Abstract
Several studies have reported that the chewing habit of smokeless tobacco (SLT) has been associated with oral cancer. The aim of the present study was to evaluate the trace levels of lead (Pb) in biological samples (blood, scalp hair) of oral cancer patients and referents of the same age group (range 30–60 years). As the concentrations of Pb are very low in biological samples, so a simple and efficient ionic liquid-based microextraction in a single syringe system has been developed, as a prior step to determination by flame atomic absorption spectrometry. In this procedure, the hydrophobic chelates of Pb with ammonium pyrrolidinedithiocarbamate (APDC) were extracted into fine droplets of 1-butyl-3-methylimidazolium hexafluorophosphate [C4MIM][PF6] within a syringe while using Triton X-114 as a dispersant. Factors influencing the microextraction efficiency and determination, such as pH of the sample, volume of [C4MIM][PF6] and Triton X-114, ligand concentration, and incubation time, were studied. To validate the proposed method, certified reference materials were analyzed and the results of Pb2+ were in good agreement with certified values. At optimum experimental values of significant variables, detection limit and enhancement factor were found to be 0.412 μg/L and 80, respectively. The coexisting ions showed no obvious negative outcome on Pb preconcentration. The proposed method was applied satisfactorily for the preconcentration of Pb2+ in acid-digested SLT and biological samples of the study population. It was observed that oral cancer patients who consumed different SLT products have 2–3-fold higher levels of Pb in scalp hair and blood samples as compared to healthy referents (p < 0.001). While 31.4–50.8 % higher levels of Pb were observed in referents chewing different SLT products as compared to nonconsumers (p < 0.01).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oral cancer is the sixth most common cancer in the world and is a common malignancy among peoples who have consumed different tobacco products (Khandekar et al. 2006; Oji and Chukwuneke 2007; Kazi et al. 2010a). The annual estimated incidence is ∼300,000 new cases (Khandekar et al. 2006); two thirds of these cases are occurring in developing countries (Ferlay et al. 2004). Oral cancer is characterized by a high rate of morbidity and mortality (Boyle and Ferlay 2005). Both tobacco smoking (cigarettes, cigars, and pipes) and chewing smokeless tobacco (SLT) products with and without other ingredients have been shown to increase the risk of developing oral cancer (Accortt et al. 2005; Kazi et al. 2010b; Rodu and Cole 2002). The International Agency for Research on Cancer recently reported that there is “sufficient evidence” that the use of SLT is carcinogenic to humans (Cogliano et al. 2004). Cancers caused by SLT products may often begin as leukoplakia or erythroplakia, which generally has a higher chance to becoming cancerous over time (Bouquot 1991).
Tobacco plant (Nicotiana tabacum) is well known for its capacity to concentrate toxic elements from its growing environment (Golia et al. 2007). Tobacco is known to contain numerous classes of carcinogenic substances such as tobacco-specific nitrosamines, which are often regarded as a major factor in smokeless tobacco-related carcinogenesis. The combined exposure to nitrosamines and other classes of organic and inorganic substances, including toxic metals, enhances the carcinogenetic effects (Stepanov and Hecht 2005). Lead (Pb) is known to be a toxic metal that accumulates in the human body throughout the lifetime (Godwin 2001). Typical symptoms of Pb poisoning are abdominal pain, anemia, headaches, chronic nephritis of the kidney, brain damage, and central nervous system disorders (Jaffe et al. 2001).
Flame atomic absorption spectrometry (FAAS) has been widely used for the determination of trace metal ions because of the relatively simple and inexpensive equipment (Citak and Tuzen 2010; Ghaedi et al. 2010). However, direct determination of metal ions at trace levels by FAAS is limited, not only due to insufficient sensitivity but also due to the matrix interference. Under these circumstances, to determine trace levels of Pb, a separation and enrichment step prior to the determinations is beneficial. Several methods have been proposed for the separation and preconcentration of trace levels of Pb including liquid–liquid extraction (LLE) (Comitre and Reis 2005), solid-phase extraction (Ghaedi et al. 2013; Gundogdu et al. 2009), cloud point extraction (Arain et al. 2013; Citak and Tuzen 2010), and liquid–liquid microextraction (LLME) (Shah et al. 2012). The use of classical extraction method requires large amounts of high purity solvents, which may also result in environmental and safety problems, due to high volatilization. Ionic liquids (ILs) consist of large organic cations like quaternary ammonium, imidazolium, or pyridinium ions combined with anions of smaller size and more symmetrical shape such as Cl−, Br−, I−, AlCl4 −, BF4 −, PF6 −, ROSO3 −, NTf2 − (bis(trifluoromethylsulfonyl) imide), triflate (trifluoromethanesulfonate), and others (Abdolmohammad-Zadeh and Sadeghi 2009). Many of the possible combinations of these ions exist as liquids at room temperature and some of them have turned out to be stable at temperatures up to 500 K (Heintz 2005). ILs are low melting point ionic compounds having unique physicochemical properties, such as broad liquid ranges, negligible vapor pressures, good thermal stabilities, nonflammability, and good extractabilities for various organic compounds and metal ions as neutral or charged complexes, as well as tunable viscosity and miscibility with water and organic solvents, which make them very attractive in separation processes (Heintz 2005; Liu et al. 2009; Marsh et al. 2004). ILs have the capability to form a wider range of intermolecular interactions than typical volatile organic solvents (Łuczak et al. 2008). Several studies have been reported in which ILs have successfully been utilized for extraction of metal ions as chelates (Haixia et al. 2007; Martinis et al. 2008). A number of new procedures with different extraction performances have been developed based on classical LLME (Baghdadi and Shemirani 2008; Martinis and Wuilloud 2010). Recently, chemists have started finding different ways to miniaturize the classical dispersive microextraction techniques (Naeemullah et al. 2013; Arain et al. 2015).
