filho.
Rights and permissions
About this article
Cite this article
Melo, K.M., Oliveira, R., Grisolia, C.K. et al. Short-term exposure to low doses of rotenone induces developmental, biochemical, behavioral, and histological changes in fish. Environ Sci Pollut Res 22, 13926–13938 (2015). https://doi.org/10.1007/s11356-015-4596-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4596-2