The objective of study is to present an efficient extraction method to avoid the centrifugation step, thermal dispersion, and usage of hazardous organic solvents. This novel approach uses two syringes: one 20 mL plastic syringe as extraction unit and another 1 or 3 mL syringe for the recovery of extractant. The proposed procedure was termed as ionic liquid-based microextraction in a single syringe system (SS-ILμE). All the variables, pH, incubation time, concentration of Triton X-114 and ammonium pyrrolidinedithiocarbamate (APDC), as well as the volume of the sample and IL, affecting the proposed procedure, have been studied and optimized. After optimization, the proposed method has been applied successfully to real samples.
Materials and methods
Chemicals and reagents
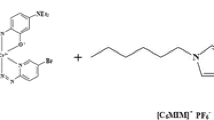
Ultrapure water obtained from ELGA LabWater system (Bucks, UK) was used throughout the work. Concentrated nitric acid (65 %) and hydrogen peroxide (30 %) were obtained from Merck (Darmstadt, Germany). All chemicals and reagents were of analytical grade. 1-Butyl-3-methylimidazolium hexafluorophosphate [C4MIM][PF6] and the nonionic surfactant, Triton X-114, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nonionic surfactant dilute solutions (0.025–0.2 % v/v) were prepared by dissolving an appropriate amount of Triton X-114 in 100 mL of distilled water; 0.1 mol/L of acetate buffer was used to control the pH of the solutions. The pH of the samples was adjusted to the desired pH (3–8) by the addition of 0.1 M HNO3/NaOH solution in acetate buffer. The certified standard solutions of Pb (1000 mg/L) and APDC were obtained from Fluka Kamica (Bush, Switzerland). HNO3 (0.2 mol/L) was used for the dilution of stock standard solution to make working standards. For the accuracy of methodology, certified reference materials (CRM) of human hair BCR 397 (Brussels, Belgium), Clincheck® control-lyophilized human whole blood (Recipe, Munich, Germany), and Virginia tobacco leaves (ICHTJ-cta-VTL-2) (Vienna, Austria) were used.
Instrumentation
A Perkin-Elmer Model AAnalyst 700 (Norwalk, CT, USA) FAAS was used. A single element hollow cathode lamp was operated at 7.5 mA at a spectral bandwidth of 0.7 nm. The analytical wavelength was set at 283.3 nm. Overall analysis was carried out with an air/acetylene flame, using 10 cm long slot-burner head. Peak heights were recorded as signals. A pH meter (Ecoscan Ion 6, Malaysia) was employed for pH adjustments. A PEL domestic microwave oven (Osaka, Japan), programmable for time and microwave power from 100 to 900 W, was used for digestion of samples.
Study population
A survey was carried out about SLT products (gutkha, mainpuri, and snuff) and the chewing and inhaling habits of both genders, age ranged 30–60 years, residing in the different cities of Sindh, Pakistan. The data of hospital-based case–control study for oral cancer patients were collected from the Nuclear Institute of Medicine and Radiotherapy (NIMRA) Jamshoro and Larkana Institute of Nuclear Medicine and Radiotherapy (LINAR), during the years 2011–2013, by collecting files and extracting important information. During a 1-year study period (2010), the information department of both hospitals recorded >5200 cases of cancers of all types, and oral cancer comprised 3.6 % of the total. Oral cancer patients were divided into subgroups according to different locations of oral cancer: lips, tongue, cheeks, floor of the mouth, hard and soft palate, sinuses, and pharynx (throat). Oral cancer patients and referents were further grouped according to their SLT chewing habits: non-SLT (NU), gutkha (GU), snuff (SU), and mainpuri users (MPU). Complete demographic information is listed in Table 1.
Physical examinations were performed to measure participant’s weight, height, blood pressure, and biochemical data. The biochemical tests of patients and referents were performed to estimate hemoglobin, red blood cells, packed cell volume, mean corpuscular hemoglobin concentration, mean corpuscular volume, and transferrin iron-binding capacity in the blood. The biochemical results and histological information are not reported in the present study. The criteria for the selection of patients were biopsy-proven oral squamous cell carcinoma prior to any treatment and patients were not taking any mineral supplements during the last 3 months. The selection criteria for the 1155 referent subjects were the same age group, socioeconomic status, dietary habits, and not taking any mineral supplement. Prior to the collection of biological samples, the referent subjects also underwent a standard routine medical examination. This study was approved by the Ethics Committee of Sindh University, working under the auspices of the Higher Education Commission of Pakistan.
Sampling of SLT products
Different brands of SLT products (n = 46) of snuff (dry and moist), gutkha, and mainpuri were purchased from local markets of the different cities of Pakistan as per their availability over a 3-year period (March 2011–January 2014). The samples were packed in their original packing and placed in prewashed dried plastic bags separately and stored at 4 °C, until tested. Five composite samples of each brand of snuff, gutkha, and mainpuri were prepared by homogenizing the mixture after removing the wrappers. Care was taken to avoid any source of contamination, and this preparation was carried out in a clean environment. All samples were dried at 80 °C. The dried samples were ground with agate mortar and pestle, sieved through nylon sieves with mesh sizes of 125 μm, and then stored in the labeled sample bottles.
Biological samples
Venous blood samples (5 mL) were collected by 7 mm heparinized lithium Vacutainer® tubes (Becton Dickinson). About 2 mL of venous blood samples were stored at −20 °C until metal analysis. The scalp hair samples were taken from the nape of the neck. The hair was tied together from 1 cm of the scalp with Teflon thread, cut with a stainless steel scissor, and pretreated as reported in our earlier work (Panhwar et al. 2013). Hair samples were put into separate plastic envelopes for each participant, tightly sealed, and attached with the identification number of the participant and questionnaire.
Microwave-assisted acid digestion (MAD)
Six replicate samples of each CRM (0.5 mL Clincheck® control-lyophilized human whole blood, 0.2 g of Virginia tobacco leaves and BCR 397 human hair) and duplicate samples of different types of SLT products (0.2 g), whole blood (0.5 mL), and scalp hair (0.2 g) were taken separately in polytetrafluoroethylene (PTFE) flasks (25 mL in capacity), which were added with 3 mL of a freshly prepared mixture of concentrated HNO3–H2O2 (2:1, v/v) and kept at room temperature for 10 min. Then, the flasks were placed in a covered PTFE container and heated at 80 % of total power (900 W) for 3–4 min. The digested samples were diluted up to 10 mL with 0.1 mol/L concentrated HNO3. A blank extraction (without sample) was carried out through the complete procedure.
Ionic liquid-based microextraction in a single syringe system
To develop the SS-ILμE system, a 20-mL plastic syringe was used as an extraction unit and another 1 mL plastic syringe was used for the injection of the ligand. Replicate 10 mL of standard solution (10–80 μg/L) whose pH was adjusted in the range of 3–8 with appropriate volume of buffer was sucked into the syringe and added with 1 mL of 0.1–0.5 % (w/v) APDC. Then, 500 μL of 0.025–0.20 % (v/v) Triton X-114 was added by an adjustable micropipette, and a cloudy solution was immediately formed. Then, 50–200 μL of IL as extractant [C4MIM][PF6] was added and slightly agitated to disperse the IL. Afterward, the piston of the syringe was slowly moved up and down to allow the recovery of IL from syringe walls. After phase separation, the aqueous phase was drawn out and the IL phase with analyte was easily recovered from the syringe. To the IL phase, 0.2 mL of acidic ethanol was added to reduce its viscosity. Samples of 100 μL were injected into FAAS through a self-made injection system of teflon funnel and Eppendorf pipette, which was connected with the capillary tubing of a nebulizer (Fig. 1).
Statistical analysis
All statistical analyses were performed using the computer program, Excel X State (Microsoft Corp., Redmond, WA, USA) and Minitab 13.2 (Minitab Inc., State College, PA, USA). Data from triplicate samples of each composite sample were expressed as means ± SD. Student’s t test was used to assess the significant difference of Pb in certified and experimentally found values. ANOVA was used to assess the significance of differences between the concentrations of Pb observed in the biological samples of patients and referent subjects, calculated by the unpaired two-sample t test. A p < 0.05 was considered as significant difference.
Results
To achieve a high recovery and enrichment factor, the influence of different parameters, such as pH, concentration of APDC, incubation time, amounts of IL, and disperser solvent, for Pb determination in biological samples by SS-ILμE was investigated and optimized.
Optimization of factors affects the SS-ILμE
The effect of pH on microextraction of Pb was investigated by using six replicate standard solutions of the analyte (10 μg/L) in the pH range of 3–8, while other parameters were at their optimum levels. Each operational desired pH value was obtained by the addition of 0.1 mol/L of HNO3/NaOH, in the presence of acetate/borate buffer. The maximum extraction efficiency was obtained at pH range 5.0–6.0. For subsequent work, pH 6.0 was selected. The concentration of APDC was used in range of 0.1 to 0.5 % (w/v). The optimum recovery of Pb is enhanced up to 0.35 % (w/v), and the APDC concentration is further increased and caused no changes in the signals. Hence, 0.35 % (w/v) of the APDC was selected for the microextraction of Pb.
The nonionic surfactant was chosen as a dispersive solvent. The solubility of “hydrophobic” IL in nonionic surfactant occurs due to the existence of significant interactions (H-bonding, dipole-induced dipole) among [C4MIM][PF6] and Triton X-114 (Zhao et al. 2008). The effect of Triton X-114 volume on Pb recovery was examined in the range of 0.025–0.20 % (v/v). The maximum recovery was obtained at 0.075 % of Triton X-114 and remained constant up to 0.125 %. It indicated that a further increase in Triton X-114 concentration results in a decrease of recovery. While at lower concentration, recovery of analyte was low because there were few molecules of Triton X-114 to disperse IL. So Triton X-114 of 0.075 % (v/v) was selected for subsequent study. The variations in recoveries against IL volume were studied in the range of 50–200 μL. The result shows that IL quantitatively extracts Pb when its volume was 100 μL. No significant changes in recoveries were observed at higher IL volume. So, 100 μL of IL was used for subsequent experimental work.
The incubation time for Pb recovery was studied in the range of 1–5 min. The optimum recovery was obtained between 2 and 3 min, so 3 min of incubation time was chosen as the optimum incubation time. In fact, when the extraction mixture (dispersant + extractant) was dispersed into the sample solution, a cloud of tiny droplets of extractant was observed, which increased the contact surface area between the two phases. Consequently, equilibrium was achieved quite quickly which is one of the main advantages of our proposed procedure. For interference study, different amounts of coexisting ions were added to standard solutions of 10 μg/L of Pb and the procedure was followed. Table 2 indicates the quantitative recovery of Pb in the presence of understudied interfering ions which proved the applicability of the proposed procedure for Pb determination in real samples.
Analytical figures of merit
In order to assess the performance of the proposed method, the following three main parameters were employed: extraction recovery (ER), enhancement factor (EF), and consumptive index (CIn). The ER was defined as the percentage of total analyte which was extracted into the IL phase:
In the above equation, m IL phase and m aq are the analyte masses in the final IL phase and the initial concentration in the sample solution, respectively. The C IL phase and C aq are the analyte concentrations in the IL phase and in the aqueous phase, respectively. The V IL phase and V aq are the concerned volumes of the phases (Cruz-Vera et al. 2009). The calibration graph for the preconcentration of Pb was linear with correlation coefficients >0.995, at the range of 10–80 μg/L. Relative standard deviation (RSD) of a minimum of six independent analyses of CRMs after SS-ILμE of Pb was <7 %. The limit of detection (LOD), calculated as the ratio of three times the standard deviation of ten blank readings to the slope of the calibration curve, was 0.412 μg/L. EF is the ratio of calibration curve slopes before and after the application of the SS-ILμE procedure and was found to be 80. Another term, CIn, is defined as:
where V s is the sample volume (in milliliters) (Cruz-Vera et al. 2009; Fang et al. 1997). The CIn obtained for the proposed method for enrichment of Pb was 0.126. Thus, CIn reflects the efficiency of sample utilization, and it is a useful tool for selecting a preconcentration method when sample amount is limited, such as in the case of body fluid analysis (Fang et al. 1997). The validity and efficiency of the analytical method were checked with certified values of human hair CRM 397, Clincheck® control-lyophilized human whole blood, and Virginia tobacco leaves (ICHTJ-cta-VTL-2) (Table 3). Paired t test was applied to compare the results obtained by SS-ILμE and showed that the t experimental value is lower than t critical (2.75) at a confidence interval of 95 % (p = 0.05), which indicated a nonsignificant difference in the obtained and certified value of Pb in CRM (Table 3). The high sensitivity and low detection limits of the present SS-ILμE method suggest that it is efficient and sensitive for the determination of very low concentrations of Pb in biological samples. A comparative study was carried out to evaluate the efficiency of SS-ILμE with other reported extraction and preconcentration methods for Pb determination, as shown in Table 4. The LOD and EF of the present study were comparatively better that those of previously reported works (Alonso et al. 2006; Arain et al. 2013; Bai et al. 2010; Gama et al. 2006; Matoso et al. 2003; Minami et al. 2005; Naseri et al. 2008; Shah et al. 2012). Therefore, the proposed method could be of key interest especially for routine analytical laboratories.
Applications
Determination of Pb in different SLT products
The pH of different types of SLT (gutkha, snuff, and mainpuri) products was highly basic, in the range of 8.2–8.9, which favors the formation of tobacco-specific amines, thus making the product potentially toxic (IARC 2007).
Multiple samples of different brands of each SLT product were analyzed, and the mean concentrations along with the standard deviation for five composite samples of each brand are provided in Table 5. The Pb level in the different brands of gutkha (G) and mainpuri (MP) were observed in the range of 1.85–5.64 and 7.44–11.9 μg/g, respectively. In brown and green moist (BM and GM) and dry brown and black snuff (DB and DBK), the concentration of Pb was found in the range of 8.46–15.7, 7.10–10.1, 4.38–6.12, and 6.05–8.64 μg/g, respectively. The concentration of Pb in different SLT products (gutkha, mainpuri, and snuff) were found in increasing order as BM > MP > GM > DBK > DB > G.
The Pb concentration in biological samples of referents and oral cancer patients
The mean concentrations with standard deviations of Pb in biological samples of referents and patients are shown in Table 6. The resulted data indicated that the concentration of Pb was significantly higher in scalp hair and blood samples of cancer patients (lips, tongue, cheeks, floor of the mouth, hard and soft palate, sinuses, and pharynx) than those of referents (p < 0.001). The Pb in scalp hair samples of male referents, NU, GU, SU, and MPU, were found in the range of 4.49–5.12, 5.70–6.21, 6.10–6.95, and 7.02–8.03 μg/g, respectively, while the range of Pb levels in scalp hair samples of male oral cancer (lips, tongue, cheeks, floor of the mouth, hard and soft palate, sinuses, and pharynx) patients were 10.5–19.7, 11.8–22.9, 11.3–19.8, 12.5–22.6, 12.9–24.6, 12.2–19.2, and 13.2–25.4 μg/g, respectively (p < 0.001) (Table 5). The same trend was observed in females. The Pb concentrations in blood samples of male referents, NU, GU, SU, and MPU, were observed in the range of 113–122, 149–161, 158–170, and 72–185 μg/L (Table 6), whereas the levels of Pb in blood samples of male with different oral cancers (lips, tongue, cheeks, floor of the mouth, hard and soft palate, sinuses, and pharynx) were found in the range of 246–302, 215–352, 221–318, 229–322, 252–362, 232–329, and 242–369 μg/L, respectively, which were significantly higher as compared to those values obtained for referents (p < 0.001) (Table 6). The same trend was observed in females.
The unpaired Student’s t test between cancerous patients and referents for different degrees of freedom was calculated at different probabilities. Our calculated t experimental exceeds that of t critical values at 95 % confidence intervals, which indicated the significant differences between the data of Pb in biological samples of oral cancer patients than referents (p < 0.001).
Discussion
A survey in Karachi indicates that 36 % of males and 44 % females chew different SLT products (Bhurgri et al. 2000). Population-based surveys from India, Pakistan, and Nepal over the past couple of decades have reported a prevalence of use of SLT products between 20 and 40 % among adolescents and adults (Gupta and Ray 2003; Qidwai et al. 2002). In Pakistan, a recent study among adolescents and adults of Karachi reported that 40 % of the population was using at least one chewable product of smokeless tobacco on a daily basis (Mazahir et al. 2006). In Pakistan and South Asian subcontinent, the popular chewing products are betel quid, betel nut, gutkha, and snuff (Johnson 2001). Oral cancer is the second most common malignancy in Pakistan. It was observed that oral cancer is now common in a younger population. According to the World Health Organization (WHO), the use of SLT products is a culturally acceptable habit in Asian countries including Pakistan (Bhurgri et al. 2006).
With regard to the habit of chewing different SLT products (gutkha and mainpuri), the referents and patients gave information that they use 2–10 packets (2–5 g) per day, kept it in the mouth for 30 min to 1 h, chewed, and mostly swallowed, and only 5 to 10 % claimed that they spitted it out, while patients suffering from different oral cancers informed that mostly at night, they kept SLT in their mouth and sleep without using any mouthwash. Therefore, it appears that more amount of SLT contents is absorbed by the buccal mucosa or posterior region of the mouth. Patients who consumed different types of snuff placed 2–3 g between the gingival and buccal mucosa, and this habit is repeated 8–15 times a day depending upon individual mood. It was observed that cancer patients, who had consumed SLT products, were not aware of the symptoms till the severity developed.
This case–control study provides data to evaluate the possible association between Pb exposure via consumption of different types of SLT products and its altered levels in the blood and scalp hair of different oral cancer patients and referents of both genders. It was observed that the Pb level varies in biological samples of referents and patients, according to the types of SLT products consumed, but the difference was not significant (p > 0.05).
The World Health Organization/Joint Expert Committee on Food Additives (WHO-JECFA) as well as the Food and Agriculture Organization/World Health Organization (FAO/WHO) has established provisional maximum tolerable daily intake (PMTDI) of Pb to be 3.6 μg/kg bodyweight/day (Baars et al. 2001). The intake of Pb via consumption of 10 g of gutkha, mainpuri, and snuff product was found in the range of 19.1–55.7, 74.3–113, and 43.1–157 μg/day/person, respectively, contributing 8.9–73 % of the PMTDI for Pb in adults (60 kg).
The present analysis, based on the dataset available on different types of oral cancer in the population of both genders, confirms that chewing SLT products could be major risk factors for oral disease. The resulted data indicated that significantly higher levels of Pb were observed in blood samples of referents GU (23.4 %), SU (35.3 %), and MPU (55.8 %) than those values obtained for referents who did not consume any SLT product (p < 0.01), while 31.4–50.8 % higher levels of Pb were observed in scalp hair samples of referents chewing different SLT products (GU, SU, and MPU) as compared to referents who did not consume any SLT products. The significantly higher levels of Pb were observed in tongue, pharynx, and hard and soft palate cancer patients as compared to referents (p < 0.001), shown in Table 6.
Many epidemiological studies have reported that oral cancer is strongly associated with tobacco and alcohol drinking (Kazi et al. 2010a; Kingsley et al. 2008). The International Agency for Research on Cancer (IARC) classified inorganic Pb as a class 2A carcinogen in 2006 (Gwini et al. 2012). Higher Pb contents were linked to cancers of the gastrointestinal, brain, breast, lung, and bladder and leukemia (Alatise and Schrauzer 2010; Gwini et al. 2012; Pasha et al. 2010; Van Wijngaarden and Dosemeci 2006). There are some lines of evidence that suggest that Pb increases the susceptibility to cancer. Pb may exert diverse toxic effects on cells, disrupting the ability of cells to develop appropriate and precise responses to genotoxic agents. It may also interfere with the ability of DNA to repair itself. By binding with histones, Pb may decrease the protection, which these proteins give to DNA, so it has direct exposure to DNA as damaging agents (Quintanilla-Vega et al. 2000; Silbergeld et al. 2000). It is reported in the literature that individuals with high levels of Pb had increased cancer-caused mortality (Lustberg and Silbergeld 2002). Substantial experimental evidence implicates oxidative stress via oxidation–reduction-inactive metal pathways for Pb, resulting in increased reactive oxygen species that lead to depletion of nitric oxide and create secondary upregulation of endothelial nitric oxide synthase (Prozialeck et al. 2008; Vaziri and Khan 2007). High exposure to Pb changes the intracellular calcium homeostasis (Silbergeld 2003). It was reported in a study that Pb induced tumors due to enhancement of cellular proliferation (Calabrese and Baldwin 1992).
The reported data of Pb in scalp hair samples of healthy human subjects were in the range of 0.22–7.26 and 4.8–5.7 μg/g, respectively (Nowak and Chmielnicka 2000; Rodushkin and Axelsson 2000). It was reported in the literature that the reference value of Pb in the blood of Nigerian referents is in the range of 18–85 μg/L (Alatise and Schrauzer 2010). On the basis of different epidemiological studies, it has been recommended that levels of blood Pb should be kept below 100 μg/L (Menke et al. 2006; Yakub and Iqbal 2010).
It was also observed in the present study that socioeconomic factors also play a role in higher mortality rates in oral cancer patients, such as poor nutrition, irregular screening, late diagnosis, and unequal access to health care due to poverty. On the other side, the cost of treatment for different types of cancer is very high, which is commonly not affordable. The local hygiene center facilities are poor in the country and there are no routine monitoring and screening carried out for those people living in small towns.
Conclusion
An efficient experimental setup for ionic liquid-based microextraction in a single syringe system is presented to determine trace levels of Pb in SLT products and biological samples. In the proposed method, the use of toxic organic extractants and dispersant solvents (i.e., chloroform, carbon tetrachloride, methanol, acetone, etc.) has been replaced with green alternative solvents such as IL and Triton X-114. Our proposed technique requires only a conventional plastic syringe as extraction unit avoiding the centrifugation step to reduce the extraction time. The present study evidenced marked significant divergences of Pb in the blood samples of oral cancer patients in comparison with the referents who consumed or not any SLT products (p < 0.001). The imbalance in Pb level in oral cancer patients could be due to change of cellular metabolism in the cancer process. Since the role of Pb in the mechanism of oral cancer development is still unclear, further detailed and comprehensive investigations are necessary.
References
Abdolmohammad-Zadeh H, Sadeghi G (2009) A novel microextraction technique based on 1-hexylpyridinium hexafluorophosphate ionic liquid for the preconcentration of zinc in water and milk samples. Anal Chim Acta 649:211–217
Accortt NA, Waterbor JW, Beall C, Howard G (2005) Cancer incidence among a cohort of smokeless tobacco users (United States). Cancer Causes Control 16:1107–1115
Alatise OI, Schrauzer GN (2010) Lead exposure: a contributing cause of the current breast cancer epidemic in Nigerian women. Biol Trace Elem Res 136:127–139
Alonso EV, Cordero MS, De Torres AG, Pavón JC (2006) Lead ultra-trace on-line preconcentration and determination using selective solid phase extraction and electrothermal atomic absorption spectrometry: applications in seawaters and biological samples. Anal Bioanal Chem 385:1178–1185
Arain SS, Kazi TG, Arain JB, Afridi HI, Brahman KD, Shah F, Arain S, Panhwar AH (2013) Simultaneous preconcentration of toxic elements in artificial saliva extract of smokeless tobacco product, mainpuri by cloud point extraction method. Ecotoxicol Environ Saf 92:289–296
Arain SS, Kazi TG, Arain AJ, Afridi HI, Baig JA, Brahman KD, Arain SA (2015) Temperature-controlled ionic liquid-based ultrasound-assisted microextraction for preconcentration of trace quantity of cadmium and nickel by using organic ligand in artificial saliva extract of smokeless tobacco products. Spectrochim Acta A Mol Biomol Spectrosc 138:387–394
Baars AJ, Theelen RMC, Janssen PJCM, Hesse JM, Van Apeldoorn MEV, Van Meijerink MCM, Verdam L, Zeilmaker MJ (2001) Re-evaluation of human-toxicological maximum permissible risk levels. RIVM Report 711701025, p 297
Baghdadi M, Shemirani F (2008) Cold-induced aggregation microextraction: a novel sample preparation technique based on ionic liquids. Anal Chim Acta 613:56–63
Bai H, Zhou Q, Xie G, Xiao J (2010) Temperature-controlled ionic liquid–liquid-phase microextraction for the pre-concentration of lead from environmental samples prior to flame atomic absorption spectrometry. Talanta 80:1638–1642
Bhurgri Y, Bhurgri A, Hassan SH, Zaidi S, Rahim A, Sankaranarayanan R, Parkin DM (2000) Cancer incidence in Karachi, Pakistan: first results from Karachi cancer registry. Int J Cancer 85:325–329
Bhurgri Y, Bhurgri A, Nishter S, Ahmed A, Usman A, Pervez S, Kayani N, Ahmed R, Hassan SH, Riaz A, Bhurgri H, Bashir I (2006) Pakistan-country profile of cancer and cancer control 1995–2004. J Pak Med Assoc 56:124–130
Bouquot J (1991) Reviewing oral leukoplakia: clinical concepts for the 1990s. J Am Dent Assoc 122:80–82 (1939)
Boyle P, Ferlay J (2005) Cancer incidence and mortality in Europe, 2004. Ann Oncol 16:481–488
Calabrese EJ, Baldwin LA (1992) Lead-induced cell proliferation and organ-specific tumorigenicity. Drug Metab Rev 24:409–416
Citak D, Tuzen M (2010) A novel preconcentration procedure using cloud point extraction for determination of lead, cobalt and copper in water and food samples using flame atomic absorption spectrometry. Food Chem Toxicol 48:1399–1404
Cogliano V, Straif K, Baan R, Grosse Y, Secretan B, Ghissassi FE (2004) Smokeless tobacco and tobacco-related nitrosamines. Lancet Oncol 5:708
Comitre ALD, Reis BF (2005) Automatic flow procedure based on multicommutation exploiting liquid–liquid extraction for spectrophotometric lead determination in plant material. Talanta 65:846–852
Cruz-Vera M, Lucena R, Cárdenas S, Valcarcel M (2009) One-step in-syringe ionic liquid-based dispersive liquid–liquid microextraction. J Chromatogr A 1216:6459–6465
Fang Z-L, Liu Z-S, Shen Q (1997) Combination of flow injection with capillary electrophoresis. Part I. The basic system. Anal Chim Acta 346:135–143
Ferlay J, Pisani P, Parkin DM (2004) Cancer incidence, mortality and prevalence worldwide. IARC Cancer Base (2002 estimates). IARC Press, Lyon
Gama EM, da Silva Lima A, Lemos VA (2006) Preconcentration system for cadmium and lead determination in environmental samples using polyurethane foam/Me-BTANC. J Hazard Mater 136:757–762
Ghaedi M, Niknam K, Taheri K, Hossainian H, Soylak M (2010) Flame atomic absorption spectrometric determination of copper, zinc and manganese after solid-phase extraction using 2,6-dichlorophenyl-3,3-bis (indolyl) methane loaded on Amberlite XAD-16. Food Chem Toxicol 48:891–897
Ghaedi M, Tashkhourian J, Montazerozohori M, Soylak M (2013) Silver nanoparticle loaded on activated carbon and activated carbon modified with 2-(4-isopropylbenzylideneamino) thiophenol (IPBATP) as new sorbents for trace metal ions enrichment. Int J Environ Anal 93:386–400
Godwin HA (2001) The biological chemistry of lead. Curr Opin Chem Biol 5:223–227
Golia E, Dimirkou A, Mitsios I (2007) Accumulation of metals on tobacco leaves (primings) grown in an agricultural area in relation to soil. Bull Environ Contam Toxicol 79:158–162
Gundogdu A, Ozdes D, Duran C, Bulut VN, Soylak M, Senturk HB (2009) Biosorption of Pb (II) ions from aqueous solution by pine bark (Pinus brutia Ten.). Chem Eng J 153:62–69
Gupta PC, Ray CS (2003) Smokeless tobacco and health in India and South Asia. Respirology 8:419–431
Gwini S, MacFarlane E, Del Monaco A, McLean D, Pisaniello D, Benke GP, Sim MR (2012) Cancer incidence, mortality, and blood lead levels among workers exposed to inorganic lead. Ann Epidemiol 22:270–276
Haixia S, Zaijun L, Ming L (2007) Ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate as a solvent for extraction of lead in environmental water samples with detection by graphite furnace atomic absorption spectrometry. Microchim Acta 159:95–100
Heintz A (2005) Recent developments in thermodynamics and thermophysics of non-aqueous mixtures containing ionic liquids. A review. J Chem Thermodyn 37:525–535
International Agency for Research on Cancer (2007) Smokeless tobacco and tobacco-specific nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 89. IARC Scientific Publications, Lyon
Jaffe EK, Martins J, Li J, Kervinen J, Dunbrack RL (2001) The molecular mechanism of lead inhibition of human porphobilinogen synthase. J Biol Chem 276:1531–1537
Johnson N (2001) Tobacco use and oral cancer: a global perspective. J Dent Educ 65:328–339
Kazi TG, Wadhwa SK, Afridi HI, Kazi N, Kandhro GA, Baig JA, Shah AQ, Kolachi NF, Arain MB (2010a) Interaction of cadmium and zinc in biological samples of smokers and chewing tobacco female mouth cancer patients. J Hazard Mater 176:985–991
Kazi TG, Wadhwa SK, Afridi HI, Kazi N, Kandhro GA, Baig JA, Shah AQ, Kolachi NF, Khan S (2010b) Evaluation of cadmium and zinc in biological samples of tobacco and alcohol user male mouth cancer patients. Hum Exp Toxicol 29:221–230
Khandekar S, Bagdey P, Tiwari R (2006) Oral cancer and some epidemiological factors: a hospital based study. Indian J Community Med 31:157–159
Kingsley K, O’Malley S, Ditmyer M, Chino M (2008) Analysis of oral cancer epidemiology in the US reveals state-specific trends: implications for oral cancer prevention. BMC Public Health 8:87
Liu R, Liu JF, Yin YG, Hu XL, Jiang GB (2009) Ionic liquids in sample preparation. Anal Bioanal Chem 393:871–883
Łuczak J, Hupka J, Thoming J, Jungnickel C (2008) Self-organization of imidazolium ionic liquids in aqueous solution. Colloids Surf A Physicochem Eng Asp 329:125–133
Lustberg M, Silbergeld E (2002) Blood lead levels and mortality. Arch Intern Med 162:2443–2449
Marsh K, Boxall J, Lichtenthaler R (2004) Room temperature ionic liquids and their mixtures—a review. Fluid Phase Equilib 219:93–98
Martinis EM, Wuilloud RG (2010) Cold vapor ionic liquid-assisted headspace single-drop microextraction: a novel preconcentration technique for mercury species determination in complex matrix samples. J Anal At Spectrom 25:1432–1439
Martinis EM, Olsina RA, Altamirano JC, Wuilloud RG (2008) Sensitive determination of cadmium in water samples by room temperature ionic liquid-based preconcentration and electrothermal atomic absorption spectrometry. Anal Chim Acta 628:41–48
Matoso E, Kubota L, Cadore S (2003) Use of silica gel chemically modified with zirconium phosphate for preconcentration and determination of lead and copper by flame atomic absorption spectrometry. Talanta 60:1105–1111
Mazahir S et al (2006) Socio-demographic correlates of betel, areca and smokeless tobacco use as a high risk behavior for head and neck cancers in a squatter settlement of Karachi, Pakistan. Subst Abuse Treat Prev Policy 1:10
Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E (2006) Blood lead below 0.48 μmol/L (10 μg/dL) and mortality among US adults. Circulation 114:1388–1394
Minami T, Sohrin Y, Ueda J (2005) Determination of chromium, copper and lead in river water by graphite-furnace atomic absorption spectrometry after coprecipitation with terbium hydroxide. Anal Sci 21:1519–1522
Naeemullah, Tuzen M, Kazi TG, Citak D, Soylak M (2013) Pressure-assisted ionic liquid dispersive microextraction of vanadium coupled with electrothermal atomic absorption spectrometry. J Anal At Spectrom 28:1441–1445
Naseri MT, Hosseini MRM, Assadi Y, Kiani A (2008) Rapid determination of lead in water samples by dispersive liquid–liquid microextraction coupled with electrothermal atomic absorption spectrometry. Talanta 75:56–62
Nowak B, Chmielnicka J (2000) Relationship of lead and cadmium to essential elements in hair, teeth, and nails of environmentally exposed people. Ecotoxicol Environ Saf 46:265–274
Oji C, Chukwuneke Fn (2007) Oral cancer in Enugu, Nigeria, 1998–2003. Br J Oral Maxillofac Surg 45:298–301
Panhwar AH, Kazi TG, Afridi HI, Shaikh HR, Arain SA, Arain SS, Brahman KD (2013) Evaluation of calcium and magnesium in scalp hair samples of population consuming different drinking water: risk of kidney stone. Biol Trace Elem Res 156:67–73
Pasha Q, Malik SA, Shaheen N, Shah MH (2010) Investigation of trace metals in the blood plasma and scalp hair of gastrointestinal cancer patients in comparison with controls. Clin Chim Acta 411:531–539
Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD (2008) The vascular system as a target of metal toxicity. Toxicol Sci 102:207–218
Qidwai W, Saleheen D, Saleem S, Andrades M, Azam S (2002) Are our people health conscious? Results of a patients survey in Karachi, Pakistan. J Ayub Med Coll Abbottabad 15:10–13
Quintanilla-Vega B, Hoover D, Bal W, Silbergeld EK, Waalkes MP, Anderson LD (2000) Lead effects on protamine–DNA binding. Am J Ind Med 38:324–329
Rodu B, Cole P (2002) Smokeless tobacco use and cancer of the upper respiratory tract. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:511–515
Rodushkin I, Axelsson MD (2000) Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part II. A study of the inhabitants of northern Sweden. Sci Total Environ 262:21–36
Shah F, Yilmaz E, Kazi TG, Afridi HI, Soylak M (2012) Vortex-assisted liquid–liquid microextraction coupled to flame atomic absorption spectrometry for lead determination: ionic liquid based microextraction using Triton X-100 as dispersant. Anal Methods 4:4091–4095
Silbergeld EK (2003) Facilitative mechanisms of lead as a carcinogen. Mutat Res Fundam Mol Mech 533:121–133
Silbergeld EK, Waalkes M, Rice JM (2000) Lead as a carcinogen: experimental evidence and mechanisms of action. Am J Ind Med 38:316–323
Stepanov I, Hecht SS (2005) Tobacco-specific nitrosamines and their pyridine-N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers 14:885–891
Van Wijngaarden E, Dosemeci M (2006) Brain cancer mortality and potential occupational exposure to lead: findings from the National Longitudinal Mortality Study, 1979–1989. Int J Cancer 119:1136–1144
Vaziri N, Khan M (2007) Interplay of reactive oxygen species and nitric oxide in the pathogenesis of experimental lead-induced hypertension. Clin Exp Pharmacol Physiol 34:920–925
Yakub M, Iqbal MP (2010) Association of blood lead (Pb) and plasma homocysteine: a cross sectional survey in Karachi, Pakistan. PLoS One 5:11706
Yousefi SR, Shemirani F (2010) Development of a robust ionic liquid–based dispersive liquid–liquid microextraction against high concentration of salt for preconcentration of trace metals in saline aqueous samples: application to the determination of Pb and Cd. Anal Chim Acta 669:25–31
Zhao FQ, Li J, Zeng BZ (2008) Coupling of ionic liquid-based headspace single-drop microextraction with GC for sensitive detection of phenols. J Sep Sci 31:3045–3049
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Arain, S.S., Kazi, T.G., Arain, A.J. et al. Estimation of lead in biological samples of oral cancer patients chewing smokeless tobacco products by ionic liquid-based microextraction in a single syringe system. Environ Sci Pollut Res 22, 12396–12406 (2015). https://doi.org/10.1007/s11356-015-4536-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4536-